
Giant malignant Chondroid Syringoma: Case Report and Literature Review
*Corresponding Author(s):
Roney Gonçalves Fechine FeitosaDepartment Of Surgery, Division Of Plastic Surgery, Escola Paulista De Medicina, Universidade Federal De São Paulo, São Paulo, Brazil
Tel:+55 1155764848,
Email:dr.an@bol.com.br
Abstract
Chondroitin syringoma, also known as cutaneous mixed tumor, is a rare type of sweat gland tumor, accounting for 0.01% to 0.1% of all primary tumors of the skin. The malignant form is extremely rare, with 41 cases described so far. It predominates in the trunk and distal extremities and affects women more frequently, with a 3: 2 rate 6,7. Patients may reach the health service with large lesions due to the fact of being slow-growing lesions and have a high rate of recurrence, requiring surgical treatment that may lead to extensive defects making complex reconstructions necessary. We present a case of malignant chondroid syringoma in an 80 years old male patient, with 29 years of evolution and unusual size. A reconstruction was performed with a muscular dorsal flap of the latissimus dorsi with good aesthetic and functional results, with no signs of recurrence after 8 months of follow-up. It is concluded that because it is a late diagnosis neoplasia, the surgical treatment ends up generating expressive defects. There for musculocutaneous should be considered as an option for reconstruction, aiming optimal functional and aesthetic restoration.
Keywords
Latissimus dorsi flap; Malignant chondroid syringoma; Mixed skin tumor; Upper limb reconstruction
Introduction
Chondroid syringoma, also known as cutaneous mixed tumor, is a rare type of sweat gland tumor, accounting for 0.01% to 0.1% of all primary tumors of the skin [1,2]. It is a benign lesion, histological and immunohistochemical similar to the benign mixed tumors of the salivary gland (pleomorphic adenoma) [3].
Most of chondroid syringomas are located in the head and neck region (80%), commonly involving the nose and malar region, and reach dimensions smaller than 3cm, although tumors up to 10cm have already been described. They occur normally in the sixth decade of life, predominantly in men, with a ratio of 2: 1 [1,4].
The malignant form is extremely rare, predominant in the trunk and distal extremities, presenting as a firm, subcutaneous nodule of slow growth [5], it affects women more frequently, with a ratio of 3: 2 [1,6] and has only about 40 cases described until now [4,6-8].
Diagnosis is made essentially by histological study, and most tumors have a high recurrence rate. It is also worth mentioning that overlapping areas of benign and malignant tumor may occur in a primarily benign tumor [9].
It frequently presents local and / or regional metastases (about 60%), especially for lymph nodes, lungs and bones; In addition, it has a mortality rate of approximately 25% after a long evolutional course.
First-line treatment is based on the surgical resection of the lesion after performing adequate staging based on imaging studies [1,6,7].
Because these tumors are rare, recurrent and insidious, these patients may reach the health service with lesion of large dimensions. This fact associated with the surgical treatment of excision may lead to the development of extensive defects requiring complex reconstruction [1,10,11]. The involvement of upper limbs is infrequent, but large tumors on this topography requires a type of reconstruction that may be challenging for the plastic surgeon [10]. Thus, the use of the dorsal musculocutaneous flap may be one of the best options to be used [10,12].
We present a case of malignant chondroid syringoma in an 80 years old male patient, with 29 years of evolution and unusual increased dimensions.
Case Report
Male patient, 80 years old, with complaint of right arm lesion with progressive growth lasting for more than 20 years. He presents a surgical history of 6 procedures for tumor`s excision, with recurrence in all episodes, and last resection was performed 7 years earlier. Physical exam showed, in the right deltoid region, lobulated tumor of approximately 20 cm, not adhered to deep planes, with central ulceration of approximately 3 cm. Brachial, radial and ulnar pulses were present and without signs of nerve compression (Figure 1). Magnetic resonance imaging of the right upper limb revealed a massive multiloculate formation of approximately 20 x 10 cm, without neurovascular bundle infiltration. Regarding muscular planes invasion, a well defined cleavage plane between the mass and the triceps` belly in the middle and lower thirds of the mass. On the middle third of the arm there was no clear cleavage plane between the mass and the triceps as well as between the anterior component of the mass and the biceps (Figure 2). Other staging exams (chest, abdomen, and pelvis tomography) showed no signs of systemic involvement. An incisional biopsy with diagnosis of chondroid syringoma was performed [13-17].
