
Impact of Information and Communication Technologies in the Neurorehabilitation of Traumatic Spinal Cord Injury (Clinical Case Report of Conus Medullaris syndrome)
*Corresponding Author(s):
Ivet B KolevaMedical University Of Sofia, Bulgaria
Email:dr.yvette.5@gmail.com
Abstract
During last years, we observe an increasing frequency of traffic accidents with neurological consequences. In case of spinal trauma at thocacic level we observe inferior paraplegia or paraparesis.
The goal of current article is to emphasize on the impact of Information and Communication Technologies (ICT) in the process of neurorehabilitation of a patient with conus medullaris syndrome, due to a traumatic Spinal Cord Injury (SCI) after fracture of the vertebra Th12.
We present a male patient of 29 years, transferred to our PRM Department one month after neurosurgical intervention (thoraco-lumbar stabilization) for an important vertebral Th12 fracture (after traffic accident).
During clinical exam (at the admission), we observed a complete inferior paraplegia with total dependence in Activities of Daily Living (ADL): impossible autonomic movements in the bed, the patient was only in lying position. X-Ray, CT scan and Magnetic Resonance Imagery (MRI) of the spine after the traffic accident demonstrated fracture with dislocation of lower thoracic vertebras. An urgent neurosurgical thoraco-lumbar stabilization was realized. The patient had urinary and bowel dysfunctions, he was with a permanent urinary catheter.
We applied a Complex Neurorehabilitation (NR) program, including preformed physical modalities (electrical stimulations), gradual verticalization, individualized physiotherapy and occupational therapy, accentuating on balance and gait training, education in Activities of Daily Living (ADL). Three months after the traumatic SCI, we began ICT-based rehabilitation with Tyro system (Lokomat and Omego).
We noticed significant efficacy of the NR-program: improvement of the range of motion of the spine and pelvis, pain relief, balance stabilization, amelioration of autonomy in ADL. At the month six after the SCI, our patient have the capacity of autonomic verticalization with a walker, the gait was possible with technical aids, but without assistance.
Authors consider that every patient with post-traumatic inferior paraplegia must be included in a longterm physiotherapeutic and ergotherapeutic program, if possible - robotic. ICT-based NR stimulates neuroplasticity and accelerates autonomy in everyday life.
Keywords
Inferior paraplegia; Information and communication technologies (ICT); Neuroplasticity; Neurorehabilitation; Paraparesis; Spinal cord injury
List of Abbreviations
ADL Activities of Daily Living
CNS Central Nervous System
CT Computer Tomography
ET Ergotherapy
ICF International Classification of Functioning, Disability and Health
ICT Information and Communication Technologies
KT Kinesitherapy
MRI Magnetic Resonance Imagery
NR Neurorehabilitation
PRM Physical and Rehabilitation Medicine
PT Physiotherapy
RoM Range of Motion
SCI Spinal Cord Injury
WHO World Health Organization
Introduction
Spinal Cord Injury
During last years, we observe an increasing frequency of traffic accidents and the resulting neurological complaints [1].
Traumatic spinal cord injury (SCI) is a neurological disorder with considerable psychologic and socio-economic repercussions for patients and family [2]. Clinical presentation of SCI depends on the location and the severity of the lesion: partial or total loss of sensory and motor function below the level of the injury [3].
Mechanisms of SCI include: Impact with persistent or transient compression; Distraction; Laceration / transection. Secondary injury of the spinal cord and subsequent deficits depend on: vascular damage, ionic imbalance, neurotransmitter accumulation (excitotoxicity), free radical formation, calcium influx, lipid peroxidation, inflammation, edema, and necrotic cell death [3].
The development of this spinal cord disease is rapid and the development of deficits depends on the adequate on-time diagnostic and subsequent therapy [4-6].
By definition, spinal cord begins at the level of the C1 vertebra (atlas) and ends at the level of L1 vertebra in the adult [7,8].The most frequent levels of SCI are cervical and thoracal. In case of thoracic vertebral fracture the most common neurological consequence is the spastic paraplegia with motor weakness and loss of sensibility in lower extremities, eventually accompagnied by development of spasticity. Conus medullaris syndrome is a consequence of spinal injuries at the level of Th12 to L2 vertebrae [9,10]. Clinical presentation comprise a combination of severe back pain and upper and lower motor neuron deficits, including: saddle anesthesia, loss of bladder reflex with urinary retention, loss of bowel reflex with incontinence, a mixed upper and lower motor neuron pattern in lower extremities with motor weakness, paresthesia and numbness [11].
