
Immune Regulatory Effects of Sinomenine on Primary Membranous Nephropathy: Based on Case Report and Network Pharmacology
*Corresponding Author(s):
Xue-Xia LiDepartment Of Nephropathy, Zhuhai Hospital Of Integrated Chinese And Western Medicine, Zhuhai, Guangdong, China
Tel:+86 15018342882,
Email:sitalisa@163.com
Abstract
PMN an organ-specific autoimmune disease, is the leading cause of nephrotic syndrome (NS) in adults worldwide. However, there is no specific treatment for PMN other than optimal supportive care in low and moderate risk PMN. Immunosuppressive therapy is restricted to patients considered at risk for progressive kidney injury.
Sinomenine (SIN), the alkaloid monomer extracted from the medicinal rhizome of Sinomenium acutum, is a kind of non-steroidal anti-inflammatory drug widely used in China to treat patients with rheumatoid arthritis (RA) and various glomerular diseases. The classic pharmacological activities of SIN are anti-inflammation, immunomodulation, promotion of histamine release, mild sedative and analgesic effects. The diverse functions of SIN make it a promising and effective drug in clinic usage. We observed the effects of SIN on the treatment of PMN. So we further systematically summarize and expound the therapeutic potentials of SIN in PMN on sigle-cell level, aiming to give enlightments for clinical applications of SIN. SIN benefit to PMN with the underlying mechanisms involving anti-inflammation, immunosuppression, regulation of cell proliferation and apoptosis, inhibition of oxidative stress and calcium overload. In all, SIN work on PMN mainly through its immune regulatory effects.
Keywords
Immune regulatory effects; Primary membranous nephropathy; Sinomenine
Background
Primary Membranous Nephropathy (PMN) an organ-specific autoimmune disease, accounting for approximately 30% to 40% of primary NS in western countries. Its incidence has been increasing in recent years. The proportion of MN was suggested to be increased from 10.4% in 2003-2006 to 24.1% in 2011-2014 from renal biopsy related studies in China [1-3].A renal biopsy study in Henan Province in 2020 suggested that the incidence of MN had surpassed immunoglobulin A nephropathy (IgAN) since 2015, accounting for 32.98% of primary glomerulonephritis (GN) [4]. PMN has a long natural history, spontaneous remission can occur in some patients, and patient outcomes are heterogeneous.Its clinical outcome is variable, with approximately one-third of cases reach spontaneous remission, while also up to one-third of them gradually progress to end-stage renal disease (ESRD) in 10-15 years [5]. However, there is no specific treatment for PMN other than optimal supportive care in low and moderate risk PMN. The need for immunosuppressive therapy in patients with PMN and the timing of its treatment have been controversial.
Zhengqingfengtongning (ZQFTN), composed of SIN, is a prescription drug for glomerulonephritis in China. We observed the effects of SIN on PMN in real world practice. Here were reported two typical cases of SIN on the treatment of resistant PMN.
Case Report
A 62-year-old otherwise healthy man was consulted at our center in December 2018 because of severe edema lasted for 2 weeks. He denied having other symptoms such as fever, fatigue, headache, nausea, muscle or joint pains, ect. Apart from edema, the rest of the physical examination was unremarkable. The patient did not refer to any relevant past medical history. He did not take any medications and had no allergies to vaccines, drugs, or food in the past. Full blood workup, including blood routine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and immunologic tests (C3, C4), antinuclear antibodies (ANA), antineutrophil cytoplasmic antibody (ANCA), immunoglobulins( IgA, IgG, IgM) , tumor indicators and cryoglobulins all were normal, except for hypoproteinemia (23.7g/L) , mass proteinuria( 6523mg/24h), hyperlipidemia and positive serum PLA2R(114.93RU/ML). Also, there were several erythrocytes in the urine analysis. The Chest X-ray showed no abnormalities. A renal biopsy was taken. In light microscopy, the glomeruli and interstitium involves thickening of the capillary wall, in sections stained with hematoxylin and eosin or with periodic acid–Schiff (PAS) reagent. For immunohistology, positive staining for IgG marks the finely granular subepithelial deposits. The IgG subclass is IgG1 and IgG4. κ and λ light-chain staining are positive. Both C3 staining and PLA2R staining were positive. For electron microscopy, immune deposit formation occurs in a subepithelial distribution, basement membrane material is laid down between the deposits and corresponds to the spikes seen on light microscopy. The patient was diagnosed PLA2R-related PMN.