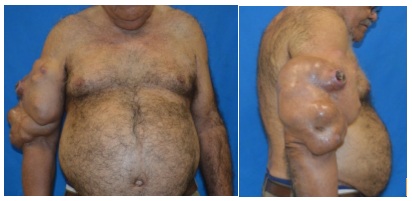 Figure 1: Magnetic resonance imaging of the right upper limb revealed a massive multiloculate formation of approximately 20 x 10 cm, without neurovascular bundle infiltration.
Figure 1: Magnetic resonance imaging of the right upper limb revealed a massive multiloculate formation of approximately 20 x 10 cm, without neurovascular bundle infiltration.
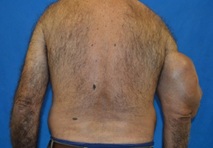 Figure 2: Anterior component of the mass and the biceps.
Figure 2: Anterior component of the mass and the biceps.
Local resection of the lesion and immediate reconstruction of the right upper limb with pedicled latissimus dorsi flap associated with split-thickness skin grafting (Figures 3 and 4) was performed. The anatomopathological result was of malignant chondroid syringoma of 26cm, ulcerated, with areas of necrosis and hemorrhage and areas with capsule and adipose tissue invasion. No angiolymphatic and perineural invasion were detected. All margins were free of disease [18-23].
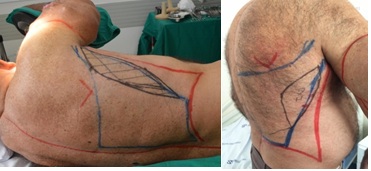 Figure 3: Right upper limb with pedicled latissimus dorsi flap.
Figure 3: Right upper limb with pedicled latissimus dorsi flap.
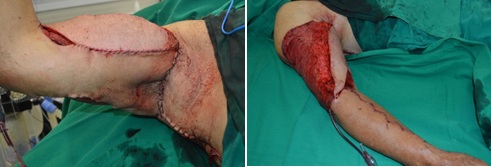 Figure 4: Split-thickness skin grafting.
Figure 4: Split-thickness skin grafting.
The patient progressed satisfactorily, leaving the hospital on the 5th postoperative day. He is currently undergoing outpatient follow-up (Figure 5), with no signs of tumor recurrence after 2 years of surgery.
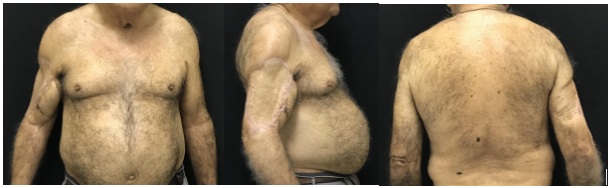 Figure 5: With no signs of tumor recurrence after 2 years of surgery.
Figure 5: With no signs of tumor recurrence after 2 years of surgery.
Discussion
The term chondroid syringoma was first used by Hirsch and Helwing in 1961 to designate a skin tumor previously known as a mixed skin tumor originating from sweat glands. Malignant Chondroid Syringoma is an extremely rare cutaneous tumor, unlike its benign form which is relatively common. According to the MEDLINE database, only 41 cases of malignant form have been reported previously since 1961 [4].
Clinically there is no distinction in appearance between the benign and malignant form. Malignant form usually presents as a non-ulcerated nodule. This type of tumor sometimes appears well circumscribed, with cystic appearance, however in other cases it may be adhered to the deep planes.
