Impact of Parity on Fracture Risk after Menopause: A Systematic Review
*Corresponding Author(s):
Georges WeryhaDepartment Of Endocrinology, University Hospital Center Of Nancy, CHU Brabois Adultes, Rue Du Morvan, 54500 Vandoeuvre Les Nancy, France
Tel:+33608335915,
Fax:+33383153440
Email:g.weryha@chu-nancy.fr
Abstract
Background
Pregnancy and breastfeeding cause temporal bone resorption and can play a role in clinical expression of osteoporosis disease. Objective was to estimate the association between parity and risk of osteoporotic fracture after menopause.
Methods
We performed a systematic review through a search of PubMed, Cochrane, Embase databases from January 1, 1980 through January 31, 2016. We included all studies that evaluated the link between parity and post-menopausal osteoporotic fracture using univariate and/or multivariate analysis.
Results
Among 29 studies, a positive effect of parity was found in 7/13 prospective, 1/7 transversal and 2/10 retrospective studies. Three studies out of 5 found that parity had a protective role against the risk for any fracture (HR = 0.94[0.90-0.99], OR = 0.41[0.28-0.61] and OR = 0.90[0.84-0.99]). For hip fracture, a protective effect was found in 5 studies out of 17 and concerned women with ≥2 children in two studies (OR = 0.75[0.62-0.91], RR = 0.5[0.32-0.79]). For vertebral fracture, 1 study out of 8 reported a significantly reduced risk in women with ≥2 children (HR = 0.44[0.26-0.76]). Wrist fracture risk was evaluated in 7 studies. One found a reduced risk in parous individuals (HR = 0.71[0.52-0.97]). An increased fracture risk was found in three studies. It’s about Asian sub-population with ≥5 children in two studies (HR = 1.65[1.06-2.56] and RR = 2.53[1.07-6.68]). In another, a positive correlation between vertebral fracture and parity was reported (OR = 1.093[1.008-1.186]).
Conclusion
Overall, pregnancy does not seem to be associated with an increased risk of osteoporotic fracture after menopause. The negative impact of ≥5 childbirth in Asiatic sub-population requires future investigations.
Keywords
ABBREVIATIONS
HR: Hazard Ratio; OR: Odds Ratio; HR: Hazard Ratio; CI: Confidence Interval; PTHrp: Parathyroid Hormone-Related Protein; BMD: Body Mass Density
INTRODUCTION
Osteoporosis is a disease essentially bound to menopause and ageing. The growing prevalence of osteoporosis is becoming an increasingly important health problem throughout the world. The estimations state a pandemic of osteoporosis in more than 200 million people in the world among which post-menopausal women represent 30% in United States and Europe. It expresses clinically after 50 years by fractures due to low energy traumas [1]. Osteoporotic fracture is due to the conjunction of minor trauma, lowered bone mass, change in trabecular bone microarchitecture and cortical porosity [2,3]. At least, 40% of osteoporotic women will suffer from one or several fragility fractures in their life [1].
Pregnancy and breastfeeding are periods of the woman’s life with important calcic loss, estimated between 200 to 300 mg daily [4]. This loss cause, in temporal way, a negative calcic balance which is stabilized by bone resorption [5]. Isolated observations of fragility fractures were especially reported in primipares. Their incidence remains underestimated and seems related to a preliminary fragility or an excessive bone resorption at the mother [6-9]. Albright and Reifenstein reported from 1948 the existence of osteoporotic fractures in two pregnant women [10].
The link between pregnancy, breastfeeding and osteoporotic fractures is not clear in the literature. The loss of bone mass during pregnancy and especially during breastfeeding varies from 1% per month to 10% during the 6 months of lactation. This phenomenon is however reversible during the weaning [5,11,12]. The accumulation of reproductive events during woman’s life plays a very important role to clinical expression of osteoporotic disease. However, acquired and environmental factors as pregnancy and lactation seem to determinate the severity of osteoporotic disease [13].
For this purpose, we performed a systematic review in order to estimate the level of association between parity and risk of osteoporotic fracture after menopause.
MATERIALS AND METHODS
Data source and search strategy
Study selection criteria
I. Having a human prospective, retrospective, case control or cross sectional design;
II. Including a female population of premenopausal, menopausal or postmenopausal status;
III. All fractures diagnosis had to be based on clinical and/or imaging studies (radiography, bone densitometry, scanner);
IV. Having result of study evaluating the risk for one or overall fractures according to parity and expressed results with univariate and/or multivariate adjusted OR (Odds Ratio) or HR (Hazard Ratio) or RR (Relative Risk) with a Confidence Interval (CI) of 95%, with or without p value.
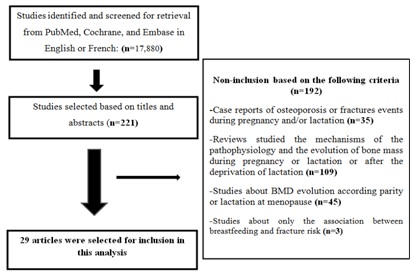
We excluded papers reporting case reports, reviews, commentaries and letters, studies about mechanisms of Pathophysiology and evolution of bone mass during pregnancy or lactation or after deprivation of lactation as well as animal’s experimental studies, studies about only Body Mass Density (BMD) evolution according to parity or lactation at menopause, studies about only the association between breastfeeding and fracture risk, studies in which the criteria of evaluation were not sufficient. Studies where fractures or low bone mass were due to hematological or endocrinological diseases (pex multiple myeloma, hyperparathyroidism, Cushing disease etc.,) or were medically induced (use of corticoids or…) were also excluded.
Data extraction and quality assessment
RESULTS
Characteristics of the included studies
Twenty nine studies presented data of association between parity and risk of fracture. Twelve studies included European population, eight studies North American population, one study South American population and two others a maghrebian population. Four studies were conducted in an Asiatic population and finally two studies in an Australian population. The follow up period varied from 1 to 29 years.