The patient received supported care as well as agiotensin receptor blockers(ARB) accompanied with atorvastatin for three month but without reduction of Proteinuria. Therapy strategy was adjusted to 60 mg of oral methylprednisolone plus intravenous cyclophosphamide(CTX) with a dose of 0.8g per month. Two months later, methylprednisolone was gradually tapered and the accumulated dose of CTX was 6.4g. NS got partial remission at the very beginning but aggravated short after. On April 2021, his edema symptom exacerbated with his Proteinuria of more than 12000mg per 24 hours. With decreased of estimated glomerular filtration rate s(eGFR) of 56ml/min. 10mg of Methylprednisolone and 1.5mg of Tacrolimus were applied. But his blood glucose level was elevated after two months of treatment. Then Tacrolimus was withdrew and he was treat with 10mg of Methylprednisolone and 120mg of ZQFTN. After two weeks of treatment, edema was finally relieved. The patient got partial remission after treatment with stable serum albumin at 35g/L and stable kidney function until now.
SIN was effective to our patient who was defined as refractory NS. However, the underlying mechanisms of SIN on PMN remain unknown, so we systematically summarize and expound the therapeutic potentials of SIN in PMN in the following study (Figure 1).
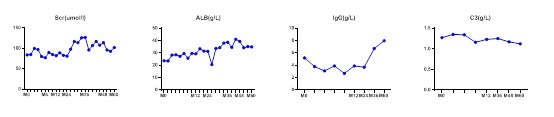 Figure 1: Variation of clinical data during 60 months of treatment.
Figure 1: Variation of clinical data during 60 months of treatment.
Changing Curve of serum Scr, ALB IgG and C3 during 60 months of the patient. To uncover the underlying possible mechanism of how SIN worked in PMN, we applied network pharmacology analysis based on real world scRNA-seq. First, we applied network pharmacology analysis to predict the potential therapeutic targets and signaling pathways of SIN for the treatment of PMN. Then we analysis the potential targets of SIN on various renal intrinsic cells, different tubular cells and diverse kidney immune cells based on single-cell RNA sequencing (scRNA-seq), aiming to give enlightenments for clinical applications of SIN.
Methods
- Targets screening of SIN
Chemical construction of SIN was obtained from traditional Chinese medicine systems pharmacology analysis [6] (TCMSP,http://lsp.nwu.edu.cn/tcmsp.php) in the format of “.mol2”, which was uploaded on to PharmaMpper database [7] (http://lilab-ecust.cn), target type defined as “(human protein Targets only(v2010,2241)”, gene symbols were classified into standard gene targets from Uniprot database (https://www.uniprot.org/) [8].
- Targets screening of PMN
Target genes of PMN were obtained from literature research based on scRNA-seq technique to explore target gens of intrinsic cells and immune cells in renal tissues of PMN patients. We found two papers related to target genes of PMN by the technique of scRNA-seq and summerized them in table 1.
|
|
Xu J et al, 2021 [9] |
Chen Z et al, 2021 [10] |
|
ScRNAseq method |
Singleron Biotechnologies |
chromium |
|
Tissue origin |
Kidney biopsy |
Kidney biopsy |
|
Sample number |
6MN, 2HC |
6 patients, 1 donor |
|
Cell number |
30313 |
14932 |
Table 1: Summary of literatures based on sc-RNA-seq in PMN.
- Common genes screening between SIN and PMN
Common genes between SIN and PMN were extracted through Excel. Venn diagrams were drown to visualize common genes by the online platform of bioinformatics (http://www.bioinformatics.com.cn).
- Gene targets PPI and enrichment analysis of SIN on PMN
Importing common genes into the STRING11.5 database (https://string-db.org) to build a PPI network model, data settings: biological species were set to "Homo sapiens", set the minimum interaction threshold to "highest confidence (>0.9)", and hide its free gene targets. Other settings are set as default to obtain the PPI network.The PPI network diagram was imported into Cytoscape 3.7.1 software for visualization afterwards, in order to clarify the core targets of the interaction between intersection gene targets. At the same time, network topology attribute analysis was conducted using Tools to obtain the top 5 core genes in "node connectivity".
