
Hearing Amplification Improves Eye Movements: A Case Study
*Corresponding Author(s):
Zoï KapoulaIris Laboratory, Physiopathology Of Motor Binocular & Vision, CNRS FRE2022, University Of Paris, France
Tel:+33 0142864039,
Email:zoi.kapoula@gmail.com
Abstract
Background: Using two different approaches, we study the possible interaction between the sound and the preparation and execution of eye movements. The first approach deals with the sensory modalities of the target to which eye movements are made, the second with the hearing amplification of the subject.
Material: Saccades and vergence were tested for a healthy 19-year-old student performing his internship in the lab. Tests with visual vs audiovisual targets were done. The normal hearing participant performed all tests with a hearing bilateral device that was adjusted in different conditions either at zero (no amplification), moderate amplification, or high amplification. Experimentations were made over a month, and the different tests were executed following a Latin square design.
Results: We found statistical differences between eye movements towards audio-visual targets and visual targets in the absence of hearing amplification. The presence of the sound component on the stimulus improved the preparation and execution of saccades and impacted the execution of vergence.
The hearing amplification has a differential impact on eye-movements towards visual targets. For vergence, it significantly reduced the amplitude of convergence. For saccades, it increased the amplitude and decreased the latency. These results could be due to an arousal state elicited by the amplifying background noise and sound coming from the participant himself.
Conclusion: This pilot study opens the investigation of the different interactions between sound and saccade and vergence eye movements. We hope that these results will inspire other studies.
Keywords
Arousal; Multi-sensoriality; Saccades; Vergence
INTRODUCTION
Eye movements are strongly linked to attention [1]. As such, they have become an easy clinical tool to examine motor control, cognition, and memory. Eye movements are easily observable and their dynamic properties are well known [2]. Eye movements are mostly studied toward visual-only targets and, in some cases, toward auditory-only targets. The saccades evoked with these two different modality stimuli do not result from the same cognitive process. Previous studies have shown that saccades for auditory targets have longer latencies than for visual targets [3,4].
The effect of sound on global performances has been the topic of many studies, with different approaches and various results.
One of these approaches is to study eye movements toward multisensory targets, including a sound component. The literature shows that reaction time toward multisensory targets is faster. These results are valid either for manual task or for eye movements [5,6]. The association between auditory and visual stimulation produces an intersensory facilitation that ultimately reduces saccade latency. This facilitation occurs when the two modalities respect a certain proximity in time and space [7,8]. Depending on the different paradigms of the studies, this time window varies between 40 and 600 ms. In a more recent study from our laboratory, both saccades and vergence eyes movements to either visual or to auditory-visual targets were investigated. These studies showed a significant differential effect of the sound on the saccades and the vergence. The addition of a sound stimulus decreases saccade latency without affecting its dynamics (velocity and duration); in contrast, for vergence, the duration decreases and the velocity increased with no effects on the latency [17].
One explanation of these results is that both auditory neural information and eye movement generation pass for instance through the superior colliculus. The superior colliculus is involved in the initiation and execution of saccades to the visual target [9]. It is also a step on the neural auditory pathway [10]. Cells in the superior colliculus respond to information coming from different sensory modalities, and behavioral changes can be seen when different sensory modalities are combined [11].
The presence of a sound component in a stimulus improves various aspects of eye movements but it is not yet known whether the intensity of this sound also has an effect. Therefore, in the present study, the first goal is to investigate whether the amplification of the sound component of an auditory-visual stimulus impacts the parameters of saccades and vergence eye movements toward this stimulus.
Another approach to study the effect of sound on global performance is to study the impact of an external background sound not linked to any stimulus in particular. In these kind of studies, selective attention [12,13], monitoring performance [14], or vigilance are measured depending on the presence of a particular background noise. The results are different and can be related to the type of sound used, but no studies have been made on eye movements.
The second goal of the study is to investigate whether the amplification of the participant’s hearing impacts the parameters of saccades and vergenceeye movements toward visual targets.
We have used a psychophysical single case study on a healthy young participant with an experimental design testing the presence of a sound prior to the visual target (visual target when there is no sound and audio-visual target with the sound) and the level of the hearing amplification of the participant (three levels: no amplification, moderate amplification and high amplification).
MATERIALS AND METHODS
Our study was assessed by the ethic committee “Ile de France II”.
Participant
This was a pilot study run over several days over a month with the voluntary cooperation of a student in optics performing its internship in the laboratory. An informed consent was obtained from the participant. The student 19 years old, was carefully screened in terms of auditory and visual function.
