
Green Synthesis, Characterization and Antimicrobial Activity of Biosynthesized Silver Nanoparticles using Ziziphusspina-Christi Leaf Extracts
*Corresponding Author(s):
Hayam AbdelkaderMicrobiology Department, College Of Science, University Of Jeddah, Saudi Arabia
Tel:0122334444/23123,
Email:hsabdelkader@uj.edu.sa
Abstract
In the current study, a simple, rapid, eco-friendly and cost-effective method was successfully developed through a green synthesis of silver nanoparticles (AgNPs) using Ziziphus spina-christi leaf extract as an antimicrobial agent. Z.spina-christi aqueous extract was used as a reducing agent for synthesizing silver nanoparticles. Z.spina-christi can reduce silver ions into silver nanoparticles within 30 min after magnetic stirring the reaction mixture at RT as observed by development of bright brown color. Biosynthesized silver nanoparticles were characterized by using different analytical techniques. The UV-Vis spectrum of silver nanoparticles revealed a characteristic peak at 420 nm. High-resolution transmission electron microscope (HRTEM) showed spherical shape monodispersed nanoparticles with the average size of 24±4 nm. Furthermore, the effective compounds responsible for the reduction of silver ions, the functional groups present in the nanoparticles mixture were investigated by Fourier transform infrared spectroscopy (FT-IR). Antibacterial activity of the biosynthesized AgNPs was studied against common human pathogens such as gram-positive (S. aureas) and gram-negative (E.coli, A. baumanni, K. pneumoniae, and P.aeruginosa) bacterial strains. Furthermore, the biosynthesized AgNPs showed reliable antifungal activity against pathogenic fungal isolates A. niger, A. flavus, P. digitatum, and F. oxysporum. This technique takes advantage of the economic and greener approach to metallic nanoparticles synthesis.
Keywords
AgNPs; Antimicrobial Activity;FT-IR;HRTEM; UV-Vis;Ziziphus spina-christi
INTRODUCTION
There is an increasing worldwide interest in the field of nanotechnology in recent years[1]. Research into nanoparticles is inevitable today, not only through implementation but also through synthesis [2].
Silver is a non-toxic, secure antibacterial inorganic agent that can kill about 650 kinds of microorganism-causing illnesses[3]. Because of their antimicrobial characteristics, there is growing interest in silver nanoparticles [4]. They are even being projected as future generation antimicrobial agents [5].
Silver nanoparticles (AgNPs) have had many notable biological roles in therapeutic field (antimicrobial, anticancer, antiparasitic, antidiabetic and antioxidant activity, bio-molecular detection and diagnostics, drug delivery, food manufacturing, farming and waste treatment [6-9].
Ziziphus spina-christi L., frequently referred to as Christ's Thorn Jujube, is a deciduous tree indigenous to hot temperate and subtropical areas, including North Africa, South Europe, Mediterranean, Australia, South, and East Asia and the Middle East [10]. Z. spina-christi L. (Family: Rhamnaceae), it was called sedr (related to the Qur'an lote trees). It was one of the most important crops in folk medicine in Saudi Arabia [11]. Z. Spina-Christi has been used as an alternative medicine for the treatment of fever, pain, dandruff, wounds and ulcers, inflammatory conditions, asthma and eye illnesses [12].
Z. spina-christi has been shown to possess antibacterial, antifungal, antioxidant and anti-hyperglycemic activity.Ziziphusplants contain strong reducing agents such as polyphenols, terpenoids, phenolic acids, alkaloids, sugars flavonoids, saponins and proteins which are key players for bio reduction of silver ions (Ag+) due to presence of several -OH group [13].
The nanoparticles inhibit bacterial cell division causing membrane destruction and enhanced cell permeability, which ultimately leads to cell death [14]. Bacterial proliferation also declines when functional groups on the surface of nanoparticles interfere with proteins of the bacterial membrane, phospholipids, lipoproteins, and lipoteichoic acids and decrease their colonization and surface adherence [15].The mechanism of antifungal activity of silver nanoparticles was also reported [16, 17].