Common genes were imported into the Metascape database [11] (http://metascape.org/gp/index.html for gene target enrichment analysis. The analysis included gene ontology (GO) functional enrichment analysis of intersecting gene targets and pathway enrichment analysis based on the Kyoto Encyclopedia of Genes and Genomes (KEGG). The top 20 results are selected based on the P-value size as the screening criteria, and visualized through the microbiological information online platform.
Results
- Gene targets of SIN and PMN
218 potential therapeutic targets for SIN were obtained through the PharmaMper database. They were imported into the Uniprot database for standardization. Finally, 208 gene targets were obtained. Through literature research, we found two ScRNA-seq studies related to PMN, which had classified the cell subpopulations of PMN kidney tissue in detail. They also conducted differential expressed genes between PMN patients and healthy controls [12,13].
- Common genes between SIN and PMN
Common genes between SIN and PMN were obtained by Excel screening. Among them, there are 18 common genes between mesangial cells and SIN, 15 common genes between endothelial cells and SIN, 37 common genes between podocytes and SIN, and 17 common genes between pericytes and SIN, as shown in table 2. After removing duplicate targets, adjust the total number of potential targets related to SIN on glomerular cells were 56(FGG ,HSP90AA1 ,STAT1 ,GSTP1 ,MIF ,PDE4D ,PIM1 ,TYMS ,ERBB4 ,CBR1 ,SYK ,HEXB ,DPP4 ,AKR1B1 ,HMGCR ,PTPN1 ,SOD2 ,PPP1CC ,KDR ,INSR ,PCK1 ,SULT1A1 ,BMP2 ,DCXR ,FKBP1A ,CTNNA1 ,CTSB ,GLO1 ,GSTA1 ,BCAT2 ,IGF1 ,BHMT ,AKR1C3 ,LYZ ,CA2 ,CTSS ,MMP7 ,FDPS ,LTA4H ,OAT ,DAPK1 ,DHFR ,NR3C1 ,LDHB ,BST1 ,DUT ,ADK ,BACE1 ,PDE4B ,PPIA ,NQO1 ,EGFR ,EIF4E ,PDE5A ,ARF4 ,FABP4).
In renal tubular cells, there were 17 common genes between proximal tubular cells and SIN. There were 11 common genes between distal tubular cells and SIN, 5 common genes between Henle cells and SIN, 8 common genes between main cells and SIN, 10 common genes between intercalated cells and SIN, 31 common genes between fibroblasts and SIN, and 49 common genes between epithelial cells and SIN, as shown in table 2. After removing duplicate targets, the total number of potential targets related to SIN on renal tubular cells was adjusted to 79 .(IMPDH2 ,LDHB ,CTSB ,GSTP1 ,PPIA ,BCAT2 ,RXRA ,CTSS ,FKBP1A ,CA2 ,DUSP6 ,PRKACA ,SULT2B1 ,ARF4 ,EEA1 ,BCL2L1 ,ABL1 ,AKR1B1 ,RAB5A ,CTNNA1 ,ADK ,MAOB ,DPP4 ,NR3C1 ,PPP1CC ,GSK3B ,QPCT ,EIF4E ,PCK1 ,FDPS ,ERBB4 ,GLO1 ,PTPN1 ,INSR ,THRA ,DAPK1 ,PDE4D ,MET ,BHMT ,SOD2 ,DHFR ,DTYMK ,AMD1 ,MMP7 ,GPI ,EGFR ,PDPK1 ,DCXR ,STAT1 ,PGR ,PLAT ,SETD7 ,CBR1 ,MIF ,ADAM17 ,GSTA1 ,FGFR1 ,CFD ,HNMT ,DCPS ,LTA4H ,AKR1C3 ,PARP1 ,BACE1 ,XIAP ,HSP90AA1 ,DUT ,AZGP1 ,KIT ,ADH1C ,SYK ,SULT1A1 ,HPN ,VDR ,SPR ,RBP4 ,PAH ,FGG ,NQO1).
