
Hybrid and Surgical First Approach for Pulmonary Valve Atresia and Ventricular Septal Defect in Small Infants: Single Center Early Results Comparison and Mid-Term Outcomes Analysis
*Corresponding Author(s):
Sara BondanzaDepartment Of Cardio Thoracic, Abdominal And Transplantation, IRCCS Istituto Giannina Gaslini, Genoa, Italy
Tel:+39 1056362333,
Email:bondanzasara@gmail.com
Abstract
Objective: To evaluate early and mid-term outcomes of patients with Pulmonary Atresia, Ventricular Septal Defect (PA-VSD), Major Aorto-Pulmonary Collateral Arteries (MAPCAs) with severely ‘diminutive’ native pulmonary arteries treated with a surgical or hybrid first approach. An initial palliative strategy is necessary to relieve cyanosis, to enhance pulmonary arteries growth and to bridge selected patients with a poor anatomy to the surgical complete repair.
Methods: We retrospectively reviewed our database to detect all the neonates diagnosed with PA-VSD, MAPCAs and severe hypoplasia of native Pulmonary Arteries (PA), who underwent surgical or hybrid first approaches between January 2005 and December 2015. First surgical palliation consisted of the placement of an aorto-pulmonary shunt (modified Blalock-Taussing Shunt). Hybrid procedure was the perventricular stenting of the Right Ventricle Outflow Tract (RVOT) through medial sternotomy.
Results: We analysed 11 patient’s data: 5 patients underwent the surgical and 6 the hybrid approach. There were no differences between the cohorts in demographic and clinical data. Surgical patients were younger at first procedure while native pulmonary arteries diameter and Nakata index were found to be lower in the hybrid group. All the procedures were successful except for one of the hybrid group; this patient underwent a surgical shunt during the same procedure. No immediate and early deaths or major complications occurred. Eight patients achieved the unifocalization with or without VSD closure at a mean age of 8.27+/-5 months. Three patients of the initial 11 died during the follow-up, one of them due to a non cardiac cause. Surviving patients were followed up for a period of 12 to 137 months.
Conclusion: Hybrid RVOT perventricular stenting provide a feasible and effective alternative compared to the standard surgical technique, for the initial management of patients with unfavourable PA anatomy. Mid and longer term outcomes of this cardiopathy and survival rates depend mainly on native pulmonary arteries size and growth.
Keywords
Congenital heart disease; Diminutive native pulmonary arteries; Hybrid palliation; Multifocal pulmonary vascularization; Neonates; Perventricular stenting; Right ventricle outflow tract
INTRODUCTION
Pulmonary Atresia (PA), Ventricular Septal Defect (VSD) and severely Hypoplastic Pulmonary Arteries is a rare and complex cardiac disease in which multifocal pulmonary vascularization exists supplied by Major Aortopulmonary Collateral Arteries (MAPCAs). The number, size, origin and course of MAPCAs are variable, such as the native pulmonary arteries size which can range from almost normal to completely absent [1,2]. In natural history of this disease there is a progressive stenosis and occlusion of MAPCAs [2,3] so, this condition requires early intervention to relieve cyanosis and to enhance the growth of the native pulmonary arteries. In cases of adequate weight and pulmonary artery size, one stage surgical unifocalization can be achieved with good results, as reported by Reddy, Abella et al., cit [2,4,5]. However, in some cases the pulmonary arteries are diminutive and therefore a staged approach is preferable.
Surgical techniques require cardiopulmonary bypass which is poorly tolerated by small infants. First surgical palliation consists of the placement of an aorto-pulmonary shunt in these patients [6,7], whilst in other cases a transannular patch is created. Stenting of the arterial duct [8] and Radiofrequency (RF) perforation of the atretic segment [9] are non-surgical alternatives, but such procedures may be particularly challenging.
Hybrid procedures on small infants have been reported [10-12] by performing a transventricular puncture and a perforation of the atretic outflow tract, followed by transluminal balloon dilation or stenting of the Right Ventricle Outflow Tract (RVOT) which showed good early results.
We analyzed early and long term outcomes of patients with PA-VSD, diminutive pulmonary arteries and multifocal pulmonary vascularization, referred to our center and treated with surgical or hybrid first approach to restore pulmonary flow.