From 41 cases of malignant chondroid syringoma described so far, 26 occurred in women, and 15 cases in men and with a ratio of 2: 7 in the benign form. The mean age in males was 49.2 years (ranging from 9 months to 70 years of age) (ie distribution by all age groups) and the most commonly affected sites were hands and feet (20 patients), followed By the head (n = 13) and the trunk (n = 8) (Table 1).
|
|
|||||||||||
|
|
Author |
Journal |
Year |
Age |
Gender |
location |
size (cm) |
Local recurrence |
Metastasis |
Treatment |
Evolution |
|
1 |
Casteleiro Roca, et al. [24] |
CIRUGÍA PLÁSTICA IBERO-LATINOAMERICANA |
2009 |
68 |
F |
Left arm |
21 |
no |
Left lung 6mm / left axillary lymph node |
Extirpation with broad margins and primary closure + chemotherapy (6 cycles with Ifosmamide, Mesna, Adriamycin and Dacarbacin). |
DFS 2 years |
|
2 |
Daniel Chang et al [25] |
J Bras Patol Med |
2007 |
54 |
M |
Sternal |
0,9 |
no |
no |
Extirpation with broad margins and primary closure |
DFS 3 years |
|
3 |
Rajesh Malik, et al. [26] |
Indian Dermatol Online J. |
2013 |
61 |
F |
scalp |
Great vegetation - pedicle of 8.0 |
no |
Local invasion until dura-mater |
Extirpation with margins and primary closure |
Death on 2nd PO - meningitis was the most likely cause |
|
4 |
Arvind Krishnamurthy, et al. [27] |
Indian J Nucl Med |
2015 |
41 |
M |
Left ear |
3 |
no |
Left cervical lymph node - simultaneous diagnosis |
Excision with margins + local flap + left neck dissection |
Not avaliable |
|
5 |
Deniz Tural, et al. [28] |
Case Reports in Oncological Medicine |
2013 |
34 |
F |
face |
1,5 |
no |
no |
Excision with 1,0 cm margins (closure not informed) |
DFS 3 year |
|
6 |
P Shashikala, et al. [29] |
Indian J DermatolVenereolLeprol |
2004 |
32 |
F |
scalp |
5 |
no |
no |
Excision with margins |
Loss of follw-up |
|
7 |
Akira Watarai, et al. [30] |
Dermatology Online Journal |
2011 |
46 |
M |
Right foot |
3 |
no |
Right Inguinal lymph node 12 years after primary tumor |
Excision with margins (t primary tumor ) + lymphadenectomy + RT and chemotherapy (metastasis) tegafur, gimeracil, and oteracil potassium |
DFS 18 months after metastasis treatment |
|
8 |
Eiji Ishimura, et al. [31] |
Câncer |
1983 |
60 |
M |
|
7 |
yes after 3 years (10 x 4cm) |
7 years bilateral cervical lymph nodes / 11 years widespread |
Tumor excision, local recurrence and cervical lymph nodes |
Death after11 years |
|
9 |
Clara Redono, et al. [32] |
Câncer |
1982 |
61 |
F |
Right foot |
2,5 |
yes - 4 previous relapses |
Inguinal 7cm, multiple pulmonary nodules, |
Chemotherapy vinblastine, vincristine, and Genoxal no response |
Palliative treatment and loss of follow-up |
|
10 |
Vivek Agrawal, et al. [33] |
The Journal of Dermatology |
1998 |
40 |
F |
scalp |
6 |
Yes - 3 previous relapses |
Bilateral occipital lymph node |
Excision with margins + local flap + excision of affected lymph nodes + RT post-op |
DFS 25 moths |
|
11 |
S Nicolaou, et al. [34] |
Australasian Radiology |
2001 |
54 |
M |
Right hand |
4,5 |
no |
no |
Excision with margins |
not informed |
|
12 |
Joa˜o Luiz, et al. [35] |
The Journal of Craniofacial Surgery |
2012 |
31 |
F |
scalp (occipital) |
Not informed |
no |
CNS local invasion |
ressection |
not informed |
|
13 |
Celia Requena, et al. [36] |
Am J Dermatopathol |
2013 |
82 |
M |
glabelar |
Not informed |
no |
iFrontal and ethmoid bone invasion |
Extensive resection with local flap (skin + duramater) + radiotherapy |