According American Spinal Injury Association (ASIA), the functional status and survival rate of these patients depend on mobility, bowel and bladder dysfunction [12,13]. Their quality of life is determined by the on-time adequate rehabilitation, adapted to the individual case.
Neurorehabilitation
According the White Book on Physical and Rehabilitation Medicine (PRM) in Europe [14], Rehabilitation is a functional therapy, based on a detailed functional assessment.Neurorehabilitation (NR) is an interdiscipline between Neurology, Neurosurgery, Physical and rehabilitation medicine [15]. The work (and the education) in this thematic field requires the coordinated activity of different members of the multidisciplinary and multi-professional neurorehabilitation team - medical specialists and health professionals [16].
Practically, NR is a combination of detailed functional assessment and subsequent complex functional therapy, oriented to modulate neuroplasticity - through stimulation or inhibition techniques. For functional assessment of patients in NR, we apply a lot of clinical and instrumental methods, specific neurological scales and the International Classification of Functioning, Disability and Health [15,17,18]. In clinical NR-practice, the goal is always functional recovery and amelioration of the quality of life of neurological and neurosurgical patients. The autonomy of patients in clinical practice requires a specific training of activities of Daily Living (ADL), especially of Grasp and Gait [19]. During the construction of the complex NR-algorithm, we apply PRM-principles - multi-disciplinary team, holistic and patient-centred approach [20,21]. For treatment, we use the SMART approach (specific, measurable, achievable, realistic and time bound) and we apply the ‘rehabilitation puzzle’ - a complex NR-programme with synergic combination of different physical modalities. In NR-clinical practice, we apply several natural physical modalities (physiotherapy /PT/ and ergotherapy /ET/; balneotherapy or balneophysiotherapy; cryo or thermo-agents; manual therapy; soft-tissue techniques) and preformed methods (functional electrostimulations, transcutaneous electroneurostimulation /TENS/, Deep Oscillation, low-frequency low intensity magnetic field, Ultrasound, Laser, etc.). We accentuate on some emerging methods, as exoskeletons, robotic NR, virtual reality [21].
The World Report on Disability, prepared by the World Health organization and the World Bank [22], defines the goals of rehabilitation: prevention of the loss of function; slowing the rate of loss of function; improvement or restoration of function; compensation for lost function; maintenance of current function. Modern rehabilitation has an integrative and holistic approach to the patient, based on the International Classification, disability and Health (ICF, 2001) and on clinical principles.
Information and communication technologies in the rehabilitation
Ultimately, contemporary information and Communication Technologies (ICT) were introduced in the rehabilitation practice. Actually, we use telerehabilitation consultations and procedures, Exoskeletons, virtual reality, robotic rehabilitation with Locomat (Hocoma system for balance and gait training) and Tyro-Motion system (for grasp and gait training). ICT permit a detailed assessment at the beginning, during and at the end of the NR-process.
Current article presents the authors’ opinion about the necessity of structuration of complex rehabilitation algorithms, including not only different traditional natural and pre-formed physical modalities, button an adapted PT and ET program with modern technology - Lokomat (Hocoma system for locomotor training), robotic rehabilitation and virtual reality [23-25].
Aim of the Article
Our objective was to underline on the potential of contemporaneous NR-methods (as robotic rehabilitation), in combination with the traditional for our country PT and electrical stimulation - in the process of recovery of SCI-patients. For illustration of the potential of ICT-based NR, we will present a clinical case with conus medullaris syndrome (consequence of SCI injury at lower thoracic level).
Clinical Case Presentation
At the admission
We present a male patient of 29 years, transferred to our PRM Department one month after neurosurgery (thoracic stabilization) for an important vertebral thoraco-lumbar fracture.