PATIENTS AND METHODS
We retrospectively reviewed the cardiovascular database of the Gaslini Institute to detect all neonates diagnosed with PA-VSD and MAPCAs, between January 2005 and December 2015. The following data was retrieved from clinical and procedural records: gender, age, weight, body mass index, Nakata index, associated extra-cardiac malformations or syndromes, age at first intervention, types of first interventions, major peri-procedural and post-procedural complications, additional interventions, follow-up duration, age at and cause of death, and outcome for survivors.
At our institution, the hybrid approach to PA-VSD was introduced in November 2007; previously, RF perforation or surgical approach was the treatment of choice. At present we select patients according to anatomical and clinical features to assess the best individual approach.
All the procedures were performed according to the guidelines of the local Ethics Committee and informed consent was obtained from the parents. Hemodynamic data was collected and multiple angiographies were performed: aortic root injection was made to detect collaterals of head and neck vessels and to document the coronary arteries anatomy. Descending aortogram by occlusion technique with a 5F Berman catheter via an antegrade venous approach was performed to demonstrate the number and location of MAPCAs (Figure 1). Then selective hand-injections in MAPCAs to delineate the multifocal perfusion of the lungs was undertaken to determine which type of pulmonary artery connection was present. Collaterals found connected to native pulmonary arteries could be coil occluded, stenotic collaterals isolated from native pulmonary arteries could be dilated.
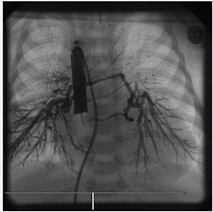
Figure 1: Antero-posterior view of descending aortogram by occlusion technique with a 5F Berman catheter.
We also performed pulmonary vein wedge injections to identify central pulmonary arteries if initial aortogram and selective injection of the MAPCAs did not demonstrate a pulmonary artery confluence (‘seagull’). The Nakata index was calculated using the formula:
RPA area (mm2) + LPA area (mm2)/BMI (m2) [13]. Values of >200 were considered optimal for repair.
Patients were successively scheduled for surgical or hybrid procedures.
Hybrid procedures were carried out in a hybrid surgical suite using high resolution biplane fluoroscopy. Access was provided through medial sternotomy, under general anesthesia. The surgeon performed the exposition of the heart and the isolation of the pulmonary arteries, and created a pursestring with 6.0 prolene at the right ventricle wall; following this, he picked the right ventricle infundibulum with an Abocatt needle 20 to 24 Gauge (Arrow) directed across the atretic pulmonary valve. Under fluoroscopy, the interventional cardiologists, advanced a short tipped stiff coronary guide wire (0.014) into the main pulmonary artery and placed it in one of the pulmonary branches or through the patent arterial duct into the descending aorta (Figure 2). A 4 or 5 French introducer sheath, was positioned 2 cm into the RVOT (a prolene marker was secured to the base of the sheath to help to arrive in the correct position). After a small contrast injection and sizing (Figure 3), one or multiple predilatations of the RVOT were performed with a coronary balloon. Finally coronary stents of different measures were implanted into the RVOT. The median diameter of coronary stents deployed was 3.5 mm, with a range of 3-4.5 mm, and the median length was 13 mm, with a range of 8-16 mm. In one case 2 stents, 3x12mm and 3.5x13 mm respectively, were positioned in overlap. Stents were deployed from just above the pulmonary valve until within the right ventricle outflow tract, restoring flow to the native pulmonary arteries (Figure 4).

Figure 2: Antero-posterior view, after medial sternotomy, of the coronary guide wire placed trough the main pulmonary artery into the right pulmonary branch.
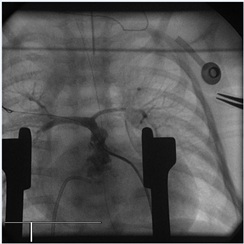
Figure 3: Antero-posterior view of the right ventricle outflow tract after balloon predilatation.
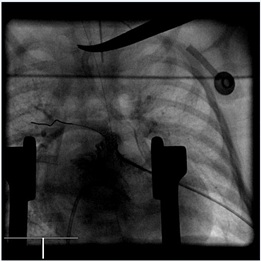
Figure 4: Antero-posterior view of the right ventricle outflow tract after stent deployment.