Hearing screening
A tonal audiometry in a sound booth was made to assess the good hearing of the participant. All the sound between 125 Hz and 8kHz was heard under a 20 dBHL level.
Visual screening
The refraction was objectively tested with an Autorefractor ARK-1 Nidek and subjectively with a Phoropter Nidek RT-600 to determine the best correction to obtain visual acuity. All subsequent measures were done with the optimum correction using the Phoropter/Trial Frame (Oculus Adult UB4, Zeiss) in free space. The visual acuity was measured with a Snellen chart projector at 5m vision for the far vision, and with a Snellen near chart for the near distance. The binocular vision was measured with the Wirt Rings Stereo Tests. The Horizontal and Vertical Phorie are measured with a prism bar. The Convergence and Divergence fusional ranges at distance and near vision were measured using rotary prisms. The amount of prism induced is gradually increased until the participant reports changes: The blur point is measured when the participant reports blurry vision, the breakpoint is measured when the participant reports double vision. The amount of prism induced is then gradually decreased, and the recovery point is measured when the participant reports single vision again. We measured the Near Point of Convergence (NPC) with a Fixation Stick and a millimeter rule. Our participant, with correction, has a visual acuity of 15/10 for both eyes. His stereo acuity with the Wirt stereo test was 50”. He has horizontal exophoria of 4 prism diopters for near vision and 1.5 pd for far vision. No vertical phoria was found. For the distance vision, the divergence ranges showed a blur point at 8 pd a breakpoint at 10 pd and a recovery point at 2 pd. The convergence ranges showed a blur point at 6 pd, a breakpoint at 26 pd and a recovery point at 6 pd. At near vision, the divergence ranges showed a blur point at 12 pd, a breakpoint at 16 pd and a recovery point at 11 pd. The convergence ranges showed a blur point at 12 pd, a breakpoint at 26 pd and a recovery point at 11 pd. Without his glasses, the participant vision was 3/10.
The tests with REMOBI were done without visual correction, but a 3/10 acuity is enough to see the LEDs.
Oculomotor tests
left saccades, right saccades, convergence and divergence were tested with protocols run at the REMOBI [15], device first described by in a previous study of our laboratory. Eye movements were recorded binocularly with the head-mounted video-oculography device [15 remove], Pupil Core (Pupil Labs, Berlin).
The REMOBI (patent US8851669, WO2011073288) is a Visio-acoustic surface placed at the eye level; 48 LEDs with nominal frequency 626 nm, 180 mCd and a diameter of 3 mm are embedded at 4 is overgence arcs. The embedded algorithms enable to test different types of eye movements lighting different sequences of LEDs. Adjacent to each LED is embedded a buzzer delivering a pure sound at 2048 Hz, the sound pressure level being 70 dB.
In this study, we used two tests, one for testing left and right saccades with the LEDS placed at the isovergence arc at 70 cm from the participants’ eyes; the other for vergence eye movements along the median plane, using the 3 central LEDs, the initial at 40 cm from the participant’s eyes, the other at 20 cm (for convergence movements), and at 150 cm for divergence movements.
Both Saccade and Vergence tests were composed by 40 trials each. During a trial, the participant first fixates at the central fixation LED, during a random time between 1200 ms and 1800 ms, then a target LED is light on for 2000 ms, following an overlap paradigm, i.e. the central fixation LED switch off 200ms after the onset of the target LED. Between trials, a blanked period of 300 ms to 700 ms was applied. The participant was instructed to fixate as quickly and as accurately as possible the target LED and to maintain the fixation.
The saccade test, was composed by 20 trials of right saccades and 20 trials of left saccades, randomly interleaved. The initial fixation LED is located at 70 cm. The angles formed by the two lateral LED targets for saccades were 20° left and right (Figure 1 A).
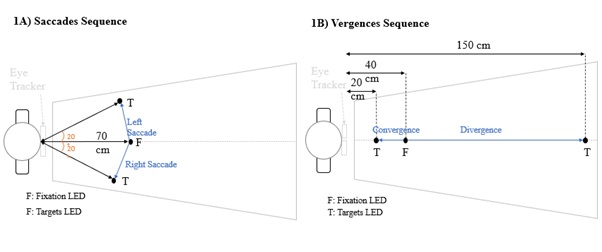 Figure 1: A) Position of the LEDs for the saccade test B) Position of the LEDs for the vergence test.
Figure 1: A) Position of the LEDs for the saccade test B) Position of the LEDs for the vergence test.