In renal immune cells, mast cells shared 12 common genes with SIN, dendritic cells share 12 common genes with SIN, plasma cells share 37 common genes with SIN, T cells share 53 common genes with SIN, and macrophages share 48 common genes with SIN, as shown in table 2. After deleting duplicate genes, immune cells shared 90 common genes with SIN(DCXR ,ERBB4 ,CBR1 ,FKBP3 ,RAP2A ,NR3C1 ,DCPS ,TGFBR1 ,SPR ,FECH ,LDHB ,GSTP1 ,PPIA ,SOD2 ,CASP3 ,PDE4B ,HMGCR ,FGFR1 ,AKR1B1 ,HPN ,GLO1 ,STAT1 ,DUSP6 ,DPP4 ,PTPN1 ,DUT ,PDPK1 ,FKBP1A ,GPI ,XIAP ,SYK ,LYZ ,CTSS ,CTSB ,EEA1 ,MMP7 ,RAB5A ,APAF1 ,CA2 ,MIF ,GSTA1 ,PCK1 ,BTK ,MAPK14 ,PDE4D ,CSNK1G2 ,KIT ,TPSB2 ,PPP1CC ,IMPDH2 ,CRABP2 ,RARG ,F11 ,ADAM17 ,SETD7 ,F10 ,AMD1 ,AKR1C3 ,FDPS ,HSP90AA1 ,MME ,CDK7 ,BCAT2 ,BHMT ,DPEP1 ,PAH ,SORD ,GSK3B ,ARF4 ,LTA4H ,GSR ,ALB ,DTYMK ,CTNNA1 ,MAOB ,PARP1 ,IGF1 ,QPCT ,PIM1 ,RBP4 ,KAT2B ,INSR ,ANG ,BCL2L1 ,MAN1B1 ,FGG ,CFD ,MMP9 ,HCK ,SDS). The Venn diagram of immune cells and SIN is shown in figure 1. The intersection of common genes between different kidney cells and SIN is shown in figure 1.
- PPI Network Analysis of SIN on PMN
PPI analysis of the intersection genes of Glomerular cells, renal tubular cells, immune cells, and SIN was performed in figure 2. As shown in table 2. By using node connectivity as the screening criteria, the top 5 targets with the highest degree were selected to identify their core gene targets, namely EGFR, HSP90AA1, IGF1, AKR1B1, GSTP1 for glomerular cells and SIN , EGFR, HSP90AA1, BCL2L1, GSK3B, AKR1B1 for renal tubular cells and SIN, ALB, HSP90AA1, CASP3, MMP9, IGF1 for immune cells and SIN.
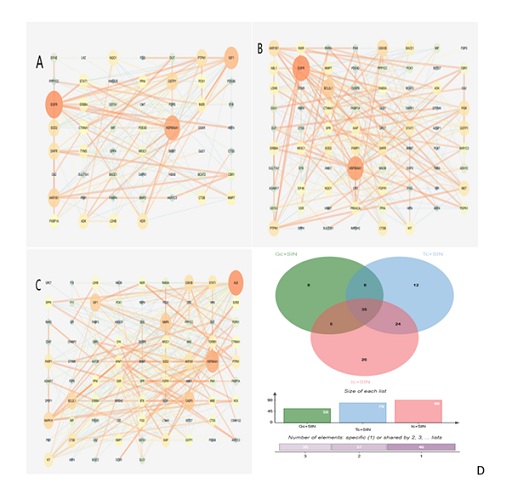 Figure 2: Potential targets among renal cells and SIN.(A)PPI of potential targets among renal glomerular cells and SIN.(B)PPI of potential targets among renal tubule cells and SIN.(C)PPI of potential targets among renal immune cells and SIN(D)Common genes among renal intrinsic cells and immune cells.
Figure 2: Potential targets among renal cells and SIN.(A)PPI of potential targets among renal glomerular cells and SIN.(B)PPI of potential targets among renal tubule cells and SIN.(C)PPI of potential targets among renal immune cells and SIN(D)Common genes among renal intrinsic cells and immune cells.