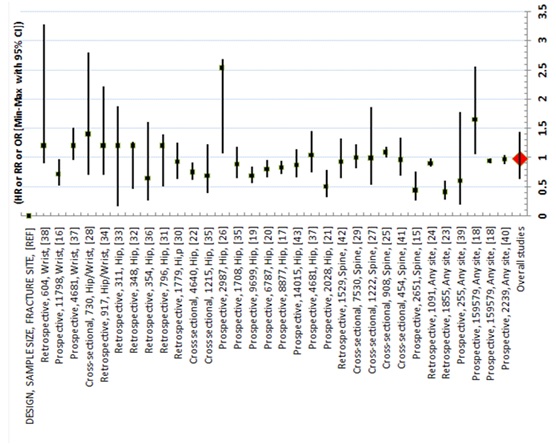
The risk of fracture was studied in 13 prospective, 7 transversal and 10 retrospective studies. Among these studies, 12 were grade B and 16 grade C. The publication of Petersen et al., [15] gives one part of prospective results (grade B) and one part of transversal results (grade C). It is about 17 studies examined the risk for hip’s fracture, 8 the risk for vertebral’s fracture, 7 the risk for wrist’s fracture and 5 the risk for all fracture (Table 1 and Figure 2).
| First author, journal, year of publication, reference, grade of recommendation | Type of the studies, setting | Effect of parity and/or lactation on fracture | Sample size, menopausal status, age in years (mean ± DS or median) | Follow-up (years) | Compared groups | Studied outcome | Results: [incidence, (HR or OR or RR (95% CI), p value] | Conclusion |
| Mori et al., Bone, [16]Grade B | Prospective, (The Study of Women's Health Across the Nation), United-State | All fracture | 2239, (1210 premenopausal and 1017 postmenopausal), (46.0±2.0) | 15.7 | - 2239 parous for 1 to 3 children | - Incidence of fractures on the whole studied population- Adjusted1 HR in compared groups for fracture risk | - 15.9%- Parity per additional childbirth:0.97(0.89-1.05),p = nd | Parity is not associated with fracture risk |
| Cauley et al., J Bone Miner Res, [17]Grade B | Prospective, (Women’s Health Initiative study), United-State | All fracture | 159579, postmenopausal, (50-79) | 8±2.6 | 144094 parous vs. 15485 nulliparous* | Adjusted2 HR for fractures risk across ethnicity (White, Black, Hispanic, Asian and American Indian) | 2-4 children: 0.94(0.90-0.99), 1.10(0.82-1.46), 0.85(0.60-1.20), 1.25(0.84-1.87), 1.09(0.35-3.42), p = nd≥5 children: 0.95(0.90-1.00), 1.13(0.84-1.54), 0.81(0.56-1.16), 1.65(1.06-2.56), 1.43(0.44-4.61), p = nd | Parity is associated to a lower risk for fracture in white parous with 2-4 children and a higher risk for fracture in asian parous with ≥5 children |
| Paganini-Hill et al., J Womens Health, [18]Grade B | Prospective, (The Leisure World Cohort Study), United-State | Hip, wrist and spine fractures | 8877, postmenopausal, (73±7.4) | 20 | 6549 parous vs. 2308 nulliparous | - Incidence of hip, wrist, spine fracture on whole studied population- Adjusted3 HR in compared groups for hip, wrist, spine fracture risk | 0.83(0.72-0.95), 0.91(nd), 0.98(nd),p = nd | Parity is associated to a significant lower risk for hip fracture |
| Taylor et al., J Am Geriatr Soc, [19]Grade B | Prospective, (Study of Osteoporotic Fractures), United State | Hip fracture | 6787, postmenopausal, (73.3±4.9) | 10.1±3.2 | 5558 parous* vs. 1229 nulliparous | - Incidence of hip fracture on whole studied population-Adjusted4 HR in compared groups (with and without BMD) for hip fracture risk | 8.9%With BMD: 1.28(1.06-1.55)Without BMD : 1.25(1.04-1.51) p = nd | Nulliparity is associated to a significant higher risk for hip fracture |
| Hillier et al., J Bone Miner Res, [20]Grade B | Prospective, (Study of Osteoporotic Fractures), United-State | Non traumatic hip, spine, and wrist fractures | 9699, postmenopausal, (72.9±5.6 for nulliparous and 71±5.2 for parous) | 3 | 7864 parous* vs. 1835 nulliparous | - Incident of hip, spine and wrist fracture on whole studied population- Adjusted5 HR in compared groups (with and without BMD) for hip, spine and wrist fracture risk | 6.1%, 4%, 6.5%With BMD : 1.44(1.17-1.78), 1.14(0.85-1.52) and 0.86(0.69-1.09)Without BMD : 1.44(1.17-1.78), 1.09(0.82-1.45) and 0.87(0.69-1.09),p = nd | Nulliparity is associated to a significant higher risk for hip fracture |
| Bjørnerem et al., J Bone Miner Res, [21]Grade B | Prospective, (Tromsø Study), Norway | Hip and wrist fractures | 4681, postmenopausal, (63.9) | 14.5 | - 4230 parous vs. 451 nulliparous* | - Incidence of hip, fragility and wrist fractures on the whole studied population- Adjusted6 HR in compared groups for hip and wrist fracture risk | 9.4%, 13.2%1.04(0.75-1.46),p = 0.89;and 1.20(0.96-1.51),p = 0.80 | -Parity is notassociated to the risk for hip and wrist fracture |
| Kauppi et al., Osteoporosis Int, [22]Grade B | Prospective, (Mini-Finland Health Survey study), Finland | Hip fracture | 2028, postmenopausal, (63.2±9.2) | 17 | 1633 parous vs. 395 nulliparous* | - Incidence of hip fracture on the whole studied population- Adjusted7 RR in compared groups for hip fracture risk | 6.5%1-2 children: 0.85(0.55-1.32)≥3 children: 0.5(0.32-0.79)p = nd | Only parity ≥3 birth is associated to a significant lower risk of hip fracture |
| Trémollieres et al., J Bone Miner Res, [23]Grade B | Prospective, (Menopause et Os Cohort Study), French | Spine and hip fractures | 2651, (756 premenopausal and 1895 postmenopausal, (54±4) | 13.4 | 2416 parous vs. 235 nulliparous* | -Incidence of fracture on the whole studied population- BMD adjusted8 HR in compared groups for spine and hip fracture risk | 15.6%2 children: 0.68(0.42-1.11) and 0.66(0.36-1.22),≥3 children: 0.44(0.26-0.76) and 0.52(0.27-1.00),p = nd | Only parity ≥3 birth is associated to a significant lower risk for spine fracture |