The vergence test, was composed by 20 trials of divergence and 20 trials of convergence also randomly interleaved. The central initial fixation LED is located at 40 cm of the participant’s eyes calling for a vergence angle of 9°; for divergence movements, the LED is located at 150 cm calling for a vergence angle of 2.5°, meaning that the required amplitude of divergence movement is 6.5°. For convergence trials, the vergence angle should change from 9° to 17.76° which is appropriate for fixating the LED at 20 cm, meaning that the amplitude of convergence movement requested is 8.76° (Figure 1B). All these values are given with a pupillary distance of 62 mm.
In our study, the participant was sitting in front of the REMOBI placed at eye level; his head was not constrained but he was instructed not to move it.
The different sensory modalities
The participant was tested with two saccade and two vergence sensory modalities: For audio-visualtargets (AV targets), i.e., the LED lighting was preceded by turning on the buzzer adjacent to that LED at 50 ms before the LED and for a duration of 100 ms. For the visual targets (V targets), no buzzer sound occurred before the LED.
Hearing Amplification (HA)
The hearing amplification devices used were two hearing aids OPN1 from Oticon. For the different tests, the level amplification was set at zero (no amplification), moderate (12,5 dB SPL of amplification), or high (28 dB SPL of amplification).
The volume delivered with higher amplification was just at the limit of discomfort for the participant; it was determined by increasing progressively the amplification until the participant signaled that the amplification was no longer tolerated. It was important to use the highest possible amplification to contrast it with the no amplification.
Hearing amplification has a double action: amplify the sound coming from the LED stimulus of the REMOBI device, and secondly, amplify the environment noise including the self-noise e. g. breath, swallowing, clothes frictions.
LATIN SQUARE DESIGN
The study was spread over 6 days. The tests sessions for audio-visual targets were done in the morning and those with V targets were done during the afternoon.
We had 6 tests for each sensory modality (audio-visual vs visual): saccades without amplification, saccade with moderate amplification, saccade with high amplification; vergence without amplification, vergence with moderate amplification and vergence with high amplification. One test session was composed of these 6 tests run with audio-visual or visual targets. In total, every day the participant made 12 tests; the study was run with a Latin square design in order to spread the order effect.
Eye Movement analysis
Data recorded with the Pupil Labs eye tracker were analyzed with AIDEAL, software developed in the IRIS laboratory (patent no 2004768, France). The vergence signal was derived by calculating the difference between the two eyes from the individual calibrated eye position signals (i.e., left eye - right eye). The beginning and end of the vergence movements were defined as the time point when the eye velocity exceeded or dropped below 5°/s: these criteria are standard and were applied automatically by the AIDEAL software; the program estimated the initial phasic component as the amplitude between these two initial points. This component does not represent the total movement that can continue for 160 ms or more. Our interest here was on the initial component which is open loop while the subsequent component is more visually driven. All parameters of vergence (amplitude, duration, average velocity) concern this phasic component. The amplitude of the phasic component was expected to be 50 to 60% of the total amplitude required.
For saccade analysis, AIDEAL treated the conjugate signal, e.g. the L+R eye position/2. The onset and the offset of the saccade were defined as the time points where the peak velocity went above or below 10% of the peak velocity; practically, this corresponded to values above or below 40°/s (as the peak velocity of 20° saccades is typically above 40°/s). The total average velocity was defined as the ratio of total amplitude in degrees divided by time in seconds. To evaluate binocular coordination of saccades, or the disconjugacy during saccadic movements, the difference in amplitude between the left and the right eye signal was calculated. The disconjugate drift, or the difference in drift amplitude during the first 80 or 160 ms of fixation, was calculated. These calculations are standard and have been used in previous experiments. Trials with blinks or other artifacts were discarded automatically by AIDEAL.
Trials from different sessions were regrouped (see below). Trials with blinks were excluded and this occurred frequently for vergence (see below). Blinks occurred more frequently for vergence movements, as such movements are known to be less stereotyped and more participant to fatigue.
Statistical analysis
A student T-test respectively compared the results between AV and V targets. One-way ANOVA tests, either toward audio-visual targets or toward visual targets, were run to compare the results with no amplification, moderate amplification, and high amplification. A post hoc test of Tukey was applied when a significant result was found.
RESULTS
Next, we present the results in two sections: the first describing the influence of the sensory modality of the stimulus (visual VS audio-visual), the second describing the effect of hearing amplification.