|
|
Gene | Degree |
Betweenness Centrality |
Closeness Centrality |
|
Glomerular |
EGFR |
22 |
0.28560816 |
0.59770115 |
|
HSP90AA1 |
19 |
0.17707672 |
0.56521739 |
|
|
IGF1 |
16 |
0.09475887 |
0.50980392 |
|
|
AKR1B1 |
12 |
0.14627831 |
0.50980392 |
|
|
GSTP1 |
11 |
0.07658736 |
0.5 |
|
|
Tubular |
EGFR |
33 |
0.31683679 |
0.59230769 |
|
HSP90AA1 |
30 |
0.19107104 |
0.55 |
|
|
BCL2L1 |
19 |
0.03742341 |
0.47239264 |
|
|
GSK3B |
15 |
0.02726655 |
0.45294118 |
|
|
AKR1B1 |
14 |
0.11638991 |
0.48734177 |
|
|
Immune cells |
ALB |
41 |
0.32843387 |
0.60714286 |
|
HSP90AA1 |
33 |
0.21624506 |
0.56291391 |
|
|
CASP3 |
24 |
0.08170864 |
0.53459119 |
|
|
MMP9 |
23 |
0.04901156 |
0.51204819 |
|
|
IGF1 |
22 |
0.03084037 |
0.49132948 |
Table 2: Core gene targets and related data in intersection gene targets.
- Go and KEGG enrichment analysis of SIN on PMN
The intersection genes between glomerular cells and SIN are mainly enriched in biological processes such as regulating kinase activity, regulating reactive oxygen species metabolism, MAPK cascade regulation, regulating MAP kinase activity, forward regulation of MAPK cascade, prostaglandin metabolism, regulation of ERK1 and ERK2 cascades, etc.KEGG enrichment analysis suggests that they are mainly involved in the PI3K-Akt signaling pathway, HIF-1 signaling pathway, FoxO signaling pathway, etc MAPK signaling pathways, etc.
The intersection genes between tubule cells and SIN are mainly enriched in biological processes such as hormone response, positive regulation of protein self phosphorylation kinase activity, transmembrane receptor protein tyrosine kinase signaling pathway, enzyme-linked receptor protein signaling pathway, and regulation of cell apoptosis signaling pathway. KEGG enrichment analysis suggests that they are mainly involved in PI3K-Akt signaling pathway, Ras signaling pathway, MAPK signaling pathway, FoxO signaling pathway, etc.
The intersection genes between immune cells and SIN are mainly enriched in biological processes such as hormone response, regulation of apoptosis signaling pathway, regulation of kinase activity, enzyme-linked receptor protein signaling pathway, negative regulation of apoptosis signaling pathway, cell response to hormone stimulation, transmembrane receptor protein tyrosine kinase signaling pathway, MAPK cascade regulation, etc. KEGG enrichment analysis suggests that they mainly participate in FoxO signaling pathway, PI3K-Akt signaling pathway MAPK signaling pathway, etc (Figure 3).
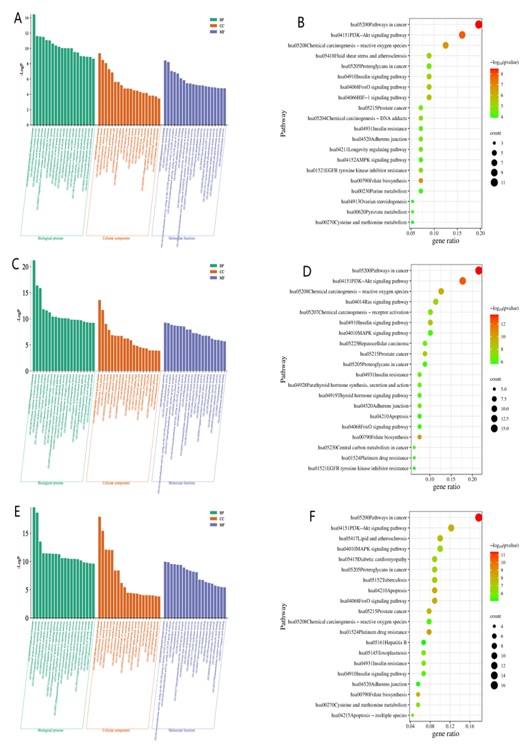 Figure 3: GO and KEGG of potential targets among renal cells and SIN.(A)GO of potential targets among renal glomerular cells and SIN.(B)KEGG pathways of potential targets among renal glomerular cells and SIN.(C)GO of potential targets among renal tubule cells and SIN.(D) KEGG pathways of potential targets among renal tubule cells and SIN.(E)GO of potential targets among renal immune cells and SIN.(F)KEGG pathways of potential targets among renal immune cells and SIN.