| Hundrup et al., Eur J Epidemiol, [24]Grade B | Prospective, Denmark, The Danish Nurse Cohort Study | Hip fracture | 14015 postmenopausal, (≥50) | 6 | 11120 parous vs 2762 nulliparous* | - Non adjusted HR in compared groups for hip fracture risk | 0.87 (0.66-1.15),p = 0.323 | Parity is notassociated to the risk for hip and wrist fracture |
| Naves et al., Osteoporosis Int, [25]Grade B | Prospective, (European Vertebral Osteoporosis Study), Spain | Vertebral and no vertebral osteoporotic fractures | 255, postmenopausal, (65±9) | 8 | - 212 Parous vs. 38 nulliparous* | - Incident of osteoporotic fracture on the whole studied population- Adjusted9 OR in compared groups for osteoporotic fracture risk | 12.1%0.60(0.20-1.78) | Parity is not associated with the risk for osteoporotic fracture |
| Petersen et al., Ann Epidemiol, [15]Grade B | Prospective, (The Danish Twin Survey Study), Denmark | Hip fracture | 1708, postmenopausal, 75 (66-99) | 29.11 | - 523 parous 1-2 birth* vs. 293 nulliparous and vs. 352 parous 3-4 birth | -Incidence of hip fracture on whole studied population- Non adjusted HR in compared groups for hip fracture risk | 18.7%Nulliparity: 1.28(0.98-1.68)3-4 children: 0.88(0.65-1.19)p = nd | Parity is not associated to the risk for hip fracture |
| Honkanen et al., Osteoporosis Int, [26]Grade B | Prospective, (Kuopio Osteoporosis Risk Factor and Prevention Study), Finland | Distal forearm fracture | 11798, (3775 premenopausal and 8023 postmenopausal), (52.3±2.9) | 5 | 10488 parous vs. 1310 nulliparous* | -Incidence of forearm fracture on whole studied population- Adjusted10 HR in compared groups for distal forearm fracture risk | 3.1%0.71(0.52-0.97),p = 0.031 | Parity is associated to a significant lower risk for distal forearm fracture |
| Fujiwara et al., J Bone Miner Res, [27]Grade B | Prospective, (Adult Health Study), Japan | Hip fracture | 2987, postmenopausal, (58.6±11.6) | 14 | 1353 parous 1-2 children* vs. 254 nulliparous and vs. 923 parous 3-4 children and vs. 403 parous ≥5 children | Adjusted11 RR in compared groups for hip fracture risk | Nulliparity: 2.31(0.60-7.76)3-4 children: 1.26(0.51-3.39)≥5 children: 2.53(1.07-6.68)p = nd | Parity ≥5 children is associated to a higher risk for hip fracture |
| Hwang et al., Osteoporos Int, [28]Grade C | Cross-sectional, (Korea National Health and NutritionExamination Survey) South Korea | Vertebral fracture | 1222 postmenopausal, (62.8±8.8) | 2 | 444 parousAdjusted12 OR in compared groups for hip fracture risk0.999 (0.537-1.861)Parity is not associated with a risk of vertebral fracture | |||
| Lambrinoudaki et al., Spine J, [29]Grade C | Cross-sectional study, Greece | Vertebral fracture | 454, postmenopausal,(56.8±7.1) | 5 | 378 parous vs. 68 nulliparous | Non adjusted OR in compared groups for vertebral fracture risk | Parity per one extra child : 0.968(0.69-1.34),p = 0.847 | Parity is not associated to the risk for vertebral fracture |
| Maghraoui et al., Bone, [30]Grade C | Cross-sectional study, Marocco | Vertebral fracture | 908, postmenopausal, (60.9±7.7) | 3 | (nd) parous vs. (nd) nulliparous* | - Percentage of vertebral fracture on the whole studied population- Non adjusted OR in compared groups for vertebral fracture risk | 42%1.093(1.008-1.186),p = 0.031 | Parity is associated to a significant higher risk for vertebral fracture |
| Allali et al., Maturitas, [31]Grade C | Cross-sectional, Marocco | Osteoporotic peripheral fracture | 730, postmenopausal, (59.4±7.6) | nd | 663 parous vs 67 nulliparous* | Adjusted13 OR in compared groups for osteoporotic peripheral fracture risk | 1-3 children: 1.40(0.70-2.80), p = 0.34-5 children: 1.10(0.53-2.28), p=0.7≥6 children: 0.85(0.39-1.80), p = 0.6 | Parity is not associated to the risk for peripheral fracture |
| Petersen et al., Ann Epidemiol, [15]Grade C | Cross sectional, (The Longitudinal Study of Aging Danish Twins 1995), Denmark | Hip fracture | 1215, postmenopausal, 80 (75-98) | 18 | -531 parous 1-2 birth* vs. 226 nulliparous and vs. 355 parous 3-4 birth | - Percentage of hip fracture on whole studied population- Non adjusted OR in compared groups for hip fracture risk | 77.3%Nulliparity: 1.18(0.69-2.02)3-4 children: 0.69(0.39-1.23)p = nd | Parity is not associated to the risk for hip fracture |
| Michaelsson et al., Am J Epidemiol, [32]Grade C | Cross sectional, case-control study,Sweden | Hip fracture | 4640, postmenopausal, (72.5±6.8 in cases and 70.5±7.7 in controls) | 3 | -3848 parous vs. 792 nulliparous* | - Percentage of hip fracture on whole studied population- Adjusted14 OR in compared groups for hip fracture risk | 28.6%1 child: 0.90(0.73-1.12),2 children: 0.75(0.62-0.91),3 children: 0.80(0.66-0.98), p = nd | Parity ≥2 children is protective for hip fracture |
