
Ibrutinib and Oral Ulcers
*Corresponding Author(s):
Julieta Ruiz- BeguerieDepartment Of Dermatology, Hospital Universitario Austral, Argentina
Tel:+541148025676,
Email:jruiz@cas.austral.edu.ar
INTRODUCTION
We report a male patient of 75 years old with the diagnosis of Mantle Cell Lymphoma who started Ibrutinib (560 mg per day) four weeks before consulting for the appearance of painful oral lesions. On oral examination he revealed 3 necrotic progressive well circumscribed very painful mucosal ulcers of about 1 cm in diameter each. A brightly erythematous area with similar appearance to major aphthous stomatitis surrounded them. The three lesions involved the non keratinized and keratinized areas including a deep round ulcer on the tip and dorsal aspect of the tongue, covered by a yellowish-grey pseudo membrane (Figures 1&2). He reported intense pain and associated dysphagia.
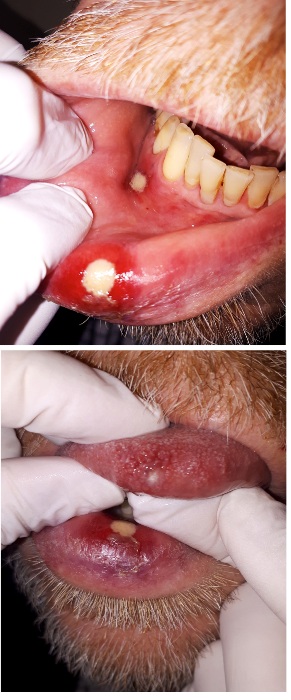 Figures 1&2: Ulcer in the tongue and lower lip.
Figures 1&2: Ulcer in the tongue and lower lip.
According to the National Cancer Institute Common Toxicity Criteria for Adverse Events, this oral toxicity was grade ≥3 and required interruption of ibrutinib [1]. Laboratory and swabs were taken to discard infectious disease. Bacterial/fungal swab cultures and in situ Polymerase Chain Reaction (PCR) screening for herpes simplex virus 1 and 2, varicella zoster virus and cytomegalovirus were negative. Additionally, blood samples for Epstein-Barr Virus (EBV), Human Parvovirus B19 (HPV-B19) and human herpes virus 6 were all negative. No neutropenia was observed during this period.
He was given oral steroids 0,5 mg /kg/d for a week while discontinuing the ibrutinib, observing the resolution of the ulcers (Figures 3&4). After 18 days, ibrutinib was reinstalled in half dose, 280 mg per day. After two days, it was observed the appearance of the same lesions as he had before. For another week the medication was suspended while observing the disappearance of all the oral lesions. Once again a new try was conducted using 280 mg per day while adding oral steroids 8 mg twice a day for the first 10 days and no appearance of new lesions was observed. Our case, would the 4th case reported in the literature so far [2].
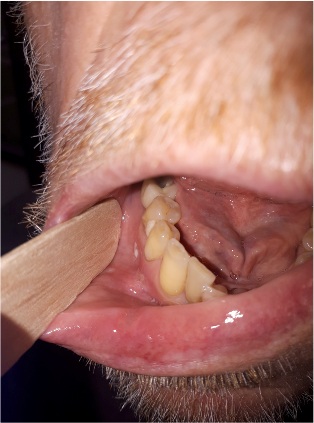
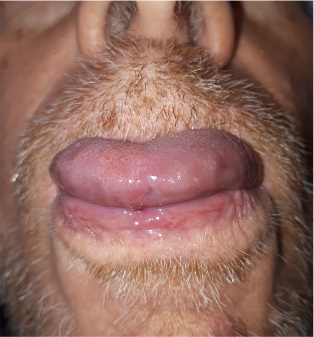 Figures 3&4: Improvement of ulcer in the tip of the tongue after 17 days without Ibrutinib.
Figures 3&4: Improvement of ulcer in the tip of the tongue after 17 days without Ibrutinib.