All the results are presented in Table 1. The amplitude, latency, duration and velocity for each movement, toward audio-visual and visual targets, and for the three levels of amplification (no amplification, moderate amplification and high amplification). Values are the means (standard deviation) and the number of movements. The value of ANOVA and the level of significance are shown.
|
Eyes movements in function of hearing amplification |
||||||
|
|
|
|
Amplitude (deg) Mean (s/d) ,N |
Latency (ms) Mean (s/d) ,N |
Duration (ms) Mean (s/d) ,N |
Avelocity (deg/ms) Mean (s/d) ,N |
|
Amplifier |
||||||
|
C |
V |
zero A |
2.41 (1.1), n=35 |
318 (98), n=51 |
56 (22), n=37 |
44 (22), n=53 |
|
moderate A |
2.62 (1.1), n=50 |
304 (84), n=58 |
58 (20), n=48 |
43 (13), n=61 |
||
|
high A |
1.91 (0.6), n= 47 |
293 (109), n= 62 |
50 (16), n=40 |
40 (15), n=58 |
||
|
ANOVA |
F=6.495, p=0.002 |
F=0.926, p=0.398 |
F=5.116, p=0.007 |
F=0.941, p=0.392 |
||
|
AV |
zero A |
3.21 (0.9), n= 45 |
300 (56), n=46 |
70 (17), n=45 |
46 (8), n=46 |
|
|
moderate A |
3.08 (1.3), n=54 |
297 (99), n= 58 |
67 (24), n=49 |
44 (16), n=63 |
||
|
high A |
3.21 (1.1), n= 47 |
305 (103), n=51 |
67 (19), n=45 |
46 (13), n=54 |
||
|
ANOVA |
F=0.238, p=0.788 |
F=0.104, p=0.901 |
F=0.349, p=0.706 |
F=0.54, p=0.584 |
||
|
D |
V |
zero A |
1.60 (0.8), n= 49 |
276 (106), n=86 |
44 (13), n=28 |
44 (15), n= 91 |
|
moderate A |
1.47 (0.3), n= 65 |
272 (78), n=103 |
38 (7), n=34 |
46 (15), n=106 |
||
|
high A |
1.43 (0.3), n=63 |
271 (120), n=93 |
39 (13), n=36 |
44 (16), n=106 |
||
|
ANOVA |
F=1.672, p=0.191 |
F=0.06, p=0.942 |
F=2.29, p=0.107 |
F=0.456, p=0.634 |
||
|
AV |
zero A |
1.72 (0.5), n=59 |
286 (75), n=77 |
38 (8), n=40 |
47 (17), n=81 |
|
|
moderate A |
1.73 (0.5), 67 |
283 (100), 101 |
41 (10), n=45 |
47 (19), n=102 |
||
|
high A |
1.75 (0.45), 55 |
307 (122), n=100 |
39(18), n= 39 |
45 (20), n=108 |
||
|
ANOVA |
F=0.045, p=0.956 |
F=1.535, p=0.217 |
F=0.527, p=0.592 |
F=0.573, p=0.564 |
||
|
LS |
V |
zero A |
18.3 (1), n=96 |
248 (32), n=94 |
66 (7), n=95 |
280 (31), n=95 |
|
moderate A |
18.7 (1.2), n= 102 |
254 (38), n= 101 |
65 (6), n=102 |
285 (24), n=100 |
||
|
high A |
18.7 (1.3), n=101 |
238 (37), n=102 |
66 (6), n= 101 |
284 (xx), n= 101 |
||
|
ANOVA |
F=4.11, p=0.017 |
F=4.9, p=0.008 |
F=0.436, p=0.647 |
F=0.973, p=0.379 |
||
|
AV |
zero A |
18.2 (1.0), n= 106 |
226 (53), n=104 |
64 (7), n=104 |
291 (27), n=105 |
|
|
moderate A |
18.5 (1.1), n=98 |
223 (34), n=96 |
65 (7), n=101 |
288 (28), n=99 |
||
|
high A |
18.5 (0.9), n=100 |
225 (48), n=100 |
64 (5), n=99 |
292 (21), n=99 |
||
|
ANOVA |
F=2.774, p=0.064 |
F=0.1, p=0.905 |
F=0.563, p=0.57 |
F=0.524, p=0.593 |
||
|
RS |
V |
zero A |
17.9 (1.0), n= 103 |
235 (29), n= 104 |
61(8), n= 105 |
240 (40), n=104 |
|
moderate A |
18.9 (1.5), n=103 |
241 (32), n= 103 |
64 (6), n= 101 |
297 (33), n= 103 |
||
|
high A |
18.7 (1.4), n=107 |
225 (38), n= 103 |
62 (6), n= 106 |
303 (29), n=106 |
||
|
ANOVA |
F=19.492, p=0 |
F=6.007, p=0.003 |
F=2.88, p=0.058 |
F=2.059, p=0.129 |
||
|
AV |
zero A |
18.7 (1.1), n=102 |
219 (47), n= 101 |
61 (7), n= 104 |
302 (34), n=101 |
|
|
moderate A |
18.4 (1.8), n=108 |
218 (31), n=107 |
63 (10), n=104 |
295 (51), n=108 |
||
|
high A |
18.6 (1.1), n=97 |
209 (28), n= 100 |
61 (5), n=97 |
296 (50), n=100 |
||
|
ANOVA |
F=1.179, p=0.309 |
F=2.459, p=0.087 |
F=2.673, p=0.071 |
F=0.682, p=0.506 |
||