| O'Neill et al., Osteoporos Int, [33]Grade C | Cross-sectional, (European Vertebral Osteoporosis Study), United-Kingdom | Vertebral deformity | 7530, postmenopausal, (67.3±7.9 in cases and 62.6±7.9 in controls) | Nd | - 6398 parous vs. 1132 nulliparous* | Adjusted15 OR in compared groups for vertebral deformity | 1.00(0.82-1.23),p = nd | Parity is not associated to the risk for vertebral deformity |
| Shin et al., J Bone Miner Metab, [34]Grade C | Retrospective, (Ansung community cohort study), Korea | Vertebral fracture | 1529, (314 premenopausal and 1215 postmenopausal), (59.1±8.7) | 2 | 687 parous ≥4 births vs. 809 parous- Percentage of vertebral fracture on the whole studied population- Age adjusted OR in compared groups for vertebral fracture risk14.8%0.93(0.65-1.32),p = ndParity is not associated to the risk for vertebral fracture | |||
| Wengreen et al., Osteoporos Int, [35]Grade C | Retrospective,Case control study,(Utah Study of Nutrition and Bone Health), United State | Hip fracture | 1779, postmenopausal, (76.7±9.1) | 5 | 1624 parous vs.155 nulliparous* | - Percentage of hip fracture on whole studied population- Adjusted16 OR in compared groups for hip fracture risk | 49.5%0.93(0.63-1.25),p = nd | Parity is not associated to the risk for hip fracture |
| Huo et al., Osteoporos Int, [36]Grade C | Retrospective,Case control study,China | Hip fracture | 354, postmenopausal, (67.1±8.3) | 2 | - 115 parous 1-2 birth* vs. 83 parous ≥5 birth and vs. 18 nulliparous | - Percentage of hip fracture on whole studied population- Adjusted17 OR in compared groups for hip fracture risk | 33.3%Nulliparity: 0.79(0.19 -3.25)≥5 children: 0.64(0.26-1.61)p = 0.49 | Parity is not associated to the risk of fracture |
| Cure-Cure et al., Int J Gynaecol Obstet, [37]Grade C | Retrospective, Columbia | All fracture | 1855, postmenopausal, (61.3±-8.3) | 5 | 1612 parous vs. 243 nulliparous* | - Percentage of fracture on whole studied population- Non adjusted OR in compared groups for all fracture risk | 22.9%0.41(0.28-0.61),p<0.000002 | Parity is associated with a significant lower risk for fracture |
| Parazzini et al., J Epidemiol Community Health, [38]Grade C | Retrospective,Cross sectional, (Italian case control study), Italia | Hip fracture | 796, postmenopausal, (66) | 10 | 632 Parous vs. 164 nulliparous* | Adjusted18 OR in compared groups for hip fracture risk | 0.8(0.5-1.4),p = 0.22 | Parity is not associated to a risk for hip fracture |
| Hoffman et al., Osteoporosis Int, [39]Grade C | Retrospective,case-control study, United-State | Hip fracture | 348, postmenopausal, (≥50) | 3 | - 233 parous vs. 115 nulliparous* | - Percentage of hip fracture on whole studied population- Adjusted19 OR in compared groups for hip fracture risk | 50%0.76(0.46-1.27),p = nd | Parity is not associated to the risk for hip fracture |
| Nguyen et al., J Clin Endocrinol Metab, [40]Grade C | Retrospective, (Dubbo Osteoporosis Epidemiology Study), Australia | Atraumatic fracture | 1091, postmenopausal, (70±7.2) | 5 | 990 parous* vs. 101 nulliparous | Adjusted20 OR in compared groups for no traumatic fracture risk | 1.10(1.01-1.19),p<0.01 | Nulliparity is associated to a significant higher risk for atraumatic fracture |
| Mallmin et al., Osteoporosis Int, [41]Grade C | Retrospective,case-control study, Sweden | Distal forearm fracture | 604, (94 premenopausal and 510 postmenopausal), (62.8±10.1) | 1 | - 515 parous vs. 89 nulliparous* | Adjusted21 OR in compared group for distal forearm fracture | 1.72(0.90-3.28),p = nd | -Parity is not associated to the risk for forearm fracture |
| Cumming et al., Int J Epidemiol, [42]Grade C | Retrospective,case-control study, Australia | Hip fracture | 311, postmenopausal, (≥65) | 2 | - 246 parous vs. 65 nulliparous* | - Percentage of hip fracture on whole studied population- Adjusted22 OR in compared groups for hip fracture risk | 55.9%0.56(0.17-1.88),p = nd | Parity is not associated to the risk for hip fracture |
| Alderman et al., Am J Epidemiol, [43]Grade C | Retrospective,case-control study, United-State | Hip and forearm fractures | 917, postmenopausal, (50-74) | 5 | - 734 parous vs. 183 nulliparous* | - Percentage of fracture on whole studied population- Adjusted23 OR in compared groups for hip and forearm fracture risk | 38.7%1.2(0.7-2.22),p = nd | Parity is not associated to the risk for hip and forearm fractures |
* Table 1 legend:
1 Adjusted for age, race/ethnicity, menopausal transition stage, body mass index, smoking status, smoking pack-years, alcohol consumption level, physical activity level, employment status, diabetes, hyperthyroidism, current use of supplementary calcium, current use of supplementary vitamin D, prior use of sex steroid hormones, prior use birth control pills, prior use of Depo-Provera injection, current or prior use of oral corticosteroids, current use of proton pump inhibitors, other bone-adverse medications, and study site
2 Adjusted for age, years since menopause, education, living with a partner, height, weight, caffeine intake, smoking, fracture history, parental fracture history, falls, current HT use (5yr), corticosteroid use (>2 years), sedative/ anxiolytics use, arthritis, depression, health status, and parity.