In the present research, we used Ziziphus spina-christi plant extracts for green synthesis of AgNPs.Up to our knowledge, there is no report on synthesis of silver nanoparticles fromZiziphus spina-christiL till date. Green synthesis of nanoparticles is an eco-friendly approach, which should be further studied for the potential synthesis of nanoparticles using different plant extracts [18].Several pathogenic gram-positive and gram-negative bacterial strains were explored for the antimicrobial activity of biosynthesized silver nanoparticles. The antifungal activity of these particles against pathogenic fungi was also evaluated.
MATERIALS AND METHODS
Materials
Ziziphus spina-christiplant were obtained from a local farm (Mekkah, KSA)identified by our expert botanical taxonomists, Silver nitrate (AgNO3), Nutrient agar, Mueller Hinton Broth (MHB), Mueller Hinton Agar (MHA) were purchased from Sigma–Aldrich (St. Louis, MO, USA). DimethylSulfoxide (DMSO) from SD Fine-Chem Limited, India.Potato Dextrose Agar (PDA) and Potato Dextrose Broth were from (Difco Laboratories, Fisher Scientific).The bacterial strains used in the present study were collected from King AbdulAziz hospital, Mekkah, KSA.All bacterial strains were identifiedmolecularly using 16s rRNA as reported by Malah[19].Mutant strains of P. aeruginosaand K. pneumoniaehave been submitted to Genbank under accession number MN121254, MN121255, MN121256, and MN121250 respectively.All fungal isolates were identifiedusing ITS rDNA sequence analysis as reported by Al-Zubaidi[20].
Preparation of aqueous leaf extract of Ziziphus spina-christi
Ziziphus leaves were extracted according to the method described by Adzu[21]. The plant was dried under shade at 25°C and the dried leaves were ground into powder. Five grams of dried powder was added to 100 ml of double-distilled deionized water stirred for 15 min at room temperature. The extract was filtered through Whatman No. 1 filter paper and was used for biosynthesis of silver nanoparticles [22].
Green synthesis of silver nanoparticles
Ziziphusleaves extract solution (1, 2, 3, 4 and 5 mL) were added separately to 10 mL of 1 mM silvernitrate solution with constant stirring for 90 min at room temperature and incubated in a dark bottle to minimize photo-activation of silver nitrate.The pH value of resulting solution was measured to be about 7.5. The color of the solution was changed to light brown immediately and then to dark brown after 90 min indicating the reduction of Ag+ to Ag0which was further confirmed by usingUV-Visible spectroscopy.The silver nanoparticles obtained by Ziziphus leaves extract were centrifuged at 15,000 rpm for 10 min and then dispersed in sterile distilled water to get rid of any clumsy biological materials.
Characterization Methods
UV visible spectroscopy was carried out on a (UV-2450, Shimadzu, Tokyo, Japan). FT-IR Spectra for silver nanoparticles solution was carried out using Fourier transform infrared spectroscopy (Thermo Scientific Smart iTR™). Scanning electron microscopy (SEM) analysis of silver nanoparticles was done using JEOL JSM 5200 SEM Electron Microscope. The shape and size of silver nanoparticles were determined by High-Resolution Transmission Electron Microscope (TEM, JEM2100, Jeol, Japan). Few drops of silver nanoparticles suspension were loaded on a carbon-coated copper grid and then allowed to air-dry on a filter paper for 5 -10 min.
Antimicrobial activity
A single colony of each test strain was grown overnight in MHB on shaker incubator with (200 rpm) at 37°C. The overnight bacterial cultures were diluted with 0.9% NaCl to obtain 0.5 McFarland standards and then plated on MH agar plates and left for 1 hour at RT. together withSterile disks were impregnated with different concentration of AgNPs (10 µg/ml, 20 µg/ml, and 30 µg/ml) were placed on the swabbed plate and incubated overnight at 37°C and the zone of inhibition was measured [23]. The antifungal activity of AgNPs was examined against the fungal isolates A. flavus,P. digitatum, F. oxysporum, and A. niger by using agar well diffusion method [24]. Fungi under test were grown on PDA liquid media at 26°C for 5 days, and then cells containing 1 × 106 colony-forming units/mL were cultured on fresh PDA solid media. 50 μl containing different concentrations of AgNPs (10 μg/mL, 20 μg/mL, and 30 μg/mL) was placed on 8 mm diameter wells on PDA media and incubated at 26°C for 5 days. Controls of Silver-free plates as well as Nystatin PDA plates as a standard antifungal control were also incubated under the same conditions.