During clinical exam (at the admission), we observed a complete inferior paraplegia with total dependence of assistance in Activities of Daily Living (ADL): impossible autonomic movements in the bed, the patient was only in lying position. X-Ray, CT scan and Magnetic Resonance Imagery (MRI) of the spine after the traffic accident demonstrated fracture with dislocation of Th12 vertebra (Figure 1). An urgent neurosurgical thoraco-lumbar stabilization was realized (Figures 1&2). The patient had permanent urinary catheter.
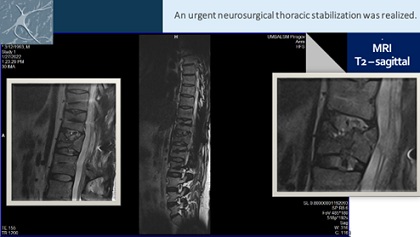 Figure 1: MRI of the spine of our patient.
Figure 1: MRI of the spine of our patient.
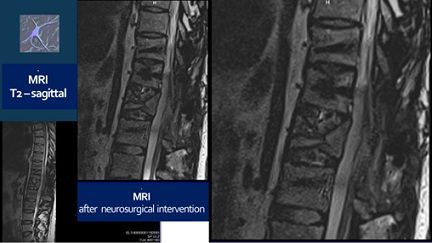 Figure 2: MRI after the neurosurgical intervention.
Figure 2: MRI after the neurosurgical intervention.
Methods
We applied a complex rehabilitation, including preformed physical modalities (electrical stimulations), gradual verticalization, individualized physiotherapeutic and occupational therapeutic program, accentuating on balance and gait training, ADL education. Three months after the traumatic Spinal Cord Injury (SCI), we began with robotic rehabilitation with Lokomat (Hocoma system) and Omego (Tyro-system), so the rehabilitation complex included electrical stimulations, physiotherapy, ergotherapy and robotic rehabilitation (Figure 3).
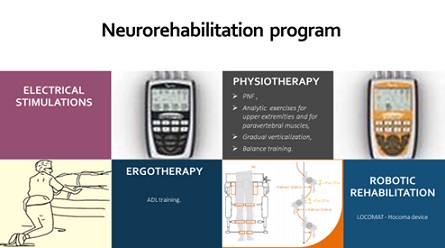 Figure 3: The Rehabilitation complex at the month 3 after the SCI.
Figure 3: The Rehabilitation complex at the month 3 after the SCI.
The individualized physiotherapeutic and ergo (occupational) therapeutic program was based on traditional principles [26-28]. We included Proprioceptive Neuro-Muscular Facilitation (PNF), analytic exercises for paravertebral and gluteal muscles and for trunk stabilization, strength training of upper extremities, gradual verticalization, training of the trunk control and of the balance, quadripedic locomotion (Figures 4-8); training in activities of daily living [29]. We emphasized on analytic exercises for upper limbs’ muscles, and for flexors and extensors of the hip joint (ilio-psoas and gluteus maximus muscles - Figure 4), on quadripedal mobility (Figure 5).
After the month 3 post-SCI, we included traditional gait training with pelvis stabilization and with a walker (Figure 8), strenght exercises for lower limbs muscles - with ICT-based device Omego (Figure 6). The robotic gait training with Lokomat Pro (Figure 7) was initiated after the trunk stabilization and the initial balance training - at the month 3 after SCI. Locomat sessions accentuated on balance and gait training (Figure 7). The robotic sessions were realized three times weekly, for one hour, during next three consecutive months - for a total of 55 sessions.
 Figure 4: Training for trunk (pelvis) stability.
Figure 4: Training for trunk (pelvis) stability.
 Figure 5: Quadrupedal training.
Figure 5: Quadrupedal training.
 Figure 6: Velo-training with Omego Plus.
Figure 6: Velo-training with Omego Plus.
 Figure 7: Gait training with Lokomat Pro.
Figure 7: Gait training with Lokomat Pro.
 Figure 8: Traditional gait training.
Figure 8: Traditional gait training.