3Adjusted for age and others variables: for hip fracture (history of fracture, BMI, diabetes, glaucoma, smoking, vitamin A supplement use, attitude and having been pregnant), for wrist fracture (history of fracture, BMI, heart attack, alcohol consumption, vitamin A supplement use, cola intake and hysterectomy), for spine fracture (history of fracture, BMI, blood pressure medication, nonprescription pain medication, smoking, exercise, and attitude
4Adjusted for total hip BMD, age, any previous fracture since age 50, history of maternal hip fracture after age 50, Parkinson’s disease, type II diabetes mellitus, lowest quartile for distant depth perception, BMI, height at age 25, walking speed, digit symbol test number completed
5 Adjusted for age, weight, height, maternal history of hip fracture, fracture of any bone after age 50, self-reported, on feet ≤ 4h/day, uses arms to stand from chair, history of diabetes, current calcium intake, current estrogen use, low frequency contrast sensitivity, resting pulse rate, and the interactions of self-reported health with on feet ≤4h/day
6 HR adjusted for age, height, BMI (body mass index), smoking, alcohol use, physical activity, history of diabetes and previous wrist or hip fracture, use of hormone replacement therapy, and length of education
7 Adjusted for age, age at last menstrual period, level of education, BMI, vitamin D status, parity, alcohol consumption, smoking history, leisure time physical activity and self-rated health
8 Adjusted for age, BMI, use of bisphosphonates, raloxifene or ranelate (past and present), use of estroprogestinic preparations (past and present), serum 25OHD levels, and number of pregnancies and presence of densitometric osteoporosis.
9 Adjustment by age, handgrip strength, femoral neck BMD, prevalent vertebral fracture and the history of falls in the follow-up
10 Adjusted for age, postmenopausal, body mass index, dairy calcium intake, hormone replacement therapy during follow-up, Wrist fracture history, parity
11 Adjusted for age, BMI, milk intake, alcohol intake, menarche, parity
12 Adjusted for age, BMI, age at menarche, duration of menopause, systolic blood pressure, GFR, PTH, 25(OH)D3, oral contraceptive use, HTN, DM, physical activity, alcohol and smoking status , number of deliveries, and age at first and last delivery
13 Adjustment for age (OR, 1.06; 95% CI, 0.97-1.15), BMI (OR, 1.01; 95% CI, 0.99-1.03), age at menopause (OR,0.96; 95% CI,0.89-1.04), time since menopause: (OR,0.94; 95% CI , 0.87-1.02),wearing veil (OR,1.02; 95% CI,0.69-1.53) and total femoral BMD (OR,0.13; 95%CI, 0.038–0.4).
14 Adjusted for age (=54, 55-59, 60-64, 65-69, 70-74, and =75 years), hormone replacement therapy (never, former, and current use), oral contraceptive use (never and ever use), and body mass index (by quintiles)
15 Adjusted for center, age, body mass index and smoking
16 Adjusted for age, education, BMI, history of estrogen use, age at menopause, history of oral contraceptive use, history of endometriosis, smoking status, vitamin D receptor genotype, lifetime physical activity, diabetes status, and history of breastfeeding
17 Adjusted for age, height, education, BMI, years lived in rural area, occupation, standing activities prior retirement, dietary calcium intake, breastfeeding and parity
18 Adjusted for age, education, BMI, smoking status and estrogen replacement therapy
19 Adjusted for hospital of recruitment, age group, and age and body mass index
20 Adjusted for age, weight parity, estrogen exposure, hysterectomy
21 Adjusted for BMI, education, daily physical activity, leisure time activity, smoking, nulliparity, duration of HRT, menopausal discomfort, age at menopause
22 Adjustment for age, Body mass index, history of hormone replacement therapy use, current use of psychotropic medications, current smoking status, current dairy product consumption, score on mental state questionnaire, current physical activity and health status (number of self-report illnesses)
23 Adjusted for attained for age, country and occupational group
Characteristics of the included population
Main results of studies (Figure 2)
| Population | References | Type of studies, Main criteria, simple size | Results: [incidence, (HR or OR or RR with 95% CI)] |
| North American | Mori et al.[16] | Prospective, Parity and all fracture, 2239 | HR = 0.97(0.89-1.05) |
| Cauley et al. [17] | Prospective, Parity and all fracture, 159579 | White (2-4 children): HR = 0.94(0.90-0.99)Asian (≥5 children): HR = 1.65(1.06-2.56) | |
| Wengreen et al. [35] | Retrospective, Parity and hip fracture, 1779 | OR = 0.93(0.63-1.25) | |
| Paganini-Hill et al. [18] | Prospective, Parity and hip fracture, 8877 | HR = 0.83(0.72-0.95) | |
| Taylor et al.[19] | Prospective, Parity and hip fracture, 6787 | HR = 0.80(0.66- 0.96) | |
| Hillier et al.[20] | Prospective, Parity and hip fracture, 9699 | HR = 0.69(0.56-0.85) | |
| Hoffman et al. [39] | Retrospective, Parity and hip fracture, 348 | OR = 0.76(0.46-1.27) | |
| Alderman et al. [43] | Retrospective, Parity and hip or wrist fracture, 917 | OR = 1.20(0.70-2.22) | |
| European | Lambrinoudaki et al. [29] | Cross-sectional, Parity and spine fracture, 454 | OR = 0.96(0.69-1.34) |
| Kauppi et al. [22] | Prospective, Parity ≥3 children and hip fracture, 2028 | RR = 0.50(0.32-0.79) | |
| Bjørnerem et al. [21] | Prospective, Parity and (hip, wrist) fracture, 4681 | HR = 1.04(0.75-1.46),HR = 1.20(0.96-1.51) | |