Figure 3: GO and KEGG of potential targets among renal cells and SIN.(A)GO of potential targets among renal glomerular cells and SIN.(B)KEGG pathways of potential targets among renal glomerular cells and SIN.(C)GO of potential targets among renal tubule cells and SIN.(D) KEGG pathways of potential targets among renal tubule cells and SIN.(E)GO of potential targets among renal immune cells and SIN.(F)KEGG pathways of potential targets among renal immune cells and SIN.
Discussion
SIN was effective to refractory PMN in our case. In order to uncover the underlying mechanism, we examined the gene expression of renal intrinsic cells and immune cells in PMN by scRNA-seq technology. It is a transcriptomic technology that measures the expression of up to thousands of genes in hundreds of single cells simultaneously, is a powerful and rapidly developing tool for characterizing individual cells and elucidating biological mechanisms at the cellular level. It offers an opportunity to comprehensively describe human kidney disease at a cellular level and plays a crucial role in identifying cell subtypes and illustrating molecular differences.
We compared common gene targets between them. We found SIN shared 56 genes with glomerular cells, 79 genes with renal tubular cells, and 90 genes with immune cells in renal tissues. At single cell level, the top three common genes were T cells, macrophages, plasma cells and Podocytes. The common gene numbers indicated that the main aspect of SIN in treating PMN is its regulatory effects on the immune system.
Through PPI analysis, we found the core genes between glomerular cells and SIN were EGFR, HSP90AA1, IGF1, AKR1B1, GSTP1. The core gene targets between renal tubular cells and SIN were EGFR, HSP90AA1, BCL2L1, GSK3B, AKR1B1, While the core gene targets between immune cells and SIN were ALB, HSP90AA1, CASP3, MMP9, IGF1. SIN can act on both renal intrinsic cells and immune cells through HSP90AA1. HSP90AA1 is a stress inducing protein that could regulate protein kinase and maintain cellular homeostasis. Researches had shown that HSP90AA1 could alleviate cell apoptosis and inflammation in cisplatin induced acute kidney injury by activating the Akt pathway [14].
Further study indicated SIN could acts on both glomerular cells and immune cells through insulin like growth factor-1(IGF1). IGF-1 is a peptide growth factor whose activity is regulated through the interactions with the IGF binding protein family (IGFBP-1 to 6). IGF-1 could be detected in the kidney of rats, and the levels of IGF-1 and IGF-1 receptors were increased in the glomerulus of diabetes rats that could lead to glomerular hypertrophy and renal fibrosis [15].
The protein encoded by CASP3 is a cysteine aspartate protease that plays a core role in the execution phase of cell apoptosis. Caspase 3 is also a key upstream regulator of the development of renal fibrosis [16]. Caspase 3 inhibitors had been proven to reduce renal interstitial fibrosis in patients with DN or obstructive nephropathy [17,18].
Matrix Metalloproteinases (MMPs) belong to the zinc dependent endoproteases family. Their functions were based on the remodeling and degradation of Extracellular Matrix (ECM) protein components [19]. Tan et al., found that MMP-9 could directly or indirectly promote the pathogenesis of renal fibrosis through osteopontin cleavage to further recruit macrophages and induce epithelial mesenchymal transition on renal tubular cells [20]. Epidermal growth factor receptor (EGFR) is a multifunctional signal transducer that plays an important role in cellular processes such as cell proliferation, survival, differentiation, migration, inflammation, and matrix homeostasis [21]. EGFR is often continuously activated in proximal tubular cells, leading to the progression of renal fibrosis during renal injury.