Table 1: Results and statistical values for the movement towards visual targets (V) and audiovisual targets (AV), when the hearing devices delivered no amplification (zero A), moderate amplification (moderate A) and high amplification (highA), for convergence (C), divergence (D), left saccade (LS) and right saccade (RS). Mean, s/d and number of observations are given. The statistical analyses were made with a one-way parametric ANOVA to study the influence of the amplification, in function of AV or V targets separately.
Influence of the sensory target modality: visual vs audiovisual targets
For vergence, the presence of the buzzer prior to the LED target significantly amplified the amplitude of the convergence and increased its duration. For divergence, the duration was significantly decreased despite the fact that the amplitude increase was not statistically significant (Figure 2).
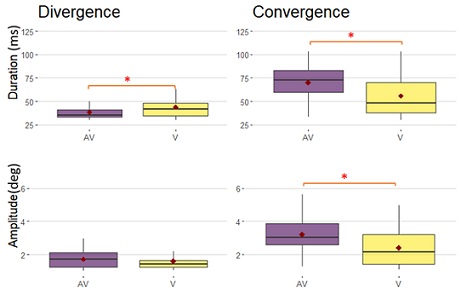 Figure 2: Boxplots of duration and amplitude for divergences and convergences, only with the hearing devices with no amplification (zero amplification), for AV and V paradigms. Red points represent the mean. Significant differences are noticed by the asterix.
Figure 2: Boxplots of duration and amplitude for divergences and convergences, only with the hearing devices with no amplification (zero amplification), for AV and V paradigms. Red points represent the mean. Significant differences are noticed by the asterix.
For saccades, the buzzer significantly reduced the latency for both directions (left and right). For left saccades, the average velocity increased and the duration significantly decreased. For right saccades, the amplitude increased significantly. In summary, the buzzer affected predominantly the execution of vergence, while for saccades it affected both the latency and the execution (Figure 3).
 Figure 3: Boxplots of latency and amplitude for left and right saccades, only with the hearing devices with no amplification (zero amplification), for AV and V paradigms. Red points represent the mean. Significant differences are noticed by the asterix.
Figure 3: Boxplots of latency and amplitude for left and right saccades, only with the hearing devices with no amplification (zero amplification), for AV and V paradigms. Red points represent the mean. Significant differences are noticed by the asterix.
INFLUENCE OF HEARING AMPLIFICATION
In this part, we compare the influence of different levels of hearing devices amplification. A parametric ANOVA was run with one factor (amplification) three levels (no amplification, moderate amplification and high amplification).
Toward audio-visual targets
The ANOVA showed no statistically significant effect of hearing amplification for vergence, for saccades, and for any of the eye movement parameters.
Toward visual targets
The ANOVA run separately for saccades and vergence showed that the level of hearing amplification devices significantly influences parameters of convergence and of saccades.
We next applied the post-hoc Tukey Test.
For convergence, the higher amplification level significantly reduced the amplitude (t=-3.535, p=0.00164) compared to moderate amplification. High amplification reduced the duration compared to moderate amplification (t=-3.050, p=0.00783) and no amplification (t=-2.390, p=0.04785). For divergence there was no significant effect (F=1.6722, p =0.1908) (Figure 4).