STATISTICAL ANALYSIS
Data is described as absolute and relative frequencies for categorical variables, while means, Standard Deviation (SD), medians, and range are used for continuous variables.
Non parametric analysis (Mann-Whitney tests) for continuous variables and the Chi square or Fisher's exact test for categorical variables were used to measure differences between groups. A p-value less than 0.05 were considered statistically significant; all p-values were based on two-tailed tests. Statistical analysis was performed using SPSS for Windows (SPSS Inc., Chicago, Illinois USA).
RESULTS
Eleven patients attended our Institution with the diagnosis of PA-VSD and MAPCAs during the study period. We divided patients into 2 groups according to the first therapeutic approach received. The surgical cohort consisted of 5 patients: 4 of them underwent a modified Blalock-Taussing shunt (mBT) and one of them received an outflow patch. The hybrid cohort consisted of 6 patients who underwent perventricular transcatheter balloon pulmonary valvuloplasty and/or stenting of the RVOT; with one of them undergoing concomitant placement of an mBT shunt. In our cohort we identified 2 genetic anomalies in the hybrid group (1 deletion of 22q11 and one anomaly of chromosome 8) and one duodenal atresia in the surgical group.
Table 1 shows the baseline demographic and clinical characteristics, the preoperative risk factors, the age at the procedures and the follow up duration for the patients. There were no differences between the cohorts in gender, weight, height, BMI and transcutaneous Oxygen saturation levels, while native pulmonary arteries diameter and Nakata index were found to be lower in hybrid group. Patients scheduled for surgical treatment were younger at first approach than patients underwent hybrid procedures.
|
Characteristics |
ALL |
Surgical |
Hybrid |
P value |
|
|
N=11 |
N=5 |
N=6 |
|||
|
Weigh at first procedure, gr, Mean ± SD |
3224±749 |
2800±474 |
3267±602 |
0.33 |
|
|
Median (range) |
3100 (2300, 5000) |
2600 (2300, 3400) |
3150 (2500, 4300) |
||
|
Heigh at first procedure, cm |
51.5±3.8 |
49.4±2.79 |
52.3±3.2 |
0.13 |
|
|
50 (47, 59) |
48 (47, 54) |
52 (49, 58) |
|||
|
BMI, m2 |
0.12±0.1 |
0.11±0.1 |
0.12±0.1 |
0.66 |
|
|
0.12 (0.10, 0,14) |
0.11 (0.10, 0,13) |
0.12 (0.10, 0,13) |
|||
|
Native PA diameter, mm |
2.3±1.58 |
3.4±1.34* |
1.67±0.52 |
0.05 |
|
|
2 (0, 5) |
4 (2, 5) |
2 (1, 2) |
|||
|
Nakata Index, mm2 /m2 |
113.3±111 |
178.4±126** |
41.5±22.8 |
0.03 |
|
|
57 (12, 356) |
193 (52, 356) |
52 (12, 63) |
|||
|
Age at 1st procedure, days |
33.7±30.8 |
16.6±22.8* |
34.5±19.7 |
0.05 |
|
|
25 (2, 120) |
7 (2, 57) |
26 (12, 60) |
|||
|
Age at 2nd procedure, months |
8.27±5 |
5.15±2.36 |
12.25±6.55 |
0.2 |
|
|
7 (2, 20) |
6 (2, 7) |
12.5 (4, 20) |
|||
|
Age at 3rd procedure, years |
4.33±2.8 |
5.67±3.2 |
3 |
1 |
|
|
4 (1, 8) |
7 (2, 8) |
3 |
|||
|
O2 Sat pre-procedure,% |
76.7±6.7 |
78±9.1 |
74.2±2 |
0.79 |
|
|
75 (70, 90) |
75 (70, 90) |
75 (70, 75) |
|
||
|
O2 Sat post first procedure,% |
84±6.3 |
85±7.1 |
81.67±2.6 |
0.66 |
|
|
|
80 (80, 100) |
80 (80, 95) |
80 (80, 85) |
|
|
|
Final O2 Sat ,% |
90.5±9.2 |
94.75±9.8 |
87±9.2 |
0.49 |
|
|
|
90 (80, 100) |
99.5 (80, 95) |
84 (80, 100) |
|
|
|
Follow- up, months |
72.9±40 |
91.5±49 |
62.5±42.4 |
0.49 |
|
|
|
73 (12, 137) |
96.5 (36, 137) |
67 (12, 103) |
|
Table 1: Demographic and Clinical Characteristics.
