
Giant Proliferating Pilomatricoma and Superficial Angiomatous Proliferation: Case Report and Review of the Literature
*Corresponding Author(s):
Yong KangDepartment Of Pathology, Robert Wood Johnson Banarbas Health/Monmouth Medical Center, Long Branch, New Jersey, United States
Tel:+1 7329237364,
Email:yk489@rwjms.rutgers.edu
Abstract
Giant proliferating pilomatricoma is a rare variant of pilomatricoma. It is a benign tumor that has the potential for local recurrence. Superficial angiomyxomas are also rare, distinctive, benign, cutaneous soft tissue lesions. The combination of both is exceedingly rare and there are three reported cases in literature to our knowledge. Here we report a case of giant proliferating pilomatricoma with superficial angiomyxomatous proliferation occurring on the posterior thorax of a 83-year-old woman. Further studies are needed to clarify whether this is a true collision tumor.
Keywords
Proliferating pilomatricoma; Superficial angiomyxoma; β-catenin
INTRODUCTION
Pilomatricoma is a benign cutaneous neoplasm with differentiation towards the hair matrix, inner sheath of the hair follicle and hair cortex. Pilomatricoma usually presents as a slow growing, firm nodule on the head, upper limbs, neck, trunk and lower limbs in decreasing order of frequency. It occurs predominantly in children and young adults [1-3]. Proliferating pilomatricoma is a proliferative variant of pilomatricoma [4-5]. The subtype is primarily composed of a large, lobular proliferation of basaloid cells in association with small to large foci of transitional cells and shadow cells. Superficial angiomyxoma has rarely been reported to be associated with pilomatricoma. In the current report, we describe a case of a giant proliferating pilomatricoma associated with superficial angiomatous proliferation. This tumor was unusually large and the rapid growth raised clinical suspicion of malignancy. The pathogenesis of such an association will be discussed in this report.
CASE PRESENTATION
A 83 year-old woman presented with an enlarging mass on the thoracic area of her back. The mass was noted several years prior and slowly growing since. Recent rapid growth raised clinical suspicion of malignancy. The physical examination revealed a 13 x 10 cm firm, exophytic, and reddish-brown mass with overlying bullous skin in posterior thorax (Figure 1). She denied any prior surgery or trauma to the affected area. There was no family history of similar lesions or known genetic syndromes. Macroscopic examination showed a well-circumscribed, nodular, dull white mass, measuring 12 x 9 x 4.7 cm. Sectioning of the mass found significant ossification of the involved tissue. Microscopic examination revealed a mutilobular proliferation of basaloid cells with mild nuclear atypia and brisk mitotic activity in association with adjacent areas containing cornified material, shadow cells, calcification and ossification (Figure 2A-2C). On β-catenin staining of the pilomatricoma, the basaloid cells exhibited prominent membranous staining (Figure 2D). The transitional cells and shadow cells did not express β-catenin at all.
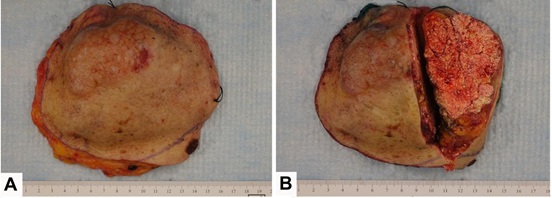
Figure 1: A: Gross macroscopic image of giant proliferating pilomatricoma; B: The sectioned tumor shows diffuse calcification after complete excision.