Results
We noticed significant efficacy of the NR-programme: improvement of the range of motion of the spine and extremities, pain relief, balance stabilization, amelioration of autonomy in ADL. At the month 6 after the SCI the patient have the capacity of autonomic verticalization and gait -with walker, but without assistance. Next figures demonstrate the positive changes of different parameters, evaluated by the machine (Lokomat report): increase of distance and training time (Figure 9), gait velocity (Figure 10). For us, the most important improvements were: the reduction of the Body weight support (Figure 11) and of the Lokomat Guidance force (Figure 12) - objective indicators of the recovery of patient’s autonomic gait. Figure 13 demonstrates the observed significant increase of the active range-of-motion (RoM) of principal joints of lower extremities (hip and knee).
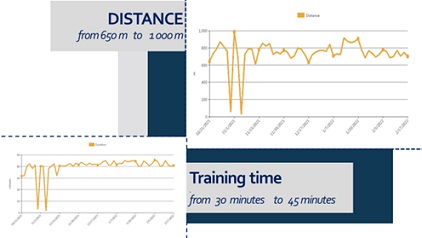 Figure 9: Patient’s report - Gait distance evaluation and increase of training time
Figure 9: Patient’s report - Gait distance evaluation and increase of training time
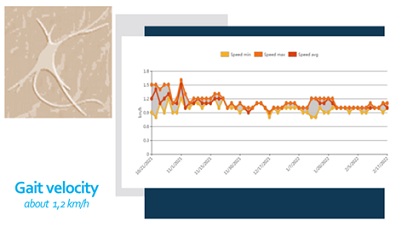 Figure 10: Patient’s report of the Locomat training - Gait velocity.
Figure 10: Patient’s report of the Locomat training - Gait velocity.
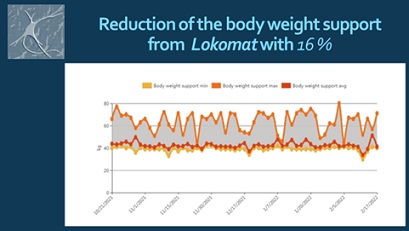 Figure 11: Patient’s report: Reduction of the body weight support by the machine.
Figure 11: Patient’s report: Reduction of the body weight support by the machine.
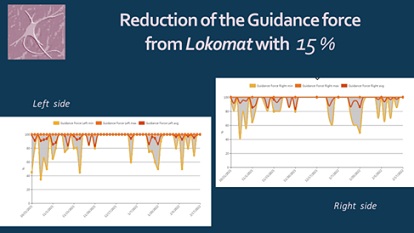 Figure 12: Patient’s report - Reduction of the guidance force from Lokomat.
Figure 12: Patient’s report - Reduction of the guidance force from Lokomat.
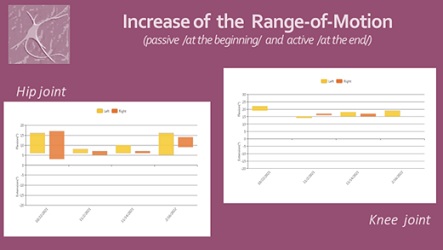 Figure 13: Patient’s report: Amelioration of the Range of Motion in hip and knee joints.
Figure 13: Patient’s report: Amelioration of the Range of Motion in hip and knee joints.
Discussion
“Any man could be the sculptor of his own brain.”: Santiago Ramón y Cajal
According Merriam Webster dictionary: “neuroplasticity is the adult brain’s ability to adapt”. Neuroplasticity allows the neurons to compensate for injury and disease and to adjust their activities in response to new situations or to changes in their environment [30,31]. The “aim” of neuroplasticity is to optimize neural networks during phylogenesis, ontogenesis and physiological learning, and in case of a brain disease [32]. Neuroplasticity is the pathophysiological basis for treatment of the medullar lesions through physical training and rehabilitation, including goal-directed activities [33].
Many NR tools have the potential to rewire cerebral functions and to excite the formation of new connections and pathways, respectively to stimulate the brain reorganization and adaptation to the ‘new’ situation (appearance of a damaged locus in the cerebral or medullar tissue), in other terms - to help functional recovery through potentiation of use-induced and use-dependent neuroplasticity (Figure 14).