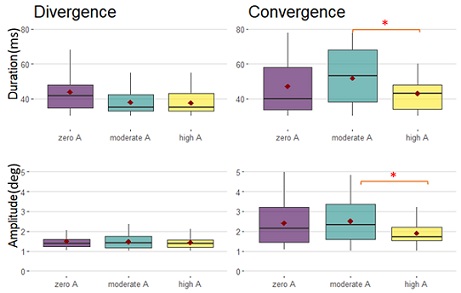 Figure 5: Boxplots of duration and amplitude for divergence and convergence, only for the V paradigm, for zero, moderate and high amplification. Red points represent the mean. Significant differences are noticed by the asterix.
Figure 5: Boxplots of duration and amplitude for divergence and convergence, only for the V paradigm, for zero, moderate and high amplification. Red points represent the mean. Significant differences are noticed by the asterix.
For left saccades, the ANOVA showed that moderate amplification significantly increased the amplitude compared to no amplification (t=2.648, p=0.0232), while high amplification significantly reduced the latency compared to moderate amplification (t=-3.090, t=0.0062).
For the right saccades, hearing amplification with moderate (t=5.566, p<1e-05)) or high amplification (t=5.252, p<1e-05), significantly increased saccade amplitude; moreover, high amplification significantly reduced the latency compared to moderate amplification (t=-3.433, p=0.00191) (Figure 5).
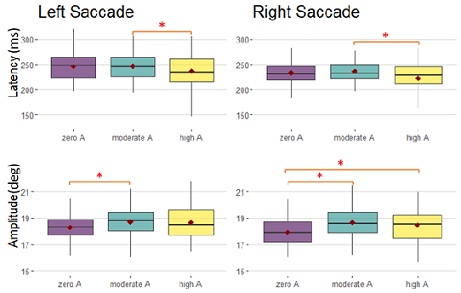 Figure 4: Boxplots of latency and amplitude for divergence and convergence, only for the V paradigm, for zero, moderate and high amplification. Red points represent the mean. Significant differences are noticed by the asterix.
Figure 4: Boxplots of latency and amplitude for divergence and convergence, only for the V paradigm, for zero, moderate and high amplification. Red points represent the mean. Significant differences are noticed by the asterix.
In summary, hearing amplification impacts only movement to visual targets. Thiseffect is more impressive for saccades, as it increases the amplitude and decreases the latency. For vergence, the hearing amplification reduces the amplitude of convergence only.
Overall, the study demonstrates differential effects of sound and hearing amplification for saccades and vergence.
DISCUSSION
Sound affects the execution of vergence and improves both the preparation and the execution of saccades
Our results show that sound effects the latency of the saccades. Latencies are shorter for audio-visual versus visual targets, consistent with the literature [3,4,7]. Moreover, with an accompanying sound stimulus, the amplitude of the saccades to the right increases significantly. Given that saccades to the right were hypometrics, the presence of sound in stimulating saccades to the right leads to an improvement in terms of accuracy, which is not reported in the above cited studies. To our knowledge, there is no literature that reports the effect of multi-sensory stimuli on the amplitude and velocity of the saccades.
We postulate that the sound from the buzzer provides both a warning stimulus, thereby facilitating saccades preparation, and a multisensory integration that could be responsible for the amplitude increase. This interpretation is in line with previous studies [3]. Noticeably, our sound appears 50 ms prior to the LED target, and such temporal configuration as shown by Frens, et al. produces a more dramatic decrease in saccade latency [16].
Our study shows, in agreement with the prior study that there are also effects from the buzzer on vergence. Importantly [17], this effect was present only for amplitude in convergence. Perhaps, the attention deployment mechanism involved during vergence preparation is not influenced predominantly by the sensory modality of the target, but sound can modify its execution. In other words, the sound could predominantly affect the premotor brainstem circuitry of the vergence command (mesencephalic reticular formation), rather than the cortical parietal frontal circuit involved to prepare the movement [18].Whether such observations are specific to this participant or general remains to be shown. The general agreement with the prior study [17] is consolidating the present findings.
Hearing amplification improves differentially saccades and vergence to visual targets
Concerning the hearing devices amplification, no significant effect was found for audio-visual targets. This means that when the sensory modality of the target includes an external sound, the internal hearing amplification is useless; all eye movements are programmed and executed similarly regardless of the hearing amplification. Amplifying the sound further by the hearing devices does not have any influence on the eye movement parameters. In other words, the external buzzer sound completely drives the eye movement preparation and execution circuit. Prior studies found similar results. Gabriel, et al. showed that the eccentricity effect for auditory target, i.e the fact that the latency of saccade decreases with the increasing target eccentricity, is independent of the intensity of the target [19]. A study of Arndt, et al. on audiovisual saccades suggested a two-stage model where the intensity of the auditory stimulus of a bimodal target does not affect the process of crossmodal integration, but is rather integrated in a different, more peripheral stage. In this model, the intensity of the auditory stimulus affects the reaction time of saccades toward a bimodal target because the auditory modality is integrated faster during the early stage of processing; however, it then no longer affects neural coactivation [20]. As our auditory stimulus is sent 50 ms before the LED, the possible additional effect of the intensity of the auditory buzzer stimulus (amplified by the hearing devices) is no longer pertinent as the benefit was already maximal.