not informed |
|
14 |
Hayato Takahashi, et al. [37] |
Am J Dermatopathol |
2004 |
22 |
F |
halux E |
2,2 |
sim |
invasaoossea |
amputação com margem de 3cm |
SED 20 meses |
|
15 |
James C Steinmetz, et al. [38] |
Journal of the American Academy of Dermatology |
1990 |
59 |
M |
escápula D |
4 |
não |
linfonodal mediastinal, disseminada |
biópsia excisíonàl + amplia;ao de margens |
obito 9 semanas apos a cirurgia |
|
16 |
Hirsch and Helwig [39] |
|
|
50 |
F |
face |
nãoinformado |
não |
não |
|
SED 18 meses |
|
17 |
Sharvill [40] |
Am J Dermatopathol |
1986 |
39 |
F |
punho D |
2 |
sim apos 36 meses |
não |
|
sem informa;áo apos a recorrência local |
|
18 |
Rosborough |
|
|
83 |
F |
braço E |
3 |
nao |
linfonodalaxilar |
|
SED 17 meses |
|
19 |
Matz, et al. |
|
|
80 |
F |
courocabeludo |
5 |
sim |
linfonodal cervical, disseminada |
|
óbito apos 84 meses |
|
20 |
Schremmer |
|
|
55 |
M |
dedo |
3 |
nao |
linfonodalaxilar, pulmonar |
|
nãoinformado |
|
21 |
Hilton, et al. [41] |
|
|
14 |
F |
bra;o E |
3,7 |
sim |
linfonodalaxilar |
|
SED 17 anos |
|
22 |
Lucas and Nordby |
|
|
74 |
F |
mao E |
nãoinformado |
sim |
não |
|
SED 24 meses |
|
23 |
Webb and Stott [42] |
|
|
52 |
F |
coxa D |
10 |
sim |
linfonodal inguinal |
|
SED 18 meses |
|
24 |
Botha and Kahn [43] |
|
|
15 |
F |
orelha E |
nãoinformado |
sim |
não |
|
SED 6 anos |
|
25 |
Dissanayake and Salm [44] |
|
|
79 |
F |
sacro |
8 |
nao |
pulmonar |
|
óbito apos 1 ano |
|
26 |
Harrist et al. (2 cases) [45] |
|
|
33 |
M |
pe E |
nãoinformado |
nao |
pulmonar |
|
perdeuseguimento |
|
27 |
|
|
|
70 |
M |
pe E |
8 |
sim |
linfonodal inguinal, ossea, pulmonar |
|
óbito apos 7 anos |
|
28 |
DeMoraes et al. [46] |
|
|
23 |
F |
perna D |
4 |
nao |
supraclavicular, pulmonar |
|
nãoinformado |
|
29 |
Shvili and Rothern [40] |
|
|
44 |
F |
nádegas |
5 |
nao |
linfonodal inguinal, disseminada |
|
obito apos 6 meses |
|
30 |
Hermann, et al. [47] |
Skeletal Radiologia |
1987 |
13 |
F |
courocabeludo |
1,4 |
sim - 3 recidivas |
linfonodal cervical, óssea |
|
obito apos 34 meses |
|
31 |
Clark [48] |
Conn Med. |
1987 |
74 |
F |
coxa D |
8 |
nãoinformado |
nãoinformado |
|
nãoinformado |
|
32 |
S Vohra et al [49] |
The Foot |
1996 |
39 |
M |
Hilux D |
3 |
nao |
nao |
amputa;ao parcial do halux |
SED 6 meses |
|
33 |
Consuelo Sa ? Nchez Herreros, et al. [50] |
Dermatol Surg |
2011 |
93 |
F |
nariz |
2,5 |
nao |
nao |
cirurgia micrografia de Mohs + reconstrua;áo com retalho fronta; |
SED 48 meses |
|
34 |
John L Kiely, et al. [51] |
Thorax |
1997 |
50 |
F |
mao E |
nãoinformado |
sim |
pulmonar 17 anos apos primario |
exerese do primário, sem teto das metástases |
nãoinformado |
|
35 |
Sun TB, et al. [52] |
Journal of the Formosan Medical Association |
1996 |
9 |
M |
Pe D |
|
sim 3 vezesem 10 anos |
óssea, apos 1 anodisseminada |
exegese do tumor, amputa;ao transitai |
obito 36 meses apos as primeiras metástases e amputa;ao |
|
36 |
Barnett MD, et al. [53] |
Am J Clin Oncol |
2000 |
34 |
M |
Pe D |
|
sim |
|
|
|
|
37 |
Medina Henriquez JA et al. [54] |
Scand J PlastReconstr Surg Hand Surg. |
2001 |
37 |
M |
Mao |
|
não |
não |
amputa;ao + retalho antebraquial reverso |
SED 5 anos |
|
38 |
Menéndez RH et al. [55] |
J Neurosurg Spine |
2015 |
63 |
F |
|
|
|
subdural (T9) |
resseccao da meta + radioterapia |
SED 2 anos |
|
39 |
Solomonov A, et al. [56] |
Respiration |
2001 |
65 |
M |
parede abdominal |
|
|
|