| Trémollières et al. [23] | Prospective, Parity ≥3 children and spine fracture, 2651 | HR = 0.44(0.26-0.76) | |
| Hundrup et al. [24] | Prospective, Parity and hip fracture, 14015 | HR = 0.87(0.66–1.15) | |
| Naves et al.[25] | Prospective, Parity and osteoporotic fracture, 255 | OR = 0.60(0.20-1.78) | |
| Petersen et al. [15] | Prospective, Parity and hip fracture, 1708 | HR = 0.88(0.65-1.19) | |
| Petersen et al. [15] | Cross sectional, Parity and hip fracture, 1215 | OR = 0.69(0.39-1.23) | |
| Michaelsson et al. [32] | Cross sectional, Parity ≥2 children and hip fracture, 4640 | OR = 0.75(0.62-0.91) | |
| Honkanen et al. [26] | Prospective, Parity and wrist fracture, 11798 | HR = 0.71(0.52-0.97) | |
| O'Neill et al. [33] | Cross-sectional, Parity and spine fracture, 7530 | OR = 1.00(0.82-1.23) | |
| Parazzini et al. [38] | Retrospective, Parity and hip fracture, 796 | OR = 0.80(0.50-1.40) | |
| Mallmin et al. [41] | Retrospective, Parity and wrist fracture, 604 | OR = 1.72(0.90-3.28) | |
| Asiatic | Hwang et al. [28] | Cross-sectional, Parity ≥3 children and spine fracture, 1222 | OR = 0.99(0.53–1.86) |
| Shin et al.[34] | Retrospective, Parity and spine fracture, 1529 | OR = 0.93(0.65-1.32) | |
| Huo et al.[36] | Retrospective, Parity and all fracture, 354 | OR = 0.64(0.26-1.61) | |
| Fujiwara et al. [27] | Prospective, Parity ≥5 children and hip fracture, 2987 | RR = 2.53(1.07-6.68) | |
| Maghrebian | Maghraoui et al. [30] | Cross-sectional, Parity and spine fracture, 908 | OR = 1.09(1.008-1.18) |
| Allali et al.[31] | Cross-sectional, Parity and peripheral fracture, 730 | OR = 1.40(0.70-2.80) | |
| South American | Cure-Cure et al. [37] | Retrospective, Parity and all fracture, 1855 | OR = 0.41(0.28-0.61) |
| Australian | Nguyen et al. [40] | Retrospective, Parity and all fracture, 1091 | OR = 0.90(0.84-0.99) |
| Cumming et al. [42] | Retrospective, Parity and hip fracture, 311 | OR = 0.56(0.17-1.88) |
Table 2: Association of fracture risk and parity according the ethnicity.
Abbreviation: HR = Hazard Ratio; OR = Odds Ratio; RR = Relative Risk
Association between parity and hip fracture risk: Data concerning hip fracture risk were included in 17 studies [15,18-24,27,31,32,35,36,38,39,42,43]. Five studies [18-20,22,32] found a statistically significant reduced risk for hip fracture in parous compared to nulliparous (IC 95% variation of RR = [0.32-0.79], HR = [0.56-0.96] and OR = [0.62-0.98]). This result was confirmed by multivariate analysis in all studies. This reduced risk concerned especially women with two or more children in two studies [22,32] with an OR = 0.75[0.62-0.91] and RR = 0.5[0.32-0.79]. However, an increased risk for hip fracture was found in one prospective study concerned women with five or more children in Japanese postmenopausal population [27].
Association between parity and vertebral fracture risk: Eight studies evaluated the association of vertebral fracture to parity [16,18,20,28-30,33,34]. One of them [23] found a significant reduced risk for vertebral fracture in French women with three or more children (OR = 0.44[0.26-0.76]). However, one study found a significant increase risk for vertebral fracture in post-menopausal Moroccan parous population with an OR = 1.093[1.008-1.186] [30].
Association between parity and wrist fracture risk: Seven studies examined the risk for wrist fracture [18,20,21,26,31,41,43]. One of these studies [26] found that the risk for wrist fracture was reduced in parous compared to nulliparous (IC 95% variation of HR = 71[0.52-0.97]). This result was confirmed by a multivariate analysis.
DISCUSSION
The impact of pregnancy on the risk of osteoporotic fracture in postmenopausal women is controversial. This systematic review analyzed the results of 29 selected clinical studies concerning the relationship between the risk of osteoporotic fracture at menopause and parity during the reproductive period of women.
Only three studies, two prospectives [17,27] and one observational [30] associated parity to an increased risk of osteoporotic fracture after menopause. They involved, respectively, Asian-American, Japanese and North African populations. In all three studies, trabecular and cortical bone compartments were affected. The risk of fracture appeared in multivariate analysis to be associated with parity 5 or more children in the two prospective series with, respectively, HR = 1.65[1.06-2.56] (18) and RR = 2.53[1.07-6.68] [27]. This increased risk should be compared with the decrease in bone mass density, because fracture risk doubles for each point reduction in T-score [44]. Early pregnancy, its impact on the acquisition of peak bone mass [45,46], poor contraceptive use and the impact of ethnic factors and/or nutritional habits (daily calcic ration) can be discussed. This negative link between parity and the fracture risk is limited to Asian women [17,27]. In all others studies in this population, even if not negative, now beneficial link is described [28,34,36]. At least, the Moroccan study show a negative link between parity and fracture risk, but exhibit methodological pitfalls: lack of control group, primary objective limited to worsening of vertebral fracture and no evaluation of osteoporotic fracture incidence [30]. In none Asiatic women, no data were support the hypothesis of a negative relationship between multiparity and fracture risk.