The most interesting result is that for silent LED visual targets, the level of hearing amplification via the hearing devices modified many eye movement parameters. Importantly, the modulation for saccades and vergence were once again different. High amplification of the hearing devices reduces the amplitude of convergence, while moderate or high hearing amplification reduces the latency of left and right saccades and increases the amplitude of the left and right saccades. In other words, internal hearing amplification improves saccade eye movements to the external visual target, while simultaneously deteriorating the amplitude of convergence. These results are unexpected: because there is technically no external sound (no buzzer), the effects on the oculomotor movements act via different mechanisms than the eye movement target itself.
There is an important bibliography on the influence of sound on detection and cognitive tasks. This literature has many contradictory results, depending on the nature of the noise and the testing situation. Some studies found that sound elevates task engagement and improves performance if it varies rather than being continuous and the task is short enough [21,22]. In response to continuous noise, performance may improve initially because of an increase in arousal, but the initial improvement may wane as arousal falls. T. Auburn and al found that a loud noise increased the speed of detection of a sequence of two identical digits during the first twenty minutes of a task [23]. Moradi and al found that selective attention [12], measured with the DUAF test, was improved with a stress noise background. Helton and al found that, during a 12 minutes vigilance task, the intense noise of dynamic aircraft increases the task engagement and the correct detections [24].
Even if there was no specific sound from targets during the visual tests, the hearing devices also amplifies all sound coming from the participant himself: his respiration, the noise of mastication, etc. Our testing wasn’t conducted in a sound booth, which means continuous background noise from outside could be heard. We normally don’t pay attention on the background noise because it is always with us; we are more marked by the real silence of an anechoic room. However, a normal hearing person should hear an amplifying background noise well with hearing devices. The high amplification of hearing devices was set at the participant’s discomfort limit; therefore, every sound he heard at this amplification was loud for him. These sounds could induce an arousal state, leading to an improvement in performance.
The arousal theory is coherent with our results because improved performance was especially apparent with high rather than moderate amplification. Our test session time, approximately 15 minutes, corresponded with the time during which arousal has effect on performance on the precedent studies [12,23,24].
The arousal theory is coherent with the study of Meredith and Stein [20], which describes how cells from the superior colliculus change their comportment when different sensory modalities are combined.
If the arousal theory is right, one may ask why it didn’t occur during the paradigm with LEDs and sound together. One possible response is that something else countered this effect. As previously mentioned, the high amplification of the hearing devices reaches the discomfort threshold level of the participant, which could have stressed him and cancelled out the beneficial effect of the arousal.
One other explanation is that there may be a possible trade-off between externally oriented auditory attention (the buzzer) and internally oriented attention (hearing amplification). The improvement produced by the external sound (the buzzer) is already maximized, and there could be a ceiling effect in which the internal amplification cannot add any additional improvement.
The hearing amplification could also act as a warning stimulus that accelerates the preparation of the movement, which is done mostly by the cortex.
In contrast, for vergence, we demonstrated a negative effect: the amplitude of vergence is reduced when hearing amplification is high. One potential explanation for this finding could be an interaction between the internal amplification system and the perception of a closed space. Further research should be conducted to help explain this unique finding.
This case study indicates that there are differential effects between saccades and vergence, even with hearing amplification. This means that visual-auditory attention and eye-movement preparation, controlled by cortical and sub cortical circuits, are not identical for saccades and vergence. This distinction in the neurological correlates of these functions have been shown for the brainstem circuits for saccade and vergence [25]. Partial distinctiveness for cortical ocular motor circuits controlling the programming of saccades and vergence have been also suggested in the literature [26].
Although this is a preliminary pilot study, we demonstrate the important of the interaction between sensory target modalities and the hearing level of the individual. The studies mentioned above on detection, and vigilance tasks, would presumably involve execution of eye movements. The effect of noise could be mediated via the parameters of the eye movements per se. We hope it will stimulate further research in order to explore this interaction further.