ressecção + radioterapia / braquiterapia para meta |
boa resposta (?) |
|
40 |
Kiran Mishra, and Sarla Agarwal [33] |
Acta Cytologica |
1998 |
40 |
F |
courocabeludo |
5 |
nãoinformado |
linfonodal cervical |
ressecção + linfadenectomia |
nãoinformado |
|
41 |
Hong JJ, et al [57] |
Dermatol Surg |
1995 |
40 |
M |
suprapubico |
7 |
sim |
|
exerese |
|
Table 1: 41 cases of malignant chondroid syringoma.
The reported case is distinguished by the location in the proximal region of the upper limb and by the large dimensions, with extremely slow evolution in 20 years.
Malignant chondroid syringoma tend to follow an unpredictable clinical course. the reported cases, including ours, more than 34% had local recurrence. It frequently presents local and / or regional metastases (about 63%), with a tendency to invasion of adjacent structures, with cases of bone and CNS invasion. Distant metastases were mainly for lymph nodes, lungs and bones. The mortality rate is approximately 17% after a prolonged evolutive course due to disseminated metastases [9].
Diagnosis is made essentially by histological study. The panoramic view shows asymmetry and little circumscription, with small clusters of epithelial neoplasia at a certain distance from the main malignant mass. The tumor has an epithelial and non-epithelial component with a remarkable amount of mucin. The epithelial component is composed of tubular or solid aggregates of polygonal or plasmacytoid cells, ranging from relatively monomorphic to extremely pleomorphic. Variations in the size and shape of neoplastic cell aggregates are common, with large nuclei and abundant mitotic figures, and often some areas of necrosis. The stroma is usually myxoid, but it might present as chondroid and even osteoid [8]. Immunohistochemistry haven’t played a import role on differential diagnosis due to a small number of cases studied, with variable immunohistochemical results [8].
The currently recommended treatment is surgical excision with inclusion of tumor free tissue to ensure complete tumor removal [2]. Although several reported cases have presented adjuvant treatment with chemotherapy and radiotherapy, there is no evidence of its benefit [13].
Since most of the cases described are small, circumscribed nodular tumors, the necessity for flap reconstruction is not frequent. We found in the literature cases that required local flaps due to location or dimensions and amputations of distal extremities due to local bone invasion or impossibility of free margins, without any description distal flaps requirement. However, its insidious evolution makes it prone to late diagnosis especially in countries with more difficult access to health services, which leads to complex defects requiring adequate reconstruction.
Considering upper limb reconstruction, the approach requires a stable and durable solution, and the flaps used, especially in oncological surgeries, should be well vascularized, must provide bone coverage, control of infectious processes, resistance to possible complementary treatments and maintenance of function and esthetics of the limb. Free muscular transfers have been recomended for these reconstructions, however, their greatest technical difficulty and complications cannot be forgotten. Among the alternatives, we highlight the latissimus dorsi flap, being a technically simpler option, accessible and with low morbidity of the donor area.