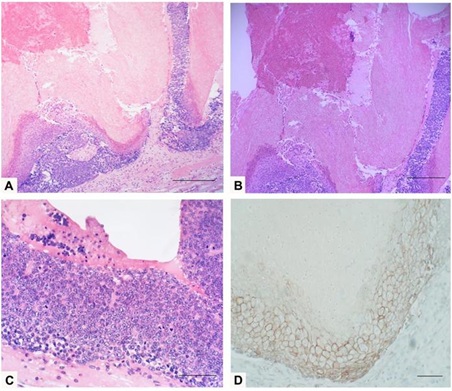
Figure 2: A: Routine hematoxylin-eosin stain shows a collection of basaloid cells at the periphery and central matrical cornification with ghost cells, scale bar: 10 µm; B: Detail of the cornified material and shadow cells with fibrotic stroma, scale bar: 10 µm; C: Lesion with area of basaloid cells with mitotic figures, scale bar: 10 µm; D: Strong membranous β-catenin immunoreactivity in basaloid cells of pilomatricoma, scale bar: 10 µm.
There was a prominent myxomatous stroma with focal bundles of collagen fibers consisting of spindle shaped cells, numerous small to medium sized blood capillaries with focal arborization (Figure 3A), which were found to be positive for CD34 (Figure 3B). Based on the immune histomorphological findings, a final diagnosis of proliferating pilomatricoma with superimposed features of a superficial angiomyxoma was rendered.
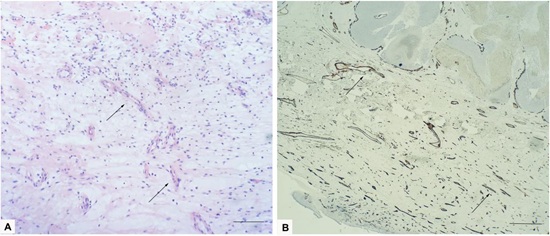
Figure 3: A: Routine hematoxylin-eosin stain shows extensive loose, myxoid connective tissue stroma with increased capillaries (black arrows), and scale bar: 10 µm; B: CD34 is expressed within spindle cells and highlighted the prominent vasculature (black arrows), scale bar: 10 µm.
DISCUSSION
Pilomatricoma, or calcifying epithelioma of Malherbe, first described in 1880 by Malherbe and Chenantais as sebaceous gland tumors, Forbis and Helwig later clarified that pilomatricomas originate from the hair follicle matrix cells [6]. It represents approximately 1% of all benign skin tumors and it is the second most common cutaneous neoplasm in childhood and youth. More than half of these tumors are located on the head, neck, or upper torso. It is a slow-growing, firm, dermal or subcutaneous neoplasm, usually measuring under 3 cm in diameter [7]. Pilomatricomas are considered benign. However, cases of pilomatricoma with a tendency for focal invasiveness and local recurrence have been reported and designated firstly as aggressive pilomatricomas [6].
Giant proliferating pilomatricoma is diagnosed based on clinical and histopathological findings. Giant pilomatricoma was first described by Krausen, et al. in 1974 [8]. Although no standard definition for giant pilomatricomas exists, these growths are generally defined as tumors larger than 5 cm [9-11]. Giant pilomatricomas are rare and represent a small proportion (<10%) of pilomatricomas [11]. Compared with more typical pilomatricomas, these large neoplasms tend to have higher rates of ulceration and secondary infection presumably caused by inadvertent, repeated external trauma. Proliferating pilomatricoma is a proliferative variant of pilomatricoma first described by Kaddu S, et al. in 1997 [4]. As compared with classical pilomatricoma, it is histopathologically characterized by relatively symmetric tumor composed of a large, lobular proliferation of basaloid cells, showing features of matrical and supramatrical cells of a normal hair follicle and exhibited variable nuclear atypia and mitotic figures. The architectural pattern is different from that of large fully developed stererotypical pilomatricomas that maintain a cystic character with basaloid cells predominantly aligned at the periphery.