Ten out of the 29 studies reported a decrease in fracture risk in menopausal women that was attributable to parity. The majority of these included a Caucasian population. The risk of vertebral and non-vertebral fractures was reduced by 6% to 50% among menopausal parous compared with nulliparous women [18-20,26,37,40]. This reduced risk appears only beyond 2 to 3 pregnancies in four studies [17,22,23,32]. A reduction in the risk of vertebral fracture more than 50% (HR = 0.44[0.26-0.76]) was reported by Trémollieres et al. [23]. In case-control studies, Michaelsson et al., [32] and Hillier et al., [20] found a reduced risk of hip fracture from 8% to 10% per child in postmenopausal women. The impact on the risk of wrist fracture was specifically assessed by Honkanen et al., [26] in a large prospective study of 11 798 pre and postmenopausal women. The risk of wrist fracture in multivariate analysis was reduced by 30% (HR = 0.71[0.52-0.97]) among women with parity compared with nulliparous. The meta-analysis of Wang et al., [47] has included 10 prospective studies about parity and fracture in postmenopausal women. This paper is in accordance with our conclusion as it reports that increasing number of parity is associated with linearly reduced hip fracture risks among women. The osteoporosis fracture and hip fracture risks of parous women with at least one live birth were reduced by 11% and 26% respectively. The risk reduction for hip fracture was 12% for each one increased live birth.
These beneficial effects on all bone compartments are partly explicable by pathophysiological mechanisms and mechanical changes in bone structure. Bone loss during pregnancy and lactation are associated with hypersecretion of Parathyroid Hormone-Related Protein (PTHrp) and a fall in estrogen impregnation [48-50]. Increasing parity leads to functionally iterative estrogen deficiency. This promotes increased bone diameter by increasing periosteal apposition and endosteal resorption [51-54]. Mechanical resistance in torsion and flexion of a hollow cylinder increases exponentially with its diameter when the amount of material remains constant. This applies to the diaphyseal bone that undergoes a beneficial transformation during pregnancy, with no change in bone mass density or a slight decrease [53,55].
However, conclusions from the included studies should be examined carefully because of important heterogeneity in studies. Studies differed considerably in their sampling profiles, follow-up times, and the extent of exposure to the risk of fracture. Regardless of the bone site and the population studied, the problem of reproducibility is addressed most often in retrospective studies data. In generally data in this synthesis of the literature suggest that multiparity do not generally appear to be deleterious to bone during the menopause.
The results of these studies provide no evidence for an association between pregnancy and an increased risk of osteoporotic fracture. Referring to the aggregate risk without stratification according to the number of pregnancies, the majority of studies in this systematic review agree that there is no significant influence of parity on fracture risk after the menopause either by population or bone site [15,16,21,24,25,28,29,31,33-36,38,39,41,42]. In agreement to these results, Henderson et al., [56] reported that postmenopausal osteoporotic risk is not affected by the occurrence of multiple pregnancies during lactation in multiparous women with more than five children. Our results confirm also the findings of Karlsson et al., [11] who reported a neutral or beneficial effect of pregnancy on osteoporotic risk after the menopause. A doubt remains for Asian women in whom osteoporotic risk increase as been described for five pregnancies or more. Additional studies are needed to clarify this point.
CONCLUSION
According to this review, pregnancy does not seem to be associated with an increased risk of osteoporotic fracture after menopause. Most of the studies agree on the absence of a significant effect, regardless of the population and the bone site. However, a non-negligible number of studies (especially Americans, Europeans and Australians) report a protective effect in bone by increased bone diameter due to increased periosteal apposition and endosteal resorption. Contraceptive use and the impact of ethnic factors and/or nutritional habits (daily calcic ration) could also be discussed in the studies on Asiatic or African population where a negative impact was reported. According to our analysis, the types of sample chosen and the study designs used explain at least partly these differences. Other powerful and rigorous studies are required to elucidate the link between parity and osteoporotic risk especially in Asian population. They will provide a basis on which to recommend preventive practices.
DECLARATION
Authors’ contributions
Competing interests
REFERENCES
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL (1992) Perspective. How many women have osteoporosis? J Bone Miner Res 7: 1005-1010.
- Silva MJ, Gibson LJ (1997) Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone 21: 191-199.
- Currey JD (1999) What determines the bending strength of compact bone?. J Exp Biol 202: 2495-2503.
- Prentice A (2000) Calcium in pregnancy and lactation. Annu Rev Nutr 20: 249-272.
- Ensom MH, Liu PY, Stephenson MD (2002) Effect of pregnancy on bone mineral density in healthy women. Obstet Gynecol Surv 57: 99-111.
- Black AJ, Reid R, Reid DM, MacDonald AG, Fraser WD (2003) Effect of pregnancy on bone mineral density and biochemical markers of bone turnover in a patient with juvenile idiopathic osteoporosis. Bone Mineral Research 18: 167-171.
- Peris P, Guañabens N, Monegal A, Pons F, Martínez De Osaba MJ, et al. (2002) Pregnancy associated osteoporosis: the familial effect. Clin Exp Rheumatol 20: 697-700.
- Anai T, Tomiyasu T, Arima K, Miyakawa I (1999) Pregnancy-associated osteoporosis with elevated levels of circulating parathyroid hormone-related protein: a report of two cases. J Obstet Gynaecol Res 25: 63-67.
- Timsit MA (2005) Bone demineralization and osteoporosis of pregnancy. Rev Rhum 72: 725-732.
- Albright F, Reifenstein EC (1948) The Parathyroid Glands and Metabolic Bone Disease. Journal of Bone & Joint Surgery 31: 881-882.
- Karlsson MK, Ahlborg HG, Karlsson C (2005) Female reproductive history and the skeleton-a review. BJOG 112: 851-856.
- Bezerra FF, Mendonça LM, Lobato EC, O'Brien KO, Donangelo CM (2004) Bone mass is recovered from lactation to postweaning in adolescent mothers with low calcium intakes. Am J Clin Nutr 80: 1322-1326.
- Kovacs CS and Kronenberg HM (1997) Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18: 832-872.
- Laboratoire d'Enseignement et de Recherche sur le Traitement de l'Information Médicale (2000) Guide d'analyse de la littérature et gradation des recommandations. ANAES, Paris, France.