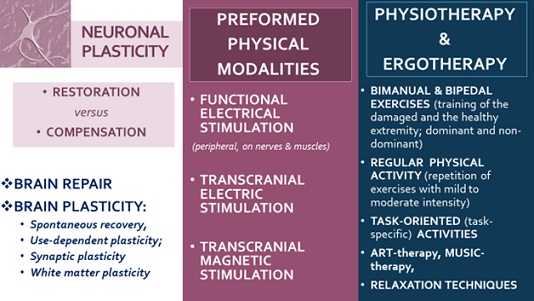 Figure 14. Physical medicine for neuroplasticity.
Figure 14. Physical medicine for neuroplasticity.
In cases with motor weakness, lack of mobility, and reduced range of motion (active or passive) we stimulate neuroplasticity through: Active exercises (including underwater training), Proprioceptive Neuro-Muscular Stimulation (PNF); Grasp training and goal-oriented activities; Balance and gait training; Functional electrostimulations; Exoskeletons (with Hybrid-assistive limb); Virtual reality training. Paralelly, we apply inhibitory techniques - oriented to pain reduction and regulation of the increased muscle tone (spasticity or rigidity): cryotherapy or thermotherapy; balneotherapy with mineral waters and peloids; manual therapy (joint tractions, mobilizations and manipulations); relaxing massage; Post-Isometric Relaxation (PIR), stretching techniques; TENS, Deep oscillation, Magnetic field, Lasertherapy and Laserpuncture. In every clinical case, we consider the balance between stimulation and inhibition, crucial for the clinical result and the efficacy of rehabilitation. Rehabilitation with modern methods of evaluation and contemporary therapeutic methods is an important patient’s right, recognized by United Nations in the Convention on the Rights of persons with disabilities [34].
Conclusion
Systematic neurorehabilitation with modern information technologies ameliorates the quality of life of patients, using the activity-dependent neuronal plasticity.
Our results demonstrated positive effects of ICT-application on the neuroplasticity, functional recovery, autonomy and quality of life of SCI-patients.
We consider that every patient with post-traumatic inferior paraplegia must be included in a longterm physiotherapeutic and ergotherapeutic program, if possible - with robotic rehabilitation.
Author’s Contribution
IK and BY prepared the full text of the publication. IK and NT are medical doctors, IK is specialist in Physical and Rehabilitation medicine (PRM) and in Neurology, NT is PRM-trainee; they described the clinical exposition and elaborated the neurorehabilitation program. BY and DP are physiotherapists; DP prepared the photos of the patient. RY is IT-specialist and facilitated the data interpretation. All authors approved the final text of the article.
Acknowledgement
No financial support. No conflict of interests. No ethical issues.
References
- WHO (2014) Global Status Report on noncommunicable diseases. WHO, Geneva, Switzerland. 2014. Page no: 298.
- WHO (2013) Spinal Cord Injury : Fact sheet N°384 (19 November 2013). WHO, Geneva, Switzerland.
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2019) Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol 10: 282.
- Harrop JS, Hunt GE, Vaccaro AR (2004) Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus 16: 4.
- Cho TA (2015) Spinal cord functional anatomy. Continuum (MinneapMinn). Continuum (Minneap Minn) 21: 13-35.
- Stein DM, Pineda JA, Roddy VT, Knight WA (2015) Emergency neurological life support: traumatic spine injury. Neurocrit Care 27: 170-180.
- Liu A, Yang K, Wang D, Li C, Ren Z, et al. (2017) Level of conus medullaris termination in adult population analyzed by kinetic magnetic resonance imaging. Surg Radiol Anat 39: 759-765.
- Khan YS, Lui F. Neuroanatomy, Spinal Cord. 2022 Jul 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32644482.
- Bican O, Minagar A, Pruitt AA (2013) The spinal cord: a review of functional neuroanatomy. Neurol Clin 31: 1-18.
- Brouwers E, van de Meent H, Curt A, Starremans B, Hosman A, et al. (2017) Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal Cord 55: 886-890.
- Mudgal P, Gaillard F, Hapugoda S (2014) Conus medullaris syndrome. Reference article, Radiopaedia.org.
- Wilson JR, Cadotte DW, Fehlings MG (2012) Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J Neurosurg Spine 17(1 Suppl): 11-26.