Data analysis
The fungal mycelium growth was recorded on PDA plates containing AgNPs and antifungal standard (Nystatin).When mycelial growth on Silver-free plates reached the edge of the petridish, radial inhibition rate was calculated according to the following equation.
Inhibition rate (%) = R−r/R
Where R is the fungal radial growth on the control silver free plate and r is the radial of fungal growth on culture plate inoculated with AgNPsor Nystatin.
RESULTS AND DISCUSSION
Effect of incubation period
Effect of incubation period on the change of the color of biosynthesized AgNPs was achieved indicating the successful reduction of silver metal ions. The intensity of the dark brown color was directly proportional to the incubation time of reaction mixture.The rate of silver ions reduction was going slowly during the first 15 min, as indicated by the low absorbance values at wavelength 420 nm and color intensity.Significant increase in the absorbance together with color intensitywas revealed after a time periods up to 90 min as shown in (Figure 1).
 Figure 1: Formation of AgNPs using Ziziphus spina-christi leaf extract within time.The bright brown color of the reaction mixture is indicating the formation of nanoparticles.
Figure 1: Formation of AgNPs using Ziziphus spina-christi leaf extract within time.The bright brown color of the reaction mixture is indicating the formation of nanoparticles.
UV-Vis spectroscopy
Ziziphus spina-christi plant-mediated synthesis of silver nanoparticles was analyzed using UV-Vis. spectroscopy within reaction duration regarding the surface plasmon resonance (SPR) of the synthesized silver nanoparticles. (Figure 2) shows the absorption spectra of the synthesized silver nanoparticles at a different time interval (15 to 90 min). The absorption peaks obtained at 420 nm can be due to the surface plasmon resonance excitation as reported by Obaid[25]. It was observed that the concentration of 1mM of AgNO3 displayed good optical properties and stability and resulted in the creation of well-dispersed silver nanoparticles as evidenced by color and UV-Vis plots. On the behalf of UV-Vis data, it was cleared that Z. christi extract was successfully capable of reducing silver metal ions rapidly after 15 min from initiation reaction indicating the formation of nanoparticles as reported by Ahmed and Ikram[26].
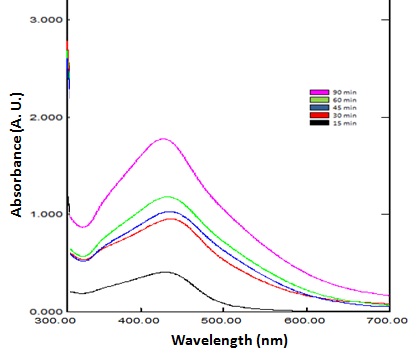 Figure 2: UV-visible absorption spectrum of biosynthesized AgNPs by the reduction of Ziziphusextracts solution at different incubation time (15, 30, 45, 60, and 90 min).The surface plasmon resonance peaks of silver nanoparticles were shown at 420 nm.
Figure 2: UV-visible absorption spectrum of biosynthesized AgNPs by the reduction of Ziziphusextracts solution at different incubation time (15, 30, 45, 60, and 90 min).The surface plasmon resonance peaks of silver nanoparticles were shown at 420 nm.
SEM, TEM and FTIR
The surface morphology and formation of nanoparticles was analyzed using SEM.(Figure 3A and3B) shows that the biosynthesized nanoparticles were sphericalin shape with different particle size.TEM measurements gave further insight into the morphology and size details of the silver nanoparticles. DLS histogram indicated most of the particles were in the size range between 10 and 42.3 nm and possesses an average size of 24± 4 nm (Figure 3C)which was in well agreement with the data obtained by TEM imaging.