Oral mucositis is a side effect of chemotherapy, head and neck radiotherapy and targeted therapy, affecting over 75% of high-risk patients. Firstly, there is a DNA damage and liberation of proinflammatory factors that result in a thinning of the epithelium through tissue injury and cell death, culminating with the destruction of the oral mucosa [3,4]. Sore mouth and oral ulcers can lead to severe pain and difficulty with sleeping, talking, eating and drinking, which may need strong painkillers such as opioids. In severe cases, hospitalization and even parenteral nutrition may be necessary. These complications may disrupt cancer therapy, which may reduce survival. Cancer patients have weakened immune systems due to their treatment and are less able to fight infections. Often times mucositis serves as a gateway to open wounds, such as ulcers, that increase the risk of bacterial/fungal infections or even sepsis.
There are five stages of the oral mucositis development, according to Sonis and colleagues [3,4].
• Initiation: DNA damage caused by chemotherapy or radiotherapy results in the loss of the ability to proliferate in the basal cells of the epithelium producing Reactive Oxygen Species (ROS)
• Primary damage response: radiotherapy, chemotherapy, ROS, and DNA strand breaks, they all contribute to the activation of transcription factors such as nuclear factor kappa beta (NF-K β), and sphingomyelinases. All this leads to the up regulation of pro-inflammatory cytokines such as tumor necrosis factor alpha -TNF-α, nitric oxide, ceramide, and matrix metalloproteinases, resulting in the thinning of the epithelium through tissue injury and cell death, culminating with the destruction of the oral mucosa
• Signal amplification: some of the molecules in the previous phase can lead to the exacerbation and prolonging of tissue injury through positive or negative feedback (e.g. TNF-α can positively feedback on NF-Kβ thus inducing more pro-inflammatory cytokine production)
• Ulceration: bacteria colonize ulcers and their cell wall products infiltrate the submucosa (the connective tissues beneath the oral mucosa), activating tissue macrophages, which results in further production of pro-inflammatory cytokines, inflammation, and pain.
• Healing: signaling from the extracellular matrix of the submucosa results in epithelial proliferation and differentiation, and thus a thickening of the epithelium. The local oral flora is reinstated.
Ibrutinib, an irreversible oral inhibitor of Bruton’s Tyrosine Kinase (BTK) that is approved since 2013 by the FDA for the treatment of several B-cell malignancies, inhibits IL2- inducible T-cell Kinase (ITK), which regulates T-cell proliferation and may decrease Th-1 immune responses to fungal and other intracellular pathogens Via a unique mechanism of action [5], ibrutinib inhibits B cell signaling pathways that regulate the survival, proliferation, adhesion, and homing of cancerous cells [6,7]. Most serious infections in patients with lymphomas treated with ibrutinib have been related to B-cell dysfunction; however, invasive aspergillosis, pneumocystis pneumonia, and other invasive fungal infections have been reported [8]. Commonly reported adverse events include fatigue and diarrhea. Dermatological toxicities have also been reported in 2–27% of treated patients mainly mild-to-moderate rash, neutrophilic panniculitis, progressive hair loss and nail changes, skin infections, bruising, petechiae and purpuric eruption [12-15]. The pathogenesis of ibrutinib-associated dermatological toxicities remains unclear [14]. It could be comparable with a localized adaptive cellular immune response where ibrutinib-conjugated peptides would be presented to host immune cells via a major histocompatibility complex, leading to a T-cell-driven immune response and bystander tissue destruction [14]. Furthermore, although ibrutinib demonstrates high selectivity for BTK, it also applies off-target effects on other kinases, including irreversible binding to Epidermal Growth Factor Receptor (EGFR) or HER2 [15]. This mechanism could partly explain the pathogenesis of ibrutinib- associated oral ulcers as our patient. Oral aphthous-like ulcers have also been reported in hematological patients with concomitant neutropenia. However, no correlation with neutropenia could be established in our patients who presented a normal range of neutrophils. In addition, an infectious origin remains very unlikely, because the lesions clearly improved with corticosteroids and bacterial as well as viral swabs were negative in all cases. In conclusion, ibrutinib can trigger severe ulcerative stomatitis that may require temporary discontinuation of the drug.