ACKNOWLEDGEMENT
Lindsay Ward, UCSF improved the English, Dr. Francois Daniel, performed the visual examination of the participant. Funding of the study, ANRT, bourse CIFRE, M. Chavant candidate Ph.D.
REFERENCES
- Rizzolatti G, Riggio L, Dascola I, Umiltá C (1987) Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25: 31-40.
- Leigh RJ, Kennard C (2004) Using saccades as a research tool in the clinical neurosciences. Brain 127: 460-477.
- Lueck CJ, Crawford TJ, Savage CJ, Kennard C (1990) Auditory-visual interaction in the generation of saccades in man. Exp Brain Res 82: 149-157.4.
- Zambarbieri D, Schmid R, Magenes G, Prablanc C (1982) Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res 47: 417-427.
- Diederich A, Colonius H (2004) Bimodal and trimodal multisensory enhancement: Effects of stimulus onset and intensity on reaction time. Perception & Psychophysics 66: 1388-1404.
- Colonius H, Arndt P (2001) A two-stage model for visual-auditory interaction in saccadic latencies. Perception & Psychophysics 63: 126-147.
- Diederich A, Colonius H (2008) Crossmodal interaction in saccadic reaction time: separating multisensory from warning effects in the time window of integration model. Exp Brain Res 186: 1-22.8.
- Colonius H, Diederich A (2010) The optimal time window of visual-auditory integration: a reaction time analysis. Front Integr Neurosci 4.9.
- Lee C, Rohrer WH, Sparks DL (1988) Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332: 357-360.
- Middlebrooks JC, Knudsen EI (1984) A neural code for auditory space in the cat’s superior colliculus. J. Neurosci 4: 2621-2634.11.
- Meredith MA, Stein BE (1986) Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. Journal of Neurophysiology 56: 640-662.12.
- Moradi G, Omidi L, Vosoughi S, Ebrahirmi H, Alizadeh A, et al. (2019) Effects of noise on selective attention: The role of introversion and extraversion. Applied Acoustics 146: 213-217.
- Hockey GR (1970) Effect of Loud Noise on Attentional Selectivity.
- Pearson RG, Shelnutt JB, Casey SM (1976) Combined tracking and monitoring performance over 7 hours under noise. Ergonomics 19: 355-356.15.
- Kapoula Z, Morize A, Daniel F, Jonqua F, Orssaud C (2016). Objective evaluation of vergence disorders and a research-based novel method for vergence rehabilitation. Transl Vis Sci Technol 5: 8.
- Frens MA, Van Opstal AJ (1995) A quantitative study of auditory-evoked saccadic eye movements in two dimensions. Exp Brain Res 107: 103-117.
- Kapoula Z, Pain E (2020) Differential effect of sound on saccades, vergence and combined eye movements. Frontier in integrative neuroscience. Under revision. Master thesis of Erwann PAIN, University Pierre et Marie Curie.
- Kapoula Z, Isotalo E, Müri RM, Bucci M.-P, Rivaud-Péchoux S (2001) Effects of transcranial magnetic stimulation of the posterior parietal cortex on saccades and vergence. Neuro Report 12: 4041-4046.
- Gabriel DN, Munoz DP, Boehnke SE (2010) The eccentricity effect for auditory saccadic reaction times is independent of target frequency. Hearing Research 262: 19-25.20.
- Arndt PA, Colonius H (150) Two stages in crossmodal saccadic integration: evidence from a visual-auditory focused attention task. Exp Brain Res 150: 417-426.
- Davies DR, Parasuraman R (1982) The psychology of vigilance. (Academic Pr, 1982).22.
- Poulton EC (1979) Composite model for human performance in continuous noise. Psychological Review 86: 361-375.
- Auburn TC, Jones MD, & Chapman AJ (1987) Arousal and the Bakan vigilance task: The effects of noise intensity and the presence of others. Springers 6: 196-206.24.
- Helton WS, Matthews G, Warm JS (2009) Stress state mediation between environmental variables and performance: The case of noise and vigilance. Acta Psychologica 130: 204-213.
- Mays LE, Gamlin PD (1995) Neuronal circuitry controlling the near response. Current Opinion in Neurobiology 5: 763-768.26.
- Tzelepi A, Lutz A Kapoula Z (2004) EEG activity related to preparation and suppression of eye movements in three-dimensional space. Exp Brain Res 155: 439-449.
Citation: Chavant M, Kapoula Z (2020) Hearing Amplification Improves Eye Movements: A Case Study. J Clin Stud Med Case Rep 7: 093.
Copyright: © 2020 Zoï Kapoula, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