The pathogenesis of pilomatricomas has not been completely elucidated. β-catenin is a downstream effector in the Wnt signaling pathway, acting as a signal for differentiation and proliferation. β-catenin gene mutation has been shown to be an important regulator of the development and differentiation of hair follicles. Previous studies have implicated the WNT/β-catenin signaling pathway in the oncogenesis of pilomatricomas with β-catenin mutations found in approximately 75% of cases. The mutations are adjacent to or abolished well-known regulatory phosphorylation sites of β-catenin, which leads to activation of Wnt signaling pathway and slow degradation of cytoplasmic β-catenin and its nuclear translocation, which then results in blocked apoptosis, abnormal differentiation, cellular proliferation and finally oncogenesis. Although most of the studies observed that the nuclear staining of β-catenin [12,13], Park, et al. observed that a prominent membranous immunoreactivity in the basaloid cells. There was definitely no evidence of nuclear positivity, so they suggested that β-catenin is primarily involved in cell-cell adhesion rather than cellular proliferation during the pathogenesis of pilomatricoma [14]. Bcl-2 has been reported to play a role in the pathogenesis of pilomatricomas through regulating the apoptotic progression of basaloid cells to shadow cells [15].
Superficial angiomyxomas are rare, distinctive, benign, cutaneous soft tissue lesions with a predilection for the trunk, head, neck, and genital region. They were first described by Allen et al. in 1988 and also have been called cutaneous myxoma [16]. Definitve epithelial components are present in approximately 25% of tumors [16]. Toth, et al. reported a case demonstrating that a superficial angiomyxoma with epithelial-lined structures occurring in retropharynx [17]. Calonje, et al. analyzed the clinicopathologic and immunohistochemical features in a series of 39 cases of superficial angiomyxoma and reported that approximately 20% of cases of the primary lesion or its recurrence contained epithelial structures, including epidermoid cysts, thin strands of squamous epithelium, and small buds of basaloid cells [18]. None of the publications reported pilomatricoma as an associated finding of superficial angiomyxoma. However, Perez Tato, et al. report a case of a 4-cm truncal mass with histologic findings of a superficial angiomyxoma with trichofolliculoma [17]. Al-Brahim and Radhi reported 3 cases of superficial angiomyxoma with co-occurring histologic features of a pilomatricoma in the absence of any known familial or other underlying pathology. All the three cases presented with variable-sized pedunculated, asymptomatic skin papules overlying firm deeply situated nodule. The first of these cases was an adult male with a 6.7 cm cystic mass on the anterior chest wall. The second and third cases were in pediatric patients with 1.5 cmsolitary lesions on the back and left arm, respectively. Microscopic examination demonstrated similar features in all three cases. The nodular lesions composed of a myxid stroma and spindle cells with prominent vasculature. The subcutaneous lesions showed typical features of pilomatricoma. While acknowledging that secondary myxoid changes are often seen in many mesenchymal tumors, Al-Brahim and Radhi suggest that pilomatricomas may originate from the epithelial components of angiomyxomas [19]. Recently Hawkes JE reported a case of 9 cm pedunculated mass on the anterior aspect of chest wall from an adult male, which was diagnosed with giant pilomatricoma with angiomyxoid stroma [20].
We report the case of a follicular neoplasm on the thoracic back of an elderly patient with histologic features consistent with a giant proliferating pilomatricoma with angiomyxomatous proliferation, although there were insufficient features to make a diagnosis of superficial angiomyxoma per se. Our report, in conjunction with other studies, suggests that an alternate hypothesis to that proposed by Al-Brahim and Radhi could be that superficial angiomyxomas or angiomyxoid stroma may represent a secondary finding of preexisting epithelial or hair follicle tumors. The membranous expression of β-catenin in this report implied that the complicated interactions between the epithelial cells and mesenchymal cells mediated by β-catenin may induce the myxoid changes, but the delicate mechanisms need more investigation. Nevertheless, we acknowledge that the association of pilomatricomas with angiomyxoid stroma may be purely coincidental and recognize that neither case reports nor retrospective histologic analyses are able to decipher the temporal, etiologic association of these tumors.
CONCLUSION
We present this case of a giant proliferating pilomatricoma occurring on the back, given the atypical location and patient’s age, rare proliferating histology and unusal angiomyxoid stroma, and large size. It is unclear whether proliferating pilomatricomas are associated with superficial angiomyxoma due to the low number of cases reported.