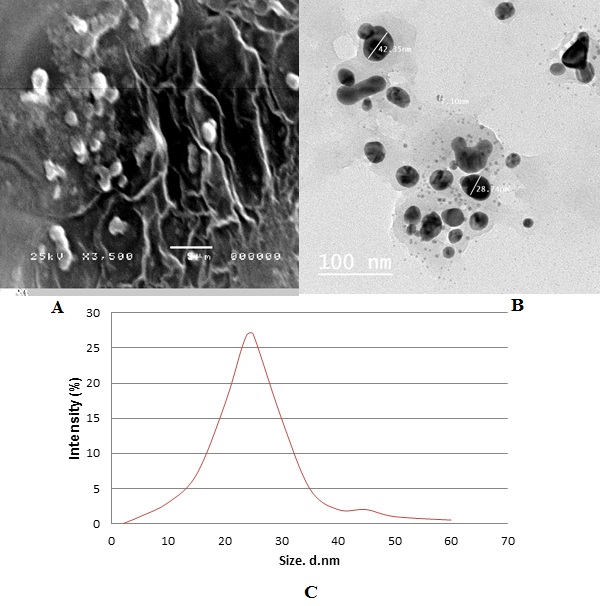 Figure 3: (A) SEM micrograph of biosynthesized silver nanoparticles using Ziziphus spina-christi leaf extract magnified 350x. (B) TEM micrograph of biosynthesized silver nanoparticles, the scale bar corresponds to 100 nm. (C) DLS histogram of biosynthesized AgNPs indicates size distribution by number.
Figure 3: (A) SEM micrograph of biosynthesized silver nanoparticles using Ziziphus spina-christi leaf extract magnified 350x. (B) TEM micrograph of biosynthesized silver nanoparticles, the scale bar corresponds to 100 nm. (C) DLS histogram of biosynthesized AgNPs indicates size distribution by number.
FTIR analysis was carried out to identify the biomolecules for capping and efficient stabilization of the metal nanoparticles synthesized. The FTIR spectrum of silver nanoparticlesshowed broad band at 3300 cm-1 corresponds to O-H stretching H-bonded alcohols and phenols(Figure 4).
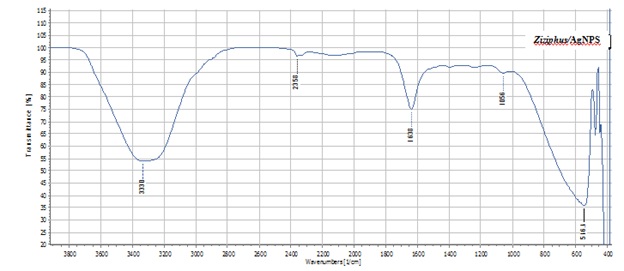 Figure 4: FITR spectrum of silver nanoparticles from Ziziphus spina-christi.
Figure 4: FITR spectrum of silver nanoparticles from Ziziphus spina-christi.
The sharp band at 1638 cm-1 corresponds to secondary amides C=Oconjugated carbonyl's (C=). The peak at 1056 cm-1 showed the bond stretch for C-O.Whilethe stretch for AgNPswas found around 516 cm-1[27]. The presence of Carbonyl groups or conjugated carbonyl's (C=) proved that flavanones or terpenoids could be absorbed on the surface of metal nanoparticles. The FT-IR results indicate that the secondary structures of proteins were not affected as a consequence of reaction with Ag+ ions or binding with AgNPs.
Dynamic Light Scattering (DLS)
The physicochemical characterization of prepared nanomaterials is an important factor in the study of biological activities using radiation scattering techniques [28,29]. DLS tests the light from a laser that passes through a colloid and relies mostly on Rayleigh scattering from the nanoparticles that are suspended [30]. The modulation of the scattered light intensity is then measured as a function of time and the hydrodynamic scale of the particles can be calculated [31]. To determine any nanomaterial's toxic potential, its solution characterization is necessary [32].Therefore, DLS is used mainly to assess particle size and size distributions in watery or physiological solutions [33].The average of particle size used in this study was 24±4 nm. (Figure 3A,B and C) revealed that the nanoparticles arespherical in shape and not uniformly distributed (polydispersed).These results were in agreement with the values obtained by TEM measurements as shown in (Figure 3B).
Antimicrobial activity
In the current study, the antimicrobial activity of the synthesized AgNPs against five pathogenic bacteria (A. baumanni, S. aureas, E.coli, K. pneumoniae, and P.aeruginosa) and four isolates of identified fungi (A. flavus, F. oxysporum,P. digitatum,and A. niger) was investigated, and antimicrobial activity of AgNPs have been compared to antimicrobial agent by antibacterial and antifungal drugs as Gentamycine and Nystatine, respectively. The synthesized AgNPs were proved to have antimicrobial activity against all the tested microorganisms (Table 1 and 2).