Mean ± SD , Median (range)
*Surgical vs Hybrid p=0.05; ** Surgical vs Hybrid p=0.03
Legend: PA: Pulmonary Arteries; BMI: Body Mass Index; O2 Sat: Oxigen Saturation levels.
The study did not experience any incidence of death or major complications during or immediately after the first procedures in any of our patients and oxygen saturation levels improved in patients across both the groups. All the procedures were successfully performed with one exception. One patient within the hybrid group had a failed attempt of dilation of the RVOT without stenting and underwent immediate surgical mBT-shunt during the same session. One late mBT shunt occlusion occurred in a patient within the surgical cohort 2 years from the date of the procedure; percutaneous reperfusion was unsuccessfully attempted, and other surgical options were not considered feasible. The patient died 2 years later.
Eight of our 11 patients underwent a second procedure of unifocalization at the mean age of 8.27+/-5 months.
In 2 patients within the surgical group unifocalization was performed and the Ventricular Septal Defect (VSD) was left open, while in 2 cases it was possible to close the VSD.
Four patients of the hybrid group underwent unifocalization. In one patient the unifocalization was performed and VSD was successfully closed while in 3 cases the VSD was left open. One of them had a late conduit thrombosis and died 2 years later.
Another patient of the hybrid cohort died due to a non-cardiac cause and the last one is still waiting for a second procedure. Our patients were followed up for a period of 12 to 137 months. Three patients from the initial 11 died (27.2%): 1 within the surgical group (20%) and 2 within the hybrid group (33.3%), (p=0.11). Four patients achieved complete repair with VSD closure (3 surgical and 1 hybrid). Further procedures in these patients consisted of a deferred VSD closure, conduit substitution, biological pulmonary valve implantation and MAPCAs percutaneous occlusion. Three patients after unifocalization still have their VSDs open and 2 of them underwent percutaneous multiple dilatations of the pulmonary arteries. Two patients in follow up had no more surgical options available and 1 is still awaiting further procedures. The data is summarized in the flow-chart.
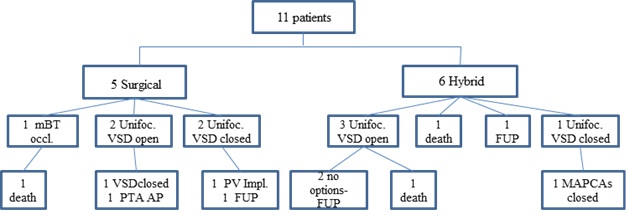
Flow-chart: mBT occl. = Modified Blalok Taussing Shunt occlusion; Unifoc = Unifocalization; VSD = Ventricular Septal Defect; PTA AP: Percutaneous Angioplasty of Pulmonary Arteries; FUP = Follow-Up; PV Impl.= Pulmonary Valve Implantation; MAPCAs = Major Aortopulmonay Arteries Collaterals
DISCUSSION
The management of patients with PA-VSD and MAPCAs with Hypoplastic Native Pulmonary Arteries is still challenging. Early age intervention is often required and often complicated by low weight. When native pulmonary arteries are severely hypoplastic, multistage approaches are preferable to promote the growth of these arteries [14]. When pulmonary arteries are completely absent the only option is to perform palliative procedures of MAPCAs dilatation where possible but the prognosis for such patients is unfavorable [15].
Percutaneous approaches such as radiofrequency perforation are possible, but are burdened with a high risk of perforation especially if a long muscular atretic segment is present. Ductal stenting is another option but sometimes unfavorable ductal anatomy, such as long and tortuous ducts, makes this procedure complex with the risk of uncompleted stenting [8]. In small low weight infants percutaneous procedures are also complicated by access problems.