The success rate with the latissimus dorsi flap for diverse reconstructions ranges from 72% to 95%. As a result of its vascularization, various shapes and sizes of this flap might be executed, depending on the location, cause and defect to be repaired. The skin island might reach dimensions up to 35 cm x 12 cm while still maintaining direct closure of the donor area. This flap can still be used in a functional way, restoring the elbow flexion or extension movement [10,12].
Conclusion
The chondroid syringoma of the upper limb usually have a late diagnosis, making extensive lesions a common presentation, which leads to aggressive oncologic surgical treatment, which results in expressive defects. Thus, the use of the latissimus dorsi flap is often used mainly because of the intrinsic characteristics of possibility of good functional and aesthetic restoration.
References
- Kakitsubata Y, Theodorou SJ, Theodorou DJ, Nakahara M, Yuki Y, et al. (2009) Giant chondroid syringoma presenting as a growing subcutaneous mass in the upper arm: MRI findings with pathologic correlation. Joint Bone Spine 76: 711-714.
- Sungur N, Uysal A, Gumus M, Kocer U (2003) An Unusual Chondroid Syringoma. Dermatol Surg 29: 977-979.
- Roca PC, Barro AV, Franco MC, Otero JP, Villar FM (2009) Cirugía Plástica Ibero-Latinoamericana. Cir plást Iberolatinoam 35: 73-78.
- Askari K, Ghorbani G, Yousefi N, Saadat SMS, Saadat SNS, et al. (2014) Chondroid Syringoma of the Forearm: A Case Report of a Rare Localization. Indian J Dermatol. Set Out 59: 507-509.
- Chang D, Shaletich C, Zerbini MCN (2007) Siringomacondróide maligno: relato de caso e revisão da literatura. J Bras Patol Med Lab Jun 43: 191-194.
- Andrade P, Reis JP, Tellechea O (2011) Tumores Sudoríparos - Revisão de 10 anos. Revista SPDV 69: 599-607.
- Obaidat NA, Alsaad KO, Ghazarian D (2007) Skin adnexal neoplasms-part 2: An approach to tumours of cutaneous sweat glands. J Clin Pathol 60: 145-159.
- Narasimha A, Kalyani R, Harendra ML, Kumar TN, Suresh A (2013) Supreeth. Giant chondroid syringoma with divergent diferentiation: Cyto-histo-immuno correlation. International Journal of Applied and Basic Medical Research. Jul-Dez 3: 129-131.
- Franco JPA, Zacaron LH, Lima RB, D’Acri AM, Martins CJ (2013) Case for diagnosis. Chondroid syringoma in an unusual location. An Bras Dermatol 88: 997-999.
- Mebazaa A, Trabelsi S, Denguezli M, Sriha B, Belajouza C, et al. (2006) Chondroid syringoma of the arm: An unusual localization. Dermatology Online Journal 12: 14.
- Adkinson JM, Chung KC (2014) Flap Reconstruction of the Elbow and Forearm: A Case-Based Approach. Hand Clin 30: 153-163.
- Araújo HJ, Batista KT (2008) Reconstrução do membro superior após cirurgia oncológica com retalho fasciomiocutâneo do grande dorsal. Rev Bras Cir Plást 23: 347-349.
- Yavuzer R, Basterzi Y, Sari A, Bir F (2003) Chondroid syringoma: a diagnosis more frequent than expected. Dermatol Surg 29: 179-181.
- Andrade P, Reis JP, Tellechea O (2011) Tumores Sudoríparos - Revisão de 10 anos. Revista SPDV 69: 599-607.
- Narasimha A, Kalyani R, Harendra ML Kumar, TN Suresh, et al. (2013) Giant chondroid syringoma with divergent differentiation: Cyto-histo-immuno correlation. Int J Appl Basic Med Res 3: 129-131.
- Kakitsubata Y, Theodorou SJ, Theodorou DJ, Nakahara M, Yuki Y, et al. (2009) Giant chondroid syringoma presenting as a growing subcutaneous mass in the upper arm: MRI findings with pathologic correlation. Joint Bone Spine 76: 711-714.