SIN can act on renal intrinsic cells and immune cells through the PI3K-Akt signaling pathway, FoxO signaling pathway, and mitogen activated protein kinase (MAPK) signaling pathway. The PI3K/Akt signaling pathway is a classic signaling pathway that participates in various physiological and pathological processes such as cell survival and apoptosis, proliferation and differentiation. Researches had shown that in hypoxic induced renal fibrosis experiments, the activation of the PI3K/Akt pathway is closely related to the progression of renal fibrosis. Inhibiting PI3K activation can reduce the accumulation of extracellular matrix (ECM), while inhibiting Akt can reduce the biomarkers of myofibroblasts in obstructive nephropathy [22]. Song Jian et al., pointed out that renal fibrosis caused by adenine in chronic kidney disease model rats was associated with excessive activation of the PI3K/Akt signaling pathway activated by EGFR. In addition, Liu et al., [23] had confirmed that the PI3K/Akt signaling pathway plays a crucial role in regulating ECM accumulation in DKD. Fox is a protein superfamily that contained over 100 members. Among them, fork head box transcription factor (Fox) O is a subfamily of the Fox protein family, composed of four members in mammals, namely FoxO1, FoxO3, FoxO4, and FoxO6. They share a highly conserved DNA binding domain, namely the fork head binding domain. Fox family O subfamily 1(FoxO1) is the most representative member of the FoxO family [24,25]. Research has shown that the FoxO1 is closely related to renal fibrosis and can inhibit myofibroblast (MF) activation and subsequent extracellular matrix (ECM) production. In addition, FoxO1 can regulate the occurrence and development of renal fibrosis through various signaling pathways, including STAT, SIRT1, and Wnt/ β- Multiple signaling pathways such as Catenin and PI-3K/Akt regulate the occurrence and development of renal fibrosis. The mitogen activated protein kinase (MAPK) signaling pathway is an important information transmission chain for cell growth, differentiation, stress, and inflammatory responses, including three pathways p38MAPK, ERK1/2, and JNK.
The imbalance of the MAPK signaling pathway was also associated with acute and chronic kidney disease [26]. Animal models of chronic kidney injury had shown that inhibiting p38 MAPK could significantly reduce renal function damage, inflammatory response, and RF severity [27]. The Ras signaling pathway acts on renal tubular cells. Research had shown that MAPK and phosphatidylinositol 3-kinase (PI3K) signaling pathways were Ras effector pathways, where RAS could activate the MAPK and PI3K signaling pathways through a series of reactions, resulting in changes such as renal fibrosis [28]. In addition, the progression of SRNS is related to the activation of the Ras/Raf/ERK 1/2 signaling pathway.
Through the HIF-1 signaling pathway acting on glomerular cells, the HIF-1 signaling pathway plays a role in regulating apoptosis, autophagy, and other aspects of renal tubular epithelial cells. HIF-1 is the main hypoxic inducer, and under certain conditions, HIF-1 α the high expression of genes has a fibrogenic effect on the kidneys [29]. Research has shown that glomerular injury can cause the loss of capillaries around the renal tubules, thereby reducing the oxygen supply to the interstitium, leading to chronic interstitial and tubular cell hypoxia, which can accelerate renal fibrosis [30]. The main medium of hypoxia response is hypoxia inducible factor 1 (HIF-1) and its oxygen sensitive component HIF-1 α. HIF-1 regulates multiple genes, some of which are closely related to tissue fibrosis. Kimura Bangzi et al., [31] pointed out through research that HIF-1 α. It seems to be a key factor in the progression of renal fibrosis.
Conclusion
Here, SIN was found in our study to act on PMN through various renal intrinsic cells, tubular cells and different immune cells, suggesting that SIN can be used as a broad-spectrum for treating PMN. Immune regulatory effects of Sinomenine on primary membranous nephropathy. These data indicated that SIN work on PMN mainly through immune regulatory. Our study not only provides a new method for finding the molecular targets through network pharmacology-based investigation and single cell sequencing, but also provides potential molecular targets for treating PMN in future.
Funding statement
Project supported by the Research Fund of Zhuhai Hospital of Integrated Chinese and Western Medicine (202203).
Conflict of Interests
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao XX, Peng C, Zhang H, Qin LP (2012) Sinomenium acutum: a review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol 50: 1053-1061.
- Wang Q, Li XK (2011) Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol 11: 373-376.
- Hou JH, Zhu HX, Zhou ML, Le WB, Zeng CH, et al. (2018) Changes in the Spectrum of Kidney Diseases: An Analysis of 40,759 Biopsy-Proven Cases from 2003 to 2014 in China. Kidney Dis (Basel) 4: 10-19.
- Hu R, Quan S, Wang Y, Zhou Y, Zhang Y, et al. (2020) Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep10: 10994.
- Lai WL, Yeh TH, Chen PM, Chan CK, Chiang WC, et al. (2015) Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc 114: 102-111.
- Ru J, Li P, Wang J, Zhou W, Li B, et al. (2014) TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 6: 13.
- Wang X, Shen Y, Wang S, Li S, Zhang W, et al. (2017) PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res 45: 356-360.
- The UniProt Consortium (2023) UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res 51: 523-531.