REFERENCES
- Wells NJ, Blair GK, Magee JF, Whiteman DM (1994) Pilomatrixoma: A common, benign childhood skin tumour. Can J Surg 37: 483-486.
- Kranjcec I, Roganovic J, Jonjic N (2015) Pilomatrixoma: A benign appendageal tumor not uncommon in children. Acta Dermatovenerol Croat 23: 308-309.
- Kato H, Kanematsu M, Watanabe H, Nagano A, Shu E, et al. (2016) MR imaging findings of pilomatricomas: a radiological-pathological correlation. Acta radiol 57: 726-732.
- Kaddu S, Soyer HP, Wolf IH, Kerl H (1997) Proliferating pilomatricoma: A histopathologic simulator of matrical carcinoma. J Cutan Pathol 24: 228-234.
- Kondo RN, Pontello Junior R, Belinetti FM, Cilião C, Vasconcellos VR, et al. (2015) Proliferating pilomatricoma - case report. An Bras Dermatol 90: 94-96.
- Kaddu S, Soyer HP, Cerroni L, Salmhofer W, Hödl S (1994) Clinical and histopathologic spectrum of pilomatricomas in adults. Int J Dermatol33 10: 705-708.
- Julian CG, Bowers PW (1998) A clinical review of 209 pilomatricomas. J Am Acad Dermatol 39:191-195.
- Krausen AS, Ansel DG, Mays BR Jr (1974) Pilomatrixoma masquerading as a parotid mass. Laryngoscope 84: 528-535.
- Souto MP, Matsushita Mde M, Matsushita Gde M, Souto LR (2013) An unusual presentation of giant pilomatrixoma in an adult patient. J Dermatol Case Rep 7: 56-59.
- Mundinger GS, Steinbacher DM, Bishop JA, Tufaro AP (2011) Giant pilomatricoma involving the parotid: Case report and literature review. J Craniomaxillofac Surg 39: 519-524.
- Lozzi GP, Soyer HP, Fruehauf J, Massone C, Kerl H, et al. (2007) Giant pilomatricoma. Am J Dermatopathol 29 : 286-289.
- Chan EF, Gat U, McNiff JM, Fuchs E (1999) A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 21: 410-413.
- Hassanein AM, Glanz SM (2004) β-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol 150: 511-516.
- Park SW, Suh KS, Wang HY, Kim ST, Sung HS (2001) β-catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol 145:624-629.
- Agoston AT, Liang CW, Richkind KE, Fletcher JA, Vargas SO (2010) Trisomy 18 is a consistent cytogenetic feature in pilomatricoma. Mod Pathol 23: 1147-1150.
- Allen PW, Dymock RB, MacCormac LB (1988) Superficial angiomyxomas with and without epithelial components. Report of 30 tumors in 28 patients. Am J Surg Pathol 12: 519-530.
- Perez Tato B, Saez AC, Fernandez PR (2008) Superficial angiomyxoma with trichofolliculoma. Ann Diagn Pathol 12:375-377.
- Calonje E, Guerin D, McCormick D, Fletcher CD (1999) Superficial angiomyxoma: Clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol 23: 910-917.
- Al-Brahim N, Radhi JM (2010) Cutaneous angiomyxoma and pilomatricoma: A new combination. Ann Diagn Pathol 14:328-330.
- Hawkes JE, Woodcock J, Christensen LC, Duffy KL (2015) Giant pilomatricoma with angiomyxoid stroma: Unusual presentation of a benign tumor. JAAD Case Rep 1: 169-171.
Citation: Yu K, Singh N, Maghari A, Kang Y (2020) Giant Proliferating Pilomatricoma and Superficial Angiomatous Proliferation: Case Report and Review of the Literature. J Clin Dermatol Ther 6: 042.
Copyright: © 2020 Kate Yu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