Three repeats were performed for each tested strain.
|
Bacterial strains |
Gentamycine (Standard antibacterial agent) (Mean±SD) (20 μg/ ml) |
*Diameter of inhibition zone (mm) produced by AgNPs (Mean±SD) |
||
|
10 µg/ml |
20 µg/ml |
30 µg/ml |
||
|
Staphylococcus aureus |
15.0±0.06 |
5.2±0.04 |
19.5±0.04 |
22.75±0.08 |
|
Acinetobacterbaumannii |
30.0±0.3 |
4.5±0.1 |
15.75±0.06 |
24.85±0.10 |
|
Pseudomonas aeruginosa |
12.0±0.1 |
7.9±0.007 |
15.0±0.08 |
16.85±0.14 |
|
Klebsiella pneumonia |
16.0±0.1 |
7.5±0.006 |
8.5±0.02 |
18.5±0.2 |
|
E.coli |
30.5±0.1 |
4.4±0.05 |
11.5±0.17 |
22.0±0.1 |
Table 1: The antimicrobial activity (inhibition zone in mm) of the biosynthesized AgNPs against test strains.
|
Fungal isolate |
Inhibition rate (%) produced by Nystatin (Standard antifungal agent) |
Inhibition rate (%) produced by AgNPs
|
|||
|
10 µg/ml |
20 µg/ml |
30 µg/ml |
|||
|
A. flavus |
96.0 |
75.0 |
88.0 |
95.75 |
|
|
F. oxysporum |
95.0 |
68.75 |
87.5 |
93.3 |
|
|
P. digitatum |
97.0 |
62.5 |
91.0 |
97.5 |
|
|
A. niger |
98.0 |
72.8 |
90.5 |
96.5 |
|
Table 2: Inhibitory rate (%) of silver nanoparticles against some pathogenic fungi.
Inhibition rates were determined based on three replicates of each experiment.The diameters of bacterial inhibition zones (mm) for AgNPs against A. baumanni, S. aureas, E.coli, K. pneumoniae, and P. aeruginosa showed strong antibacterial activity in comparison to those reported by Guzman [34]. The inhibitory effect of the AgNPs on each bacterial strain is specific and differs from each other. Our study was agreed with that obtained by results of antibacterial activities of biosynthesized silver nanoparticles evaluated from the agar well diffusion method using C. zylinicum extract mediated silver nanoparticles revealed that the AgNPs showed great antibacterial activity against both gram negative and gram positive bacterial strain where the inhibition zone diameter were (25, 22, 24, and 24 mm) for S. aureus, A. baumannii, P. aeruginosa, and K.pneumonia, respectively. As well as, our results were consistent with the results obtained by Halawani,whostudied the antibacterial properties of silver nanoparticles using Zizyphusspinachristi L aqueous leaves extract (ZSE), and found that the maximum inhibition zones of 24 mm, 23 mm, 15 mm and 17 mm against S. aureus, Acinetobacter sp. , P. aeruginosa and E. coli, respectively[35]. Figure 5: Activity of silver nanoparticles against (A)E.coli ,(B) P. aeruginosa, (C) A. baumanni, (D) K. pneumonia, (E) S. aureas, (F) A. flavus, (G) F. oxysporum, (H) P. digitatum,and (I) A. niger.
Figure 5: Activity of silver nanoparticles against (A)E.coli ,(B) P. aeruginosa, (C) A. baumanni, (D) K. pneumonia, (E) S. aureas, (F) A. flavus, (G) F. oxysporum, (H) P. digitatum,and (I) A. niger.
The inhibition rate of silver nanoparticles (AgNPs) against A. flavus, F.oxysporum, P. digitatum, and A.niger on PDA in vitrowas shown in (Table 2). Inhibition rate of P. digitatum treated with 30 μg/ml concentration of silver nanoparticles was (97.5%), followed by (96.5%) for A.niger, (95.75 %) for A. flavus, and (93.3 %) for F. oxysporium. Our results matched with that obtained by Al-Zubaidiwho indicated that the biothensizedAgNPs inhibited the growth of three different pathogenic fungi, including Fusariumoxysporum, Aspergillusflavus and Penicillin digitatum, at 10 µg/ml concentrations of AgNPs in comparison to Nystatin and the highest inhibition rate obtained was (97.3%),(93.75 %), and (91.0 %) against A. flavous, P. digitatum,andF. oxysporum, respectively.