- Xu J, Shen C, Lin W, Meng T, Ooi JD, et al. (2021) Single-Cell Profiling Reveals Transcriptional Signatures and Cell-Cell Crosstalk in Anti-PLA2R Positive Idiopathic Membranous Nephropathy Patients. Front Immunol 12: 683330.
- Chen Z, Zhang T, Mao K, Shao X, Xu Y, et al. (2021) A single-cell survey of the human glomerulonephritis. J Cell Mol Med 25: 4684-4695.
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, et al. (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10: 1523.
- Xu J, Shen C, Lin W, Meng T, Ooi JD, et al. (2021) Single-Cell Profiling Reveals Transcriptional Signatures and Cell-Cell Crosstalk in Anti-PLA2R Positive Idiopathic Membranous Nephropathy Patients. Front Immunol 12: 683330.
- Chen Z, Zhang T, Mao K, Shao X, Xu Y, et al. (2021) A single-cell survey of the human glomerulonephritis. J Cell Mol Med 25: 4684-4695.
- Zhang MY, Ma LJ, Jiang L, Gao L, Wang X, et al. (2023) Paeoniflorin protects against cisplatin-induced acute kidney injury through targeting Hsp90AA1-Akt protein-protein interaction. J Ethnopharmacol 310: 116422.
- Miyatake N, Shikata K, Wada J, Sugimoto H, Takahashi S, et al. (1999) Differential distribution of insulin-like growth factor-1 and insulin-like growth factor binding proteins in experimental diabetic rat kidney. Nephron 81: 317-323.
- Yang B, Lan S, Dieudé M, Sabo-Vatasescu JP, Karakeussian-Rimbaud A, et al. (2018) Caspase-3 Is a Pivotal Regulator of Microvascular Rarefaction and Renal Fibrosis after Ischemia-Reperfusion Injury. J Am Soc Nephrol 29: 1900-1916.
- Wen S, Wang ZH, Zhang CX, Yang Y, Fan QL (2020) Caspase-3 Promotes Diabetic Kidney Disease Through Gasdermin E-Mediated Progression to Secondary Necrosis During Apoptosis. Diabetes Metab Syndr Obes 13: 313-323.
- Wu M, Xia W, Jin Q, Zhou A, Wang Q, et al. (2021) Gasdermin E Deletion Attenuates Ureteral Obstruction- and 5/6 Nephrectomy-Induced Renal Fibrosis and Kidney Dysfunction. Front Cell Dev Biol 9: 754134.
- Cui N, Hu M, Khalil RA (2017) Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci 147: 1-73.
- Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, et al. (2013) Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93: 434-449.
- Sabbah D A, Hajjo R, Sweidan K (2020) Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Top Med Chem 20: 815-834.
- Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, et al. (2000) Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225-234.
- Liu H, Wang X, Liu S, Li H, Yuan X, et al. (2016) Effects and mechanism of miR-23b on glucose-mediated epithelial-to-mesenchymal transition in diabetic nephropathy. Int J Biochem Cell Biol 70: 149-160.
- Link W (2019) Introduction to FOXO Biology. Methods Mol Biol 1890: 1-9.
- Huang H, Tindall DJ (2007) Dynamic FoxO transcription factors. J Cell Sci 120: 2479-2487.
- Cassidy H, Radford R, Slyne J, O'Connell S, Slattery C, et al. (2012) The role of MAPK in drug-induced kidney injury. J Signal Transduct 2012: 463617.
- Wang D, Warner GM, Yin P, Knudsen BE, Cheng J, et al. (2013) Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in a murine renal artery stenosis model. Am J Physiol Renal Physiol 304: 938-947.
- Pomeroy EJ, Eckfeldt CE (2020) Targeting Ras signaling in AML: RALB is a small GTPase with big potential. Small GTPases 11: 39-44.
- Qiu Y, Huang X, He W (2020) The regulatory role of HIF-1 in tubular epithelial cells in response to kidney injury. Histol Histopathol 35: 321-330.
- Fine LG, Bandyopadhay D, Norman JT (2000) Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75: 22-26.
- Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, et al. (2008) Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: 1023-1029.
Citation: Li X-X, Lai L-N (2024) Immune Regulatory Effects of Sinomenine on Primary Membranous Nephropathy: Based on Case Report and Network Pharmacology. J Altern Complement Integr Med 10: 470.
Copyright: © 2024 Xue-Xia Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

