
Hashimoto’s Encephalopathy: A Remediable Cause of Unexplained Encephalopathy
*Corresponding Author(s):
Shafaq SaleemDepartment Of Neurology, Aga Khan University And Hospital, Karachi, Pakistan
Tel:+92 3330214197,
Email:drshafaqsaleem@gmail.com
Abstract
Hashimoto’s encephalopathy is non-vasculitic autoimmune steroid responsive encephalopathy. It is an uncommon syndrome characterized by altered level of consciousness, confusion, seizures and myoclonus. Exact pathogenesis is yet to be determined though humoral hyper-immune response is favored mechanism rather than dysthyroidism. We present a case of Hashimoto’s encephalopathy in an eighty years old lady who presented with unexplained drowsiness. Extensive workup for the cause of drowsiness was carried outto find structural, metabolic, autoimmune or para-neoplastic etiology only to reveal high titers of anti-TPO antibodies. Patient responded remarkably to high dose steroids.
Keywords
Encephalitis; Hashimotos; Steroids
Introduction
Hashimotos Encephalopathy (HE) first described in 1966 by Brain, et al. has gained prominence in the differential diagnosis of encephalopathy of unknown origin [1]. Hashimoto’s Encephalopathy (HE) is a rare neuropsychiatric syndrome, common in women, associated with serologic evidence of raised anti-thyroid antibodies with other causes of encephalopathy being excluded [2]. The relationship between HE and Hashimoto’s Thyroiditis (HT) remains uncertain, as no evidence suggests their effect on neuronal tissue. Level of circulating antibody levels don’t correlate with disease severity and cannot be followed for disease response to treatment. The exquisite response to corticosteroid therapy and its association with other autoimmune diseases points it towards a disorder of inflammatory or immunological dysfunction and earns it with name of “Steroid-Responsive Encephalopathy Associated with Autoimmune Thyroiditis (SREAAT) [2-5]. The propensity of delaying diagnosis and hence treatment lies in the variation in neurological manifestations as well as lack of well-defined diagnostic criteria.
Case Presentation
An 80 years old Asian woman, with independent mobility at home and known co-morbids of diabetes, hypertension and depression presented to emergency with complaints of progressive drowsiness since 1 week without associated fever, headache, fall, trauma or seizures. Aside from recently started sodium valproate (outside hospital) in epileptic doses with suspicion of subclinical seizure disorder, there was insignificant drug history. Upon examination she was vitally stable and general physical examination was unremarkable including lack of thyromegaly or lymphadenopathy. In neurological examination she was drowsy, responding with groaning, occasional eye opening to deep tactile stimulus and following simple single steps commands. Neck was supple. Pupils were bilaterally equal and reactive to light. Bulk and Tone was normal in all four limbs and she was able to move her limbs against gravity upon slight painful stimulus. Plantars were bilateral flexors. Gait and cerebellar examination could not be assessed.
Initiallaboratory workup showed normal complete blood count, renal and liver functions tests and electrolytes. Serum TSH and free T4 levels were normal as were serum ammonia and valproate levels. Urine and blood cultures were negative as were inflammatory markers (ESR/CRP). Ultrasound abdomen and CT pyelogram to look for infective focus came out unremarkable as well. Neurology department was consulted for unexplained drowsiness and MRI Brain, EEG and CSF studies were advised. MRI Brain showed no evidence of acute infarction, edema or demyelination; except periventricular white matter microvascular ischemic changes (Figures 1-4). Multiple EEGs were conducted showing intermittent bilateral (right more than left) fronto-temporal sharp and slow waves with intermittent generalized triphasic waves and diffuse theta and delta slowing. CSF studies showed white cell count of 1, glucose 89 mg/dl, proteins of 18 and. PCR test for multiple bacterial and viral pathogens was negative. Patient was initially started empirically on broad spectrum antibiotics in meningitic doses for few days and later shifted to non-meningitic doses as CSF studies were unremarkable. Later CSF autoimmune and serum paraneoplastic workup was sent along with serum anti TPO antibody. Attributing intermittent to frequent EEG discharges as a cause of drowsiness increasing dose of various anti-epileptics were given (leveteracetam, lacosamide and valproic acid) with minimal improvement. While paraneoplastic/ autoimmune panel and serum TPO antibody was pending; she was started with IV methylprednisolone1000mgonce a day empirically. Few days later CSF autoimmune/paraneoplastic panel came out negative while serum TPO antibodies titres came out significantly higher (401 IU/ml vs normal value < 35 IU/ml). Thyroid anti-microsomal antibody was not checked.
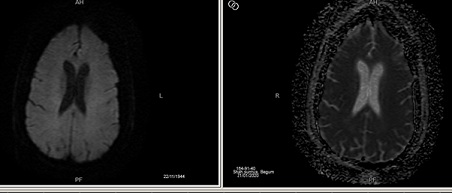 Figure 1: axial non contrast view of brain showing normal grey and white matter differentiation. No established infarct, mass effect edema or intracranial haemorrhage is detected.
Figure 1: axial non contrast view of brain showing normal grey and white matter differentiation. No established infarct, mass effect edema or intracranial haemorrhage is detected.