One of the important parameters of AgNPs against microbes is the surface area of the nanomaterials. AgNPs can sustainably release Ag+ in and out of bacteria. The size of metallic nanoparticles ensures that a significantly large surface area of the particles is in contact with the bacterial cells. Such a large contact surface is expected to enhance the extent of bacterial elimination[36]. Smaller AgNPs having a large surface area available for interaction would give more bactericidal effect than the larger AgNPs [37].The size of the nanoparticles developed in the current study was multi-size, ranging in size from 10 to 42 nm with average size 24±4 nm, therefore, the great microbial inhibition might be due to the smaller size ofAgNPs (≤24 nm) as the smaller the size of nanoparticles, the greater the inhibitory effect of microbes as reported by Zawadzka[38].
The mechanism of inhibitory action of AgNPs on microorganisms indicates inhibition of DNA replication and consequently, and the expression of the ribosomal subunit protein might be deactivated, as well as other cellular proteins and enzymes that are crucial for the manufacturing of ATP, thereby inactivating microorganisms by changes in gene expression [39-41]. Other studies have suggested that AgNPs can bind to the cell membrane that disorders cell permeability and respiration or interfere with the electron transport system of microbes [42-44]. AgNPs found to have a strong an inclination to react with sulfur and phosphorus groups present on the cell membrane proteins as well as interacting with phosphorus present in DNA and inhibition of metabolic pathway indicated another mechanism governing gene expression involving the bactericidal action of AgNPs. Specifically; the AgNPs demonstrated the adequate activity of sterilization against E. coli and S. aureus[45-48]. The Ag NPs ' antibacterial activity engaged a species-specific mechanism of multiple antioxidant genes being upregulatedas well as genes coding for pumps for metal transport, metal reduction, and ATPase. Hence, AgNPs antibacterial mechanism is linked to antioxidant depletion.
Certain studies indicate that the beneficial impact of the Ag+ ion on its antimicrobial activity through the electrostatic attraction between the microorganisms' negatively charged cell membrane and the positively charged nanoparticles [49-51]. AgNPs have not been demonstrated to trigger bacterial resistance to bacterial diseases that presently complicate antibiotic therapy. This is presumed to be caused by the reality that in contrast to antibiotics AgNPs have not their antibacterial impacts at a single particular site but at various range of targets in the organisms such as a bacterial wall, protein synthesis and DNA [52]. The synthesized AgNPs showed an extremely pronounced antifungal activity that could be likely due to the destruction of fungal membrane integrity which leads to cell death[53].
CONCLUSION
It is becoming very hard to combat infectious diseases and cure patients due to widespread of MDR, in which bacteria are developing resistance to a wide range of antibiotics, leading to severe morbidity and mortality. AgNPs are a feasible solution to antibiotics and seem to have a strong ability to overcome the emergence of bacterial MDR.From the current study, it is clear that the silver nanoparticles synthesized through the green route using leaf extract of Ziziphus spina-christiexhibited remarkable antimicrobial activity against both gram-positive and gram-negative bacterial strainsand also could be considered as a potential antifungal agent. Thus, the silver nanoparticles from leaves of Ziziphus may be used in the management of human microbial infection.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- Natarajan K, Selvaraj S, Ramachandra MV (2010) Microbial production of silver nanoparticles. Dig J Nanomater Biostruct 5:135-140.
- Gopinath V, MubarakAli D, Priyadarshini S, Priyadharsshini NM, Thajuddin, N, et al. (2012) Biosynthesis of silver nanoparticles from Tribulusterrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B Biointerfaces 96: 69-74.
- Jeong SH, Yeo SY, Yi SC (2005) The effect of filler particle size on the antibacterial properties ofcompounded polymer/silver fibers. J Mater Sci 40: 5407-5411.
- Choi O, Deng KK, Kim NJ, Ross L, Hu Z, et al. (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res 42: 3066-3074.
- Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27: 76-83.
- Dipankar C, Murugan S (2012) The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresineherbstiileaf aqueous extracts.Colloids Surf B Biointerfaces 98:112-119.