- Askari K, Ghorbani G, Yousefi N, Saadat SMS, Saadat SNS, Zargari O (2014) Chondroid Syringoma of the Forearm: A Case Report of a Rare Localization. Indian J Dermatol 59: 507-509.
- Kakitsubata Y, Theodorou SJ, Theodorou DJ, Nakahara M, Yuki Y, et al. (2009) Giant chondroid syringoma presenting as a growing subcutaneous mass in the upper arm: MRI findings with pathologic correlation. Joint Bone Spine 76: 711-714.
- Sungur N, Uysal A, Gumus M, Kocer U (2003) An Unusual Chondroid Syringoma. Dermatol Surg 29: 977-979.
- Joshua M. Adkinson, Kevin C (2014) Chung. Flap Reconstruction of the Elbow and Forearm: A Case-Based Approach. Hand Clin 30: 153-163.
- Yavuzer R, Basterzi Y, Sari A, Bir F, Sezer C (2003) Chondroid syringoma: a diagnosis more frequent than expected. Dermatol Surg 29: 179-181.
- Mebazaa A, Trabelsi S, Denguezli M, Sriha B, Belajouza C, et al. (2006) Chondroid syringoma of the arm: An unusual localization. Dermatology Online Journal 12: 14.
- Medina Henriquez JA, Navarro Garcia R, Nagel D, Foucher G (2001) Malignant chondroid syringoma of the hand: a case report. Scand J PlastReconstr Surg Hand Surg 35: 437-439.
- Roca PC, Barro AV, Franco MC, Otero JP, Villar FM (2009) Siringoma condroide maligno: a propósito de un caso. Cir.plást. Iberolatinoam Jan-Fev-Mar 35: 73-78.
- Chang D, Shaletich C, Zerbini MCN (2007) Siringomacondróide maligno: relato de caso e revisão da literatura. J Bras Patol Med Lab Jun 43: 191-194.
- Malik R, Saxena A, Kamath N (2013) A rare case of malignant chondroid syringoma of scalp. Indian Dermatol J 4: 236-238.
- Krishnamurthy A, Aggarwal N, Deen S, Majhi U, Ramshankar V (2015) Malignant chondroid syringoma of the pinna. Indian J Nucl Med. 30: 334-337.
- Tural D, Selçukbiricik F, Günver F, Kar??maz A, Serdengecti S (2013) Facial localization of malignant chondroid syringoma: a rare case report. Case Rep Oncol Med 2013: 907980.
- Shashikala P, Chandrashekhar HR, Sharma S, Suresh KK (2004) Malignant chondroid syringoma. Indian J DermatolVenereolLeprol. 70: 175-176.
- Watarai A, Amoh Y, Aki R, Takasu H, Katsuoka K (2011) Malignant chondroid syringoma: report of a case with lymph node metastasis 12 years after local excision. Dermatol Online J 17: 5.
- Ishimura E, Iwamoto H, Kobashi Y, Yamabe H, Ichijima K (1883) Malignantchondroidsyringoma. Report of a case with widespread metastasis and review of pertinent literature. Cancer 52: 1966-1973.
- Redono C, Rocamora A, Villoria F, Garcia M (1982) Malignant mixed tumor of the skin: Malignant chondroid syringoma. Cancer 49: 1690-1696.
- Agrawal V, Gupta RL, Kumar S, Mishra K, Agarwal S (1998) Malignant chondroid syringoma. J Dermatol 25: 547-549.
- Nicolaou S, Dubec JJ, Munk PL, O’Connell JX, Lee MJ (2001) Malignant chondroid syringoma of the skin: Magnetic resonance imaging features. Australasian Radiology 45: 240-243.
- Araújo JL, de Aguiar GB, do Prado Aguiar U, Mayrink D, et al. (2012) Malignant chondroid syringoma with central nervous system involvement. J Craniofac Surg 23: 514-515.
- Requena C, Brotons S, Sanmartín O, Llombart B, Traves V, et al. (2013) Malignant chondroid syringoma of the face with bone invasion. Am J Dermatopathol 35: 395-398.