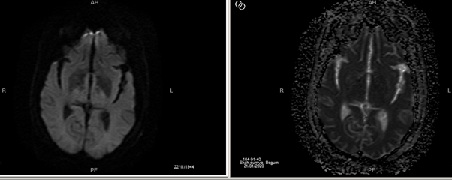 Figure 2: Axial non contrast view of brain showing hyperintensities in bilateral periventricular subcortical areas.
Figure 2: Axial non contrast view of brain showing hyperintensities in bilateral periventricular subcortical areas.
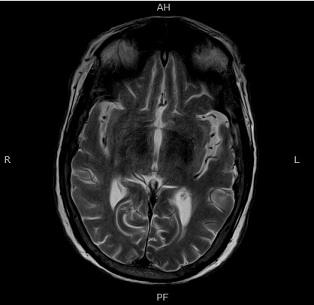 Figure 3: Axial T2 weighted image showing normal grey white matter differentiation: There is no established infarct , mass effect , edema or intracranial haemorrhage.
Figure 3: Axial T2 weighted image showing normal grey white matter differentiation: There is no established infarct , mass effect , edema or intracranial haemorrhage.
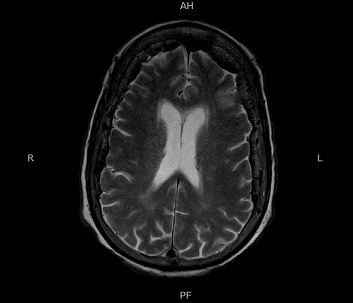 Figure 4: Axial T2 weighted non contrast image showing hyperintensities in periventricular areas suggesting white matter ischemic changes.
Figure 4: Axial T2 weighted non contrast image showing hyperintensities in periventricular areas suggesting white matter ischemic changes.
Patient started to open her eyes on verbal stimulus on 3rd day of IV methylprednisolone and soon started to make spontaneous moaning voices and move all four limb spontaneously. The patient was discharged on Nasogastric (NG) tube diet after few days on Tab Deltacortil 20mg per day. On follow-up after 5 days patient had spontaneous eye opening, NG feeding tube was removed, she was following one step commands and nodding head on communication and moving all four limbs against gravity. After 2 months Tab Deltacortil was reduced to 15mg per day for next 2 months. Currently, having passed 5 months, though she requires support for walking, she frequently utters monosyllable words for conversation. Currently, as we write the report patient is on Tab deltacortil 10mg per day with plan for further tapering on monthly interval.
Discussion
Hashimoto’s encephalitis is a rare reversible cause of encephalopathy associated with thyroid illness either indirectly as a thyroid complication or autoimmune phenomena [6]. Diagnosis of Hashimoto’s encephalitis needs high index of suspicion especially when extensive workup of drowsiness is unrevealing. It mimicks autoimmune encephalitis, paraneoplastic encephalitis, Creutzfeldt-Jakob, fronto-temporal dementia, HIV dementia, psychiatric illnesses which are progressive and difficult to manage. The diagnostic criteria has been described by Graus, et al. as follows [7]. 1. Encephalopathy with seizures, myoclonus or stroke like episodes; 2. Mild overt hypothyroid or subclinical thyroid disease; 3. MRI brain showing non-specific abnormalities; 4. Positive anti TPO antibodies; 5. Absence of other neuronal antibodies; 6. Reasonable exclusion of other alternative causes. The patient presented in this study accurately falls in the criteria with exhaustive investigations to pursue metabolic or septic encephalopathies at first, structural pathologies of brain later and to seek paraneoplastic/autoimmune encephalitis in the last revealed no lead except high TPO titres with exquisite clinical response to steroid [2,8].
A case series of 4 patients with divergent symptomatology but similar workup profile was described by Liaquat J, et al. in 2018 [9]. Age range was between 24 years to 55 years. MRI Brain findings were various ranging from non-specific white matter ischemic changes to midbrain/pons infarct. All had anti-TPO antibody and anti-microsomal antibody positive. Half of the patients had neuropsychiatric symptoms and were euthyroid. While CSF was normal in majority of cases, EEG was done in 2 patients only with non-specified cortical dysfunction. Another case series from same center describing 13 patients with Hashimoto’s encephalopathy was presented by Hashmat A, et al. [10]. Average age of diagnosis was 44 years while two third of cases were females and euthyroid. Neuropsychiatric symptoms with depression was predominant presentation. Overt seizures were present in one fourth of the cases. MRI findings showed temporal and hippocampal T2WI hyperintensities in 3out of 13 patients only. CSF protein was significantly raised in all patients. MRI findings, euthyroidisim and female gender relates to our case, while raised CSF protein and clinical presentation differs from above studies [9,10]. Thyroid functions were reported to vary in different cases with majority of the patients being euthyroid at the time of clinical presentation as in case of our patient [2].