- Vasanth K, Ilango K, Mohankumar R (2014) Anticancer activity of Moringaoleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction, Colloids Surf. B Biointerfaces 117:354-359.
- Azzazy HME, Mansour MMH, Samir TM, Franco R (2012) Gold nanoparticles in the clinical laboratory: principles of preparation and applications. Clin Chem Lab Med 50: 193-209.
- Chen XJ, Sanchez-Gaytan BL, Qian ZX, Park SJ (2012) Noblemetal nanoparticles in DNA detection and delivery.Wiley Interdiscip Rev Nanomed Nanobiotechnol 4: 273-290.
- Yossef HE, Khedr AA, Mahran MZ (2011) Hepatoprotective activity and antioxidant effects of El Nabka (Zizyphusspina-christi) fruits on rats hepatotoxicity induced by carbon tetrachloride. Nat Sci 9:1-7.
- Abalaka ME, Daniyan SY, Mann A (2010) Evaluation of the antimicrobial activities of two Ziziphus species (Ziziphus mauritiana and Ziziphus spina-christi L.) on some microbial pathogens. Afr J Pharm Pharmacol 4:135-139.
- Asgarpanah J, Haghighat E (2012) Phytochemistry and pharmacologic properties of Ziziphus spinachristi (L.) Willd.Afr J Pharm Pharmacol 6: 2332-2339.
- Rodríguez-León E, Iñiguez-Palomares R, Navarro RE, Herrera-Urbina R, Tánori J, et al. (2013) Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumexhymenosepalus extracts). Nanoscale Res Lett. 10: 318.
- Ma J, Zhang J, Xiong Z, Yong Y, Zhao X S (2011) Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J Mater Chem 21: 3350-3352.
- Arakha M, Pal S, Samantarrai D, Panigrahi TK, Mallick BC, et al. (2015).Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface.Sci Rep.6: 14813.
- Mallmann EJJ,Cunha FA, Castro BNMF, Maciel AM, Menezes EA, et al. (2015)Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop Sao Paulo 57: 165-167
- Xia ZK, Ma QH, Li SY , Zhang DQ, Cong L, et al. (2016) The antifungal effect of silver nanoparticles on Trichosporonasahii. J Microbio Immunol Infect 49: 182-188.
- Chung IM, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G (2016) Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res Lett 11: 40.
- Almalah HI, Alzahrani HA, Abdelkader, HS (2019) Green Synthesis of Silver Nanoparticles using Cinnamomum Zylinicum and their Synergistic Effect against Multi-Drug Resistance Bacteria. J Nanotechnol Res 1: 095-107.
- Al-Zubaidi S, Al-Ayafi A, Abdelkader H (2019) Biosynthesis, Characterization and Antifungal Activity of Silver Nanoparticles by Aspergillusniger Journal of Nanotechnology Research 2: 22-35.
- Adzu B, Amos S, Wambebe C, Gamaniel K (2001) Antinociceptive activity of Zizyphus spinachristiroot bark extract Fitoterapia 72: 344-350.
- Al-Jassabi S, Abdullah MS (2013) Extraction, Purification and Characterization of Antioxidant Fractions from Zizyphus spina-christi Fruits. American-Eurasian J Toxicol Sci 5: 66-71.
- Cruickshank R (1968) Medical Microbiology: A guide to diagnosis and control of infection. Edinburgh and London : E & S Livingston Ltd 888.
- Clinical and Laboratory Standards Institute (2014) Performance standards for antimicrobial susceptibility testing, 24thinf suppl Pennsylvania USA 34.
- Obaid AY, Al-Thabaiti SA, Al-Harbi LM, Khan Z (2015) Extracellular bio-synthesis of silver nanoparticles. Global Adv Res J Microbio 3: 119-126.
- Ahmed S, Ikram S (2016) Biosynthesis of gold nanoparticles: A green approach. J Photochem Photobio 161: 141-153.
- Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf B Biointerfaces 368: 58-63.
- Inagaki S, Ghirlando R, Grisshammer R (2013) Biophysical characterization of membrane proteins in nanodiscs. Methods 59: 287-300.
- Lin PC, Lin S, Wang PC, Sridhar R (2014) Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv 32: 711-726.
