Recently, multiple biologics have been approved for the treatment of chronic plaque psoriasis. One topic of interest is whether gender influences drug efficacy. Recent studies claim that gender does not affect biologics treatment response in psoriasis patients [1]. However, women are underrepresented in clinical studies that assess the efficacy and safety of new treatments.
Recently, multiple biologics have been approved for the treatment of chronic plaque psoriasis. One topic of interest is whether gender influences drug efficacy. Recent studies claim that gender does not affect biologics treatment response in psoriasis patients [1]. However, women are underrepresented in clinical studies that assess the efficacy and safety of new treatments. As a result, outcomes of these studies may be less precise when it comes to treating women. A distinct difference in men and women is hormones. For example, psoriasis symptoms tend to worsen during the post-partum period, prior to menses and at menopause when there are lower levels of estrogen and progesterone [2]. With limited gender analyses in these biologics studies, there is likely a disparity in gender-specific management when it comes to treatment options for those with plaque psoriasis. Our objective is to examine the gender ratios for recently published phase 3 biologic studies since the year 2000 to quantify this unconscious gender bias.
We conducted a literature search of various biologics in the treatment of plaque psoriasis to identify gender disparities in existing phase 3 studies. Using the PubMed database, we searched for biologics and biologic similar including but not limited to: ABP501, adalimumab, BI695501, brodalumab, certolizumab, CT_P13, etanercept, guselkumab, GP1111, GP2015, GP2017, infliximab, ixekizumab, SB2, SB4, SB5, secukinumab, ustekinumab, and tildrakizumab. The search also included the following key words: “plaque psoriasis” and “phase 3” or “phase III”. We excluded articles published before the year 2000 and those that were sub-analyses of original studies. We also reviewed citations within articles to identify relevant sources. The percentage of men and women in each study was calculated to standardize the proportion of gender. To test for gender differences, we performed t-test with adjustment for intra-study correlation.
A total of 65 studies were included in this literature review for analysis. There was a significant difference in the percentage of males (M=68.7%, SD=5.5%, R= 55.9-85.1%, N=65) and females (M=31.3%, SD=5.5%, R=14.9-44.1%, N=65) enrolled in each study (p<0.0001). All the studies had more men enrolled than females in each, with 97% having over 60% males enrolled.
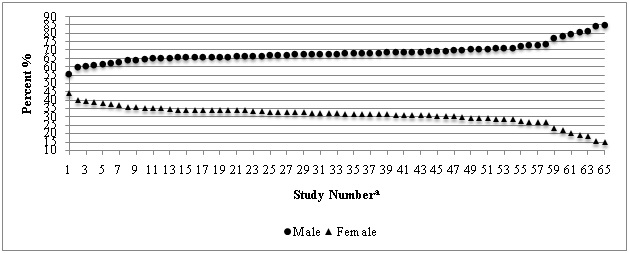 Figure 1:
Figure 1: Percent gender enrollment in psoriasis biologics clinical trials.
a. Each pair of data points represents an individual clinical trial that was included in our analyses. A total of 65 studies were included.
Our findings show a clear gender bias in biologics research. This bias may be due to various factors that hinder women from recruitment into biologics studies, one reason being that women must fulfill more stringent qualifications to be enrolled into clinical trials. For example, women often have to be on two forms of contraception, whereas men do not. The results of these major phase 3 studies that have disproportionately more men means that the treatment results may not apply to women. In order to properly assess the efficacy of a medication for males and females, future studies should look towards accounting an equal number in gender during recruitment. These findings also support that gender disparities in medical treatment exist. For example, a recent study showed that female patients are less satisfied with biological treatments for psoriasis and experienced more side effects compared to male patients [3]. Psoriasis is a complex disease, and understanding its effects in both men and women can improve the therapies available for treatment.

 Figure 1: Percent gender enrollment in psoriasis biologics clinical trials.
Figure 1: Percent gender enrollment in psoriasis biologics clinical trials.