Likewise in international literature, there is no specific MRI/CT, EEG or CSF finding to characterize the disease. Where Imaging reveals non-specific findings e.g., cerebral atrophy, focal cortical abnormality, diffuse subcortical abnormality and non-specifc subcortical focal white matter abnormality in many cases; half to two third of cases revealed no abnormality [4,11]. Most frequently observed EEG abnormality has been described as slow wave activity associated with encephalopathy [9,10,12]. A case series by Castillo P, et al. described 20 diagnosed patients with 19 patients having abnormal EEG. Majority had generalized slowing. Few of them had focal slowing, triphasic waves, epileptiform abnormalities and photomyogenic responses. Follow-up EEG in 17 patients showed resolution of EEG abnormalities. Generalized slowing, triphasic waves and focal seizure disorder was seen in our patient similar to the case series CSF fluid analysis is normal in majority (upto 80%) of patients with most frequent abnormality as raised protein with mild to moderate lymphocytic pleocytosis in minority of patients while IgG and oligoclonal bands are found in rarity [4,11].
Being known as steroid responsive encephalopathy with autoimmune thyroiditis, majority of the patients with Hashimotos Encaphalitis show improvement with steroid therapy as our patient. Other treatment modalities include treatment with immunomodulators e.g., azathioprine, cyclophosphamide, plasmapharesis and intravenous immunoglobulins in steroid resistant cases [13-15]. Response to antiepileptics has been a dilemma in patient presenting with seizures or myoclonus; seizures respond better to steroids than anti-epileptics. Optimistic short and long term prognosis can be expected. Most patients improve completely after the onset of corticotherapy, but the possibility of relapse may be as high as 12.5%-40% as pera two-year follow-up study [13].
Conclusion
We present a case of elderly lady presenting with drowsiness undergoing exhaustive investigations for structural, metabolic and septic etiology finally leading to fairly easily identifiable and treatable diagnosis of Hashimoto’s encephalopathy with good treatment response. The disease does not have characteristic imaging, EEG, CSF or thyroid profile findings hence should always be kept in list of differential diagnosis of unexplained encephalopathy.
Confict of Interest
We authors declare that there is no conflict of interest amongst authors for this case report.
References
- Brain L, Jellinek EH, Ball K (1966) Hashimoto’s disease and encephalopathy. Lancet 288: 512-514.
- Chong JY, Rowland LP, Utiger R (2003) Hashimoto encephalopathy: syndrome or myth?. Arch Neurol 60: 164-171.
- Sa´nchez Contreras A, Rojas SA, Manosalva A (2003) Hashimoto encephalopathy (autoimmune encephalitis). J Clin Rheumatol 10: 339-343.
- Castillo P, Woodruff B, Caselli R, Vernino S, Lucchinetti C, et al (2006) Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol 63: 197-202.
- Tamagno G, Celik Y, Simo´ R (2010) Encephalopathy associated with autoimmune thyroid disease in patients with Graves’ disease: clinical manifestations, follow-up, and outcomes. Neurology 10: 27.
- Chaudhuri A, Behan PO (2003) The clinical spectrum, diagnosis, pathogenesis and treatment of Hashimoto's encephalopathy (recurrent acute disseminated encephalomyelitis). Curr Med Chem 10: 1945-1953.
- Fiore AA, Pfeiffer WB, Rizvi SA, Cortes A, Ziembinski C, et al (2019) Hashimoto Encephalopathy as a complication of autoimmune thyroiditis. Med Princ Pract 28: 91-95.
- Shaw PJ, Walls TJ, Newman PK, Cleland PG, Cartlidge NE (1991) Hashimoto's encephalopathy: A steroid-responsive disorder associated with high anti-thyroid antibody titers--report of 5 cases. Neurology 41: 228-233.
- Liaqat J, Raja W, WaliW, Javed MU (2018) Neuro-Psychiatric Manifestations of Hashimoto Thyroiditis: A Case Series. Pak Armed Forces Med J 68: 369-373.
- HashmatA, Alamgir W, Arif S, Liaqat J (2019) Evaluation Of Clinical Symptomatology In Patients With Hashimoto’s encephalopathy and Association with Underling Thyroid Disease. Pak Armed Forces Med J 69: 971-976.
- Barseem NF, Helwa MA (2015) Hashimoto’s encephalopathy presenting with acute confusional state in a patient with hypothyroidism. Egyptian Pediatric Association Gazette 63: 69-73.
- Zamora EM, Labarta ML, Mallor BT, Villacampa CV, Avellanas CM, et al. (2014) Hashimoto’s encephalitis, report of a case. Medicina Intensive 38: 522.
- Gauthier AC, Baehring JM (2017) Hashimoto’s encephalopathy mimicking Creutzfeldt-Jakob disease. J Clin Neurosci 35: 72-73.
- Marshall GA, Doyle JJ (2006) Long-term treatment of Hashimoto’s encephalopathy. J Neuropsychiatry Clin Neurosci 18: 14-20.
- Kothbauer-Margreiter I, Sturzenegger M, Komor J, Baumgartner R, Hess CW (1996) Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment. J Neurol 243: 585-593.
Citation: Javed M, Saleem S, Sajjad A, Wasay M (2021) Hashimoto’s Encephalopathy: A Remediable Cause of Unexplained Encephalopathy. J Clin Stud Med Case Rep 8: 0108.
Copyright: © 2021 Maryam Javed, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