- Fissan H, Ristig S, Kaminski H, Asbach C, Epple M (2014) Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal Methods 6: 7324-7334.
- Dieckmann Y, Cölfen H, Hofmann H, Petri-Fink A (2009)Particle size distribution measurements of manganese-doped ZnS nanoparticles. Anal Chem 81: 3889-3895.
- Pleus R (2012) Nanotechnologies-Guidance on Physicochemical Characterization of Engineered Nanoscale Materials for Toxicologic Assessment ISO: Geneva, Switzerland.
- Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM (2008) Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci 101: 239-253.
- Guzman M, Dille J, Godet S (2011) Synthesis and Antibacterial Activity of Silver Nanoparticles Against Gram-Positive and Gram-Negative Bacteria. Nanomedicine 8:37-45.
- Halawani EM (2017) Rapid Biosynthesis Method and Characterization of Silver Nanoparticles Using Zizyphusspinachristi Leaf Extract and Their Antibacterial Efficacy in Therapeutic Application. J Biomater Nanobiotechnol 8: 22-35.
- Parameswari E, UdayasoorianC, Sebastian SP, Jayabalakrishnan RM (2010) The Bactericidal Potential of Silver Nanoparticles, Int Res J Biotech 1: 44-49.
- Kvitek L, Panacek A, Soukupova J, Kolar M, Ve-Cerova R, et al. (2008) Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J Physic Chem C 112: 5825-5834.
- Zawadzka K, K?dzio?a K, Felczak A, Wro?ska N, Piwo?ski I, et al.(2014) Surface area or diameter – which factor really determines the antibacterial activity of silver nanoparticles grown on TiO 2 coatings?New J Chem 3: 3275-3281.
- Yamanaka M, Hara K, Kudo J (2005) Bactericidal actions of a silver ion solution on Escherichia coli, studiedby energy filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol 71: 7589-7593.
- Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, et al. (2010)Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram negative bacteria.Nanomedicine 61:103–109.
- Xu Y, Wei MT, Ou-Yang HD, Walker SG, Wang HZ, et al. (2016) Exposure to TiO2 nanoparticles increases Staphylococcus aureus infection of HeLa cells. J Nanobiotechnology 14: 34.
- Percival SL, Bowler PG, Dolman J (2007) Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int Wound J 4: 186-191.
- Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145: 83-96.
- Zhao L, Ashraf MA (2015)Influence of silver-hydroxyapatite nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med J. 64:506-513.
- Shrivastava S, Bera T, Roy A, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18:225103.
- Yang W, Shen C, Ji Q, An H, Wang J, et al. (2009) Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 20: 085102.
- Dehkordi SH, Hosseinpour F, Kahrizangi AE (2011) An in vitro evaluation of antibacterial effect of silver nanoparticles on Staphylococcus aureus isolated from bovine subclinical mastitis. African J Biotechnology 10: 10795.
- Nagy A, Harrison A, Sabbani S, Munson RS, Dutta PK, et al. (2011) Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int J Nanomedicine 6: 1833-1852.
- Sondi I, Salopek-Sondi B (2004)Silver nanoparticles as antimicrobial agent: a case study on coli as a model for Gram-negative bacteria.J Colloid Interface Sci 275: 177-182.
- Tiwari V, Mishra N, Gadani K, Solanki PS, Shah NA, et al. (2018). Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle AgainstCarbapenem-Resistant Acinetobacterbaumannii. Front Microbiol 9:1218.
- Vijayakumar S, Vinoj G, Malaikozhundan B, Shanthi S, Vaseeharan B, et al. (2015) Plectranthusamboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim ActaA Mol Biomol Spectrosc137: 886-891.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, et al. (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16: 2346-2353.
- Nasrollahi A, Pourshamsian K, Mansourkiaee P (2011) Antifungal activity of silver nanoparticles on some of fungi. Int J Nanomedicine 1:233-239.
Citation: AbdelkaderH, Alzahrani H, Al-Ayafi A, Al-Mulah H, Al-Zubaidi S(2019)Green synthesis, Characterization and Antimicrobial activity of Biosynthesized Silver Nanoparticles using Ziziphus spina-christi leaf extracts. Adv Microb Res 3: 010.
Copyright: © 2019 Hayam Abdelkader, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