Surgical techniques consist of a modified Blalock-Taussing shunt (m-BT shunt) [6] and a transannular patch, but they require cardiopulmonary by-pass which is poorly tolerated in very small infants [16,17]. Furthermore, an m-BT shunt in very small arteries can involve complications such as early or late occlusion, distortion of pulmonary arteries shape with asymmetrical growth, stenosis of the pulmonary branches and pulmonary overcirculation.
Hybrid technology combines the achievements of both surgical and percutaneous techniques to bring benefits to patients with different congenital cardiac disease with a minimally invasive approach [18]. We attempted hybrid procedures in selected patients with extreme hypoplasia of native pulmonary arteries. These procedures allowed to avoid cardiopulmonary by-pass, eliminated percutaneous access problems, allowed antegrade pulsatile flow into the pulmonary arteries and minimized the shape distortion of pulmonary arteries. The most challenging maneuver in the procedure is the perventricular RVOT puncture due to the risk of going the wrong way into the muscular infundibulum. Other technical complications include the sheath instability and the loss of conventional radiological landmarks. To deploy a stent in the RVOT has the potential to compress a coronary artery [19,20], but the small diameters of the stents employed (stents are chosen 1.5 to 2 mm larger than pulmonary trunk) and the muscular tissue that surrounds them, minimizes this risk in small patients.
Hybrid procedures have been demonstrated to be a safe and feasible alternative to conventional approaches, a great cooperation between the surgeon and the interventional cardiologist is required. This can offer patients better opportunities to improve survival and future quality of life.
In our follow up, we also observed patients who underwent all types of approaches, inadequate growth of native pulmonary arteries, which made it impossible to proceed to VSD closure after unifocalization. Poor pulmonary vascularization and cyanosis lead to limitations in exercise and stress tolerance and required multiple palliative procedures of MAPCAs and pulmonary artery dilatation or stenting throughout life. In some patients no more surgical options were feasible and progressive cyanosis led to death.
LIMITATIONS
The retrospective approach and the small sample size were the major limitations of our study: this severe form of cardiopathy is rare; moreover, we considered only patients with very diminutive or absent pulmonary arteries and therefore application of findings outside of the specified study criteria needs to be approached with due caution.
CONCLUSION
This is the first study, to our knowledge, to compare the hybrid approach for restoring flow in native diminutive pulmonary arteries in PA-VSD and MAPCAs to the standard surgical approach. We reported the largest number of patients who underwent this hybrid procedure.
Hybrid approach is a safe and feasible alternative to mBT shunt, with a high rate of technical immediate success and low rate of procedural complications. It reduces the exposure to cardiopulmonary by-pass, which may have long-term effects, particularly in neonates; promotes a more balanced growth of the pulmonary branches and also prevents the shape distortion and future stenosis of very small native pulmonary arteries. Our experience demonstrated encouraging results to expand the use of this procedure to bridge high risk patients with diminutive pulmonary arteries to final surgical repair. However, the prognosis of this cardiopathy is poor. Mid and longer term outcomes and survival rates depend mostly on the native pulmonary arteries diameter and growth. Multiple surgical or percutaneous palliations are required to improve oxygen saturation levels, exercise tolerance and general quality of life of these patients.
DISCLOSURE
No disclosure to declare.
REFERENCES
- Griselli M, Mc Guirk SP, Winlaw DS, Stümper O, de Giovanni JV, et al. (2004) The influence of pulmonary artery morphology on the results of operations for major aortopulmonary collateral arteries and complex congenital heart defects. J Thorac Cardiovasc Surg 127: 251-258.
- Lofland GK (2000) The management of pulmonary atresia, ventricular septal defect, and multiple aorta pulmonary collateral arteries by definitive single stage repair in early infancy. Eur J CArdiothorac Surg 18: 480-486.
- Nørgaard MA, Alphonso N, Cochrane AD, Menahem S, Brizard CP, et al. (2006) Major aorto-pulmonary collateral arteries of patients with pulmonary atresia and ventricular septal defect are dilated bronchial arteries. Eur J Cardiothorac Surg 29: 653-658.