- Takahashi H, Ishiko A, Kobayashi M, Tanikawa A, Takasu H, et al. (2004) Malignant chondroid syringoma with bone invasion: a case report and review of the literature. Am J Dermatopathol 26: 403-406.
- Steinmetz JC, Russo BA, Ginsburg RE (1990) Malignant chondroid syringoma with widespread metastasis. J Am Acad Dermatol 22: 845-847.
- Hirsch P, Helwig EB (1961) Chondroid syringoma. Mixed tumor of skin, salivary gland type. Arch Dermatol 84: 835-847.
- Shvili D, Rothem A (1986) Fulminant metastasizing chondroid syringoma of the skin. Am J Dermatopathol 8: 321-325.
- Hilton JM, Blackwell JB (1973) Metastasising chondroid syringoma. J Pathol 109: 167-170.
- Webb JN, Stott WG (1975) Malignant chondroid syringoma of the thigh. Report of a case with electron microscopy of the tumour. J Pathol 116:43-46.
- Botha JB, Kahn LB (1978) Aggressive chondroid syringoma. Report of a case in an unusual location and with local recurrence. Arch Dermatol 114: 954-955.
- Dissanayake RV, Salm R (1980) Sweat-gland carcinomas: prognosis related to histological type. Histopathology 4: 445-466.
- Harrist TJ, Aretz TH, Mihm Jr MC (1981) Cutaneous malignant mixed tumor. Arch Dermatol 117:. 719-724.
- de Moraes HP, Herrera GA, Mendonca AM, Estrela RR (1986) Metastatic malignant mixed tumor of the skin. Ultrastructural and immunocytochemical characterization, histogenetic considerations and comparison with benign mixed tumors of skin and salivary glands. Appl Pathol 4: 199-208.
- Hermann G, Moss D, Norton KI, Guttenberg ME (1987) Case report 450: Skeletal metastases secondary to malignant chondroid syringoma. Skeletal Radiol 16: 657-659.
- Clark P (1987) Malignant chondroid syringoma. Conn Med 51: 569-572.
- Vohra S, Bates WA, Baithun SI (1996) A rare adnexal tumor of the hallux: Malignant chondroid syringoma. Foot 6: 175-177.
- Herreros CS, Flores PB, Murillo EdeE, Recio ED, et al. (2011) A Case of Cutaneous Malignant Mixed Tumor Treated with Mohs Micrographic Surgery, Dermatol Surg 37: 267-270.
- Kiely JL, Dunne B, McCabe M, McNicholas WT (1997) Malignant chondroid syringoma presenting as multiple pulmonary nodules. Thorax 52: 395-396.
- Sun TB, Chien HF, Huang SF, Shih TT, Chen MT (1996) Malignant chondroid syringoma. J Formos Med Assoc 95: 575-578.
- Barnett MD, Wallack MK, Zuretti A, Mesia L, Emery RS, et al. (2000) Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol 23: 227-232.
- Medina Henriquez JA, Navarro Garcia R, Nagel D, Foucher G (2001) Malignant chondroid syringoma of the hand: a case report. Scand J PlastReconstr Surg Hand Surg 35: 437-439.
- Menéndez RH, Erice SG, Bas CA, Casas G, Dillon HS (2015) Spinal cord compression secondary to metastasis of malignant chondroid syringoma: case report. J Neurosurg Spine 22: 310-313.
- Solomonov A, Rosenblatt E, Ben-Izhak O, Goralnik L, Yigla M (2001) High-dose-rate endobronchial brachytherapy in endobronchial metastatic malignant chondroid syringoma. Respiration 68: 406-410.
- Hong JJ, Elmore JF, Drachenberg CI, Jacobs MC, Salazar OM (1995) Role of radiation therapy in the management of malignant chondroid syringoma. Dermatol Surg 21: 781-785.
Citation: Feitosa RGF, Waisberg FMV, Trincado MM, Mendes JdeA, Okamoto RH, et al. (2021) Giant malignant Chondroid Syringoma: Case Report and Literature Review. J Clin Dermatol Ther 7: 094.
Copyright: © 2021 Roney Gonçalves Fechine Feitosa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