- Shavelle RM, Paculdo DR, Tran LM, Strauss DJ, Brooks JC, et al. (2015) Mobility, continence, and life expectancy in persons with Asia Impairment Scale Grade D spinal cord injuries.Am J Phys Med Rehabil 94: 180-191.
- White Book on Physical and Rehabilitation Medicine in Europe. Third edition (2018). European Journal on PRM, European PRM Bodies Alliance: European Academy of Rehabilitation Medicine, European Society of PRM, EUMS – PRM Section, European College of PRM. European Journal of Physical and Rehabilitation Medicine, 54, 2: 1-204.
- ??leva I (2009) The Bulgarian Neurorehabilitation School and the International Classification of Functioning, Disability and Health (ICF): Integrating ICF requirements into clinical practice. Journal of Biomedical and Clinical Research 1: 8-18.
- Koleva Y (2011) Neurorehabilitation in Bulgaria. In: Public health and health care in Greece and Bulgaria: The challenge of the cross border collaboration in times of financial crisis. Papazissis Publishers, Athens Page no: 906-911.
- WHO (2001) International Classification of Functioning, Disability and Health (ICF). WHO, Geneva, Switzerland.
- Koleva I (2019) Clinical neurorehabilitation: A Monograph. Sofia: Publishing house “SIMEL PRESS”, Pg no: 678.
- Koleva I, Avramescu ET (2017) Grasp and gait rehabilitation. Sofia: ‘SIMEL PRESS’, Pg no: 396.
- European PRM Bodies Alliance (2018) White Book on Physical and Rehabilitation Medicine.. European Journal of Physical and Rehabilitation Medicine 54: N 2, 156-311.
- Koleva I, Yoshinov B (2020) Koleva I (ed.). Sofia: Edition house ‘SIMEL PRESS’, Pg no: 582.
- WHO (2011) World Report on Disability. WHO, Geneva, Switzerland.
- Holden MK, Dyar Th (2002) Virtual Environment Training: A New Tool for Neurorehabilitation. Journal of neurologic physical therapy: JNPT 26: 62-71.
- Barbeau H. Locomotor Training in Neurorehabilitation: Emerging Rehabilitation Concepts. Neurorehabilitation and Neural Repair 17 : No 1, 1-11
- Huang V, Krakauer J (2009) Robotic Neurorehabilitation: a computational motor learning perspective. J Neuroeng Rehabil 6: 1-13.
- Porter S (2003) Tidy’s physiotherapy. Edinburg - London - New York - Oxford - Philadelphia - St Louis - Sydney - Toronto: Butterworth Heinemann (Elsevier Sciences), Elsevier, Amsterdam, Netherlands, Page no: 591.
- Lennon S, Stokes M (2009) Pocketbook of Neurological Physiotherapy. London: Churchill-Livingstone, Elsevier, London, Page no: 308.
- Lamprecht S, Lamprecht H (2018) Training in Neurorehabilitation. Stuttgart: Thieme Page no: 125.
- Koleva IB, Yoshinov RR, Yoshinov BR. E-book based education in Ergotherapy for students and trainees in the rehabilitation field. In: Chova LG, Lopez-Martinez A, Torres IC (ed.). Proceedings of ICERI 2019 Conference, 11th-13th November 2019, IATED Academy, Seville, Spain, Page no: 4626-4633.
- Shaffer J (2016) Neuroplasticity and Clinical Practice: Building Brain Power for Health. Front Psychol, 7: 1118.
- Kramer AF, Erickson KI (2007) Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 11(8): 342-348.
- Vaynman S, Gomez-Pinilla F (2006) Revenge of the "sit": how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res, 84: 699-715.
- Moore D, Loprinzi PD (2022) Exercise influences episodic memory via changes in hippocampal neurocircuitry and long-term potentiation. Eur J Neurosci 54: 6960-6971.
- United Nations (2020) Convention on the Rights of Persons with Disabilities.
Citation: Koleva IB, Yoshinov BR, Tzvetkova N, Petrov D, Yoshinov RR (2023) Impact of Information and Communication Technologies in the Neurorehabilitation of Traumatic Spinal Cord Injury (Clinical Case Report of Conus Medullaris syndrome). J Clin Stud Med Case Rep 10: 0143.
Copyright: © 2023 Ivet B Koleva, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