- Reddy VM, Petrossian E, McElhinney DB, Moore P, Teitel DF, et al. (1997) One-stage complete unifocalization in infants: when should the ventricular septal defect be closed? J Thorac Cardiovasc Surg 113: 858-866.
- Abella RF, De La Torre T, Mastropietro G, Morici N, Cipriani A, et al. (2004) Primary repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals: a useful approach. J Thorac Cardiovasc Surg 127: 193-202.
- Gladman G, McCrindle BW, Williams WG, Freedom RM, Benson LN (1997) The modified Blalock-Taussig shunt: clinical impact and morbidity in Fallot’s tetralogy in the current era. J Thorac Cardiovasc Surg 114: 25-30.
- Petrucci O, O’Brien S, Jacobs ML, Jacobs JP, Manning PB, et al. (2011) Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Ann Thorac Surg 92: 642-651.
- Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, et al. (1992) Stenting of the arterial duct: A new approach to palliation for pulmonary atresia. Br Heart J 67: 240-245.
- Walsh MA, Lee KJ, Chaturvedi R, Van Arsdell GS, Benson LN (2007) Radiofrequency perforation of the right ventricular outflow tract as a palliative strategy for pulmonary atresia with ventricular septal defect. Catheter Cardiovasc Interv 69: 1015-1020.
- Cools B, Boshoff D, Heying R, Rega F, Meyns B, et al. (2013) Transventricular Balloon Dilation and Stenting of the RVOT in Small Infants With Tetralogy of Fallot With Pulmonary Atresia. Catheter Cardiovascr Interv 82: 260-265.
- Butera G, Abella R, Carminati M, Frigiola A (2009) Perventricular implantation of a right ventricular-to-pulmonary artery ‘conduit’. Eur Heart J 30: 2078.
- Zampi JD, Armstrong AK, Hirsch-Romano JC (2014) Hybrid Perventricular Pulmonary Valve Perforation and Right Ventricular Outflow Stent Placement: A Case Report of a Premature, 1.3-kg Neonate With Tetralogy of Fallot and Pulmonary Atresia. World J Pediatr Congenit Heart Surg 5: 338-341.
- Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, et al. (1984) A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. J Thorac Cardiovasc Surg 88: 610-619.
- Marshall AC, Love BA, Lang P, Jonas RA, del Nido PJ, et al. (2003) Staged repair of tetralogy of Fallot and diminutive pulmonary arteries with a fenestrated ventricular septal defect patch. J Thorac Cardiovasc Surg 126: 1427-1433.
- Brown S, Eyskens B, Mertens L, Dumoulin M, Gewilling M (1998) Percutaneous treatment of stenosed major aortopulmonary collaterals with balloon dilatation and stenting: what can be achieved? Heart 79: 24-28.
- Di Donato RM, Jonas RA, Lang P, Rome JJ, Mayer JE Jr, et al. (1991) Neonatal repair of tetralogy of Fallot with and without pulmonary atresia. J Thorac Cardiovasc Surg 101: 126-137.
- Liu Y, Xu Y, Li DZ, Shi Y, Ye M (2009) Comparison of S100B and NSE between cardiac surgery and interventional therapy for children. Pediatr Cardiol 30: 893-897.
- Agrawal H, Alkashkari W, Kenny D (2017) Evolution of hybrid interventions for congenital heart disease. Expert Rev Cardiovasc ther 15: 257-266.
- Dohlen G, Chaturvedi RR, Benson LN, Ozawa A, Van Arsdell GS, et al. (2009) Stenting of the right ventricular outflow tract in the symptomatic infant with tetralogy of Fallot. Heart 95: 142-147.
- Dryzek P, Mazurek-Kula A, Moszura T, Sysa A (2008) Right ventricle outflow tract stenting as a method of palliative treatment of severe tetralogy of Fallot. Cardiol J 15: 376-379.
Citation: Bondanza S, Calevo MG, Santoro F, Marasini M (2020) Hybrid and Surgical First Approach for Pulmonary Valve Atresia and Ventricular Septal Defect in Small Infants: Single Center Early Results Comparison and Mid-Term Outcomes Analysis. J Angiol Vasc Surg 5: 034.
Copyright: © 2020 Sara Bondanza, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