Lesions can develop after several weeks or even months of treatment and can involve keratinized mucosa, which is unusual in comparison with the aphthous-like ulcers observed with other TKIs [16]. In our experience, topical or short courses of systemic corticosteroids should be recommended for managing oral ulcers and relieving pain, once an active infection and neutrophilia associated with it have been ruled out.
Oral toxicity with ibrutinib has rarely been described so far. A prospective dermatological survey did not observe any mucosal involvement in a cohort of 66 consecutive patients [15]. However, a recent pivotal study reported all-grade and high-grade stomatitis in 11% and 1% of treated patients, respectively, but the clinical aspects were not documented [9].
The first report of a case series of 3 patients with grade ≥3 stomatitis induced by ibrutinib as monotherapy (420 mg/day) for treating CLL was published last year. Lesions were reminiscent of painful oral necrotic ulcers mimicking aphthous stomatitis and required treatment interruption in all cases in the same way as our patient [17].
Ibrutinib is exclusively metabolized by cytochrome P450 (CYP) CYP3A; hence, therapy with macrolides may result in an increase in the plasma level of ibrutinib and subsequent toxicity. Hence, dose interruption or modification is needed when treatment of a patient on ibrutinib requires administration of strong or moderate CYP3A inhibitors [18]. Furthermore, it should be monitored the electrocardiogram periodically as both ibrutinib and macrolides have been linked to serious cardiac arrhythmias [19,20]. Ibrutinib is the first and only BTK inhibitor approved in the United States for the treatment of patients with Mantle Cell Lymphoma (MCL) or Chronic Lymphocytic Leukemia (CLL) who have received at least one prior therapy, as well as for CLL patients with the deletion17p (del17p) aberration and patients with Waldenstrõm’s Macroglobulinemia (WM) [21].
Mantel Cell Lymphoma (MCL) is a clinically aggressive type of B cell non- Hodgkin’s lymphoma that arises from the outer edge (mantle zone) of the lymph node, and usually occurs in older adults. The MCL uncontrolled cells can enter the lymphatic channels and blood and spread to other lymph nodes or tissues, such as bone marrow, the liver, and the gastrointestinal tract. Overall, MCL has the lowest survival rate of any of the cancers of the blood affecting B cells; patients can expect to survive, on average, for only 4-5 years after diagnosis [6,22]. MCL is typically characterized by a cytogenetic abnormality whereby short segments of chromosome 11 and 14 swap places. This translocation triggers the overproduction of cyclin D1, a cell cycle regulator, not normally expressed by healthy B cells, that contributes to the uncontrolled growth of malignant cells in MCL. 6,7Patients above 65 years, such as our patient, are typically treated with chemoimmunotherapy, such as fludarabine and cyclophosphamide Plus Rituximab (FCR); Rituximab- Cyclophosphamide, Vincristine, and Prednisone (R-CVP); Rituximab-Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP); Bendamustine-Rituximab (B-R); or Rituximab- Chlorambucil (R-C) [6,7,23,24].
CONCLUSION
Patients diagnosed with mantle cell lymphoma are likely to experience relapses after the initial treatment [25]. These are determined by a series of factors, including previous treatments, comorbidities and the performance status of the cancer patients. Ibrutinib is an irreversible oral inhibitor of BTK, currently used in a variety of hematological conditions, ranging from mantle cell lymphoma to graft versus host disease. It is relatively well tolerated by patients and has shown response in most individuals with relapsed/refractory mantle cell lymphoma. Adverse reactions often include nausea, mild or moderate diarrhea and fatigue. Oral ulcers as the patient we present in this article are a very uncommon side effect with only 4 cases in the literature. However, several cases of moderate oral mucositis have been reported, probably due to localized adaptive cellular immune response and non-selective binding to EGFR, which might in turn further inhibit keratinocyte reproduction with the consequent thinning and destruction of both keratinized and non keratinized mucosa. After ruling out infectious oral disease and other causes of oral ulcers, removal of the triggering factor such as the Ibrutinib in our case and starting a short course of systemic steroid treatment are often the first measures to be taken in these cases. In our patient, these measures resulted in rapid recovery ad-integrum, which allowed for treatment re-installment while a slow tapering of corticoid therapy was completed.
REFERENCES
- .(https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf)
- Vigarios E, Beylot-Barry M, Jegou MH, Oberic L, Ysebaert L, et al. (2019) Dose-limiting stomatitis associated with ibrutinib therapy: a case series. Br J Haematol 185: 784-788.
- 3. Sonis ST (2004) Oral mucositis in cancer therapy. J Support Oncol 2: 3-8.
- Sonis ST (2009) Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 45: 1015-1020.
- Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, et al. (2013) Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122:2539-2549.
- McKay P, Leach M, Jackson R, Cook G, Rule S, et al. (2012) Guidelines for the investigation and management of mantle cell lymphoma. Br J Haematol 159: 405-426.
- Gaykos U, Fung M, Clow F, Sun S, Faust E, et al. (2015) Development of the Bruton's tyrosine kinase inhibitor ibrutinib for B cell malignancies. Ann NY Acad SCI 1358: 82-94.
- Hilal T, Banacloche JC, Leis JF (2018) Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: linking mechanisms with infections. Blood Rev 32: 387-399.
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, et al. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369: 32-42.
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, et al. (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia NEJM 373: 2425-2437.
- Pula B, Jamroziak K (2019) Ibrutinib Therapy for Chronic Lymphocytic Leukemia Complicated with Secondary Serous Adenocarcinoma of the Peritoneum. International Journal of Hematology and Oncology 3; 186-189.
- Ransohoff JD, Kwong BY (2017) Cutaneous adverse events of targeted therapies for hematolymphoid malignancies. Clinical Lymphoma Myeloma Leukemia 17: 834-851.
- Iberri DJ, Kwong BY, Stevens LA, Coutre SE, Kim J, et al. (2018) Ibrutinib-associated rash: a single-centre experience of clinicopathological features and management. Br J Haematol 180: 164-166.
- Fabbro SK, Smith SM, Dubovsky JA, Gru AA, Jones JA (2015) Panniculitis in Patients Undergoing Treatment With the Bruton Tyrosine Kinase Inhibitor Ibrutinib for Lymphoid Leukemias. JAMA Oncol 1: 684-686.
- Bitar C, Farooqui MZ, Valdez J, Saba NS, Soto S, et al. (2016) Hair and nail changes during long-term therapy with ibrutinib for chronic lymphocytic leukemia JAMA Dermatol 152: 698-701.
- Vigarios E, Epstein JB, Sibaud V (2017) Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Supportive Care in Cancer 25: 1713-1739.
- Vigaros E, Beylot-Barry M, Jegou MH, Oberic L, Ysebaert L et al. (2019) Dose-limiting stomatitis associated with ibrutinib therapy: A case series. Br J Haematol 185: 784-788.
- de Jong J, Skee D, Murphy J, Sukbuntherng J, Hellemans P, et al. (2015) Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect 3: 00156.
- Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, et al. (2017) Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood 129: 2581-2584.
- Albert RK, Schuller JL; COPD Clinical Research Network (2014) Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med 189: 1173-1180.
- Varma, G, Johnson TP, Advani RH (2016) Bruton’s Tyrosine Kinase Inhibitors in Chronic Lymphocytic Leukemia and Lymphoma. Clinical Advances in Hematology & Oncology 4: 543-554.
- Catovsky D, Montserrat E (2011) “Chronic lymphocytic leukaemia and other B-cell disorders.” In Postgraduate Haematology. A. Hoffbrand & D. Catovsky, Eds: 530-557. Oxford: Wiley-Blackwell, USA.
- Castellino A, Santambrogio E, Nicolosi M, Botto B, Boccomini C, et al. (2017) Follicular Lymphoma: The Management of Elderly Patient. Mediterr J Hematol Infect Dis 9: 2017009.
- Thermos G, Tosios KL (2019) Ibrutinib-associated oral ulcers. Oral Oncol 100: 104445.
- Wang ML, Rule S, Martin P, Goy A, Auer R, et al. (2013) Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 369: 507.
Citation: Ruiz-Beguerie J, Yazbek RIB, Busso C, Trucco J (2020) Ibrutinib and Oral Ulcers. J Clin Dermatol Ther 6: 047.
Copyright: © 2020 Julieta Ruiz- Beguerie, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