- Petersen HC, Jeune B, Vaupel JW, Christensen K (2002) Reproduction life history and hip fractures. Annals Epidemiology 12: 257-263.
- Tremollieres FA, Pouilles JM, Drewniak N, Laparra J, Ribot CA, et al. (2010) Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX Tool. J Bone Miner Res 25: 1002-1009.
- Honkanen RJ, Honkanen K, Kröger H, Alhava E, Tuppurainen M, et al. (2000) Risk factors for perimenopausal distal forearm fracture. Osteoporos Int 11: 265-270.
- Paganini-Hill A, Atchison KA, Gornbein JA, Nattiv A, Service SK, et al. (2005) Menstrual and reproductive factors and fracture risk: the Leisure World Cohort Study. J Womens Health 14: 808-819.
- Cauley JA, Wu LL, Wampler NS, Barnhart JM, Allison M, et al. (2007) Clinical risk factors for fractures in multi-ethnic women: The Women’s Health Initiative. J Bone Miner Res 22: 1816-1826..
- Hillier TA, Rizzo JH, Pedula KL, Stone KL, Cauley JA, et al. (2003) Nulliparity and fracture risk in older women: The Study of Osteoporotic Fractures. J Bone Miner Res 18: 893-899.
- Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, et al. (2004) Long-term prediction of incident hip fracture risk in elderly white women: Study of osteoporotic fractures. J Am Geriatr Soc 52: 1479-1486.
- Kauppi M, Heliövaara M, Impivaara O, Knekt P, Jula A (2011) Parity and risk of hip fracture in postmenopausal women. Osteoporos Int 22: 1765-1771.
- Michaëlsson K, Baron JA, Farahmand BY, Ljunghall S (2001) Influence of parity and lactation on hip fracture risk. Am J Epidemiol 153: 1166-1172.
- Cure-Cure C, Cure-Ramírez P, Terán E, López-Jaramillo P (2002) Bone-mass peak in multiparity and reduced risk of bone-fractures in menopause. Int J Gynaecol Obstet 76: 285-291.
- Nguyen TV, Jones G, Sambrook PN, White CP, Kelly PJ, et al. (1995) Effects of estrogen exposure and reproductive factors on bone mineral density and osteoporotic fractures. J Clin Endocrinol Metab 80: 2709-2714.
- El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, et al. (2013) Systematic vertebral fracture assessment in symptomatic postmenopausal women. Bone 52: 176-180.
- Fujiwara S, Kasagi F, Yamada M, Kodama K (1997) Risk factors for hip fracture in Japanese Cohort. J Bone Miner Res 12: 998-1004
- Hwang IR, Choi YK, Lee WK, Kim JG, Lee IK, et al. (2016) Association between prolonged breastfeeding and bone mineral density and osteoporosis in postmenopausal women: KNHANES 2010-2011. Osteoporos Int 27: 257-265.
- Allali F, Maaroufi H, Aichaoui SE, Khazani H, Saoud B, et al. (2007) Influence of parity on bone mineral density and peripheral fracture risk in Moroccan postmenopausal women. Maturitas 57: 392-398.
- O'Neill TW, Silman AJ, Diaz MN, Cooper C, Kanis J, et al. (1997) Influence of hormonal and reproductive factors on the risk of vertebral deformity in European women. European Vertebral Osteoporosis Study Group. Osteoporos Int 7: 72-78.
- Wengreen H, Cutler DR, Munger R, Willing M (2006) Vitamin D receptor genotype and risk of osteoporotic hip fracture in elderly women of Utah: an effect modified by parity. Osteoporosis International 17: 1146-1153.
- Parazzini F, Bidoli E, Franceschi S, Schinella D, Tesio F, et al. (1996) Menopause, menstrual and reproductive history, and bone density in northern Italy. J Epidemiol Community Health 50: 519-523.
- Hoffman S, Grisso JA, Kelsey JL, Gammon MD, and O'Brien LA (1993) Parity, lactation and hip fracture. Osteoporos Int 3: 171-176.
- Cumming RG, Klineberg RJ (1993) Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int J Epidemiol 22: 684-691.
- Alderman BW, Weiss NS, Daling JR, Ure CL, Ballard JH (1986) Reproductive history and postmenopausal risk of hip and forearm fracture. Am J Epidemiol 124: 262-277.
- Huo D, Lauderdale DS, Li L (2003) Influence of reproductive factors on hip fracture risk in Chinese women. Osteoporos Int 14: 694-700.
- Bjørnerem A, Ahmed LA, Jørgensen L, Størmer J, Joakimsen RM (2011) Breastfeeding protects against hip fracture in postmenopausal women: The Tromsø Study. J Bone Miner Res 26: 2843-2850.
- Mallmin HS. Ljunghall S, Persson I, and Bergström R (1994) Risk factors for fractures of the distal forearm: A population-based case-control study. Osteoporos Int 4: 298-304.
- Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Cannata-Andia JB (2005) Determinants of incidence of osteoporotic fractures in the female Spanish population older than 50. Osteoporos Int 16: 2013-2017.
- Mori T, Ishii S, Greendale GA, Cauley JA, Ruppert K, et al. (2015) Parity, lactation, bone strength, and 16-year fracture risk in adult women: Findings from the Study of Women's Health Across the Nation (SWAN). Bone 73: 160-166
- Lambrinoudaki I, Flokatoula M, Armeni E, Pliatsika P, Augoulea A, et al. (2015) Vertebral fracture prevalence among Greek healthy middle-aged postmenopausal women: association with demographics, anthropometric parameters, and bone mineral density. Spine J 15: 86-94.
- Shin CS, Kim MJ, Shim SM, Kim JT, Yu SH, et al. (2012) The prevalence and risk factors of vertebral fractures in Korea. J Bone Miner Metab 30: 183-192.
- Hundrup YA, Ekholm O, Høidrup S, Davidsen M, Obel EB (2005) Risk factors for hip fracture and a possible effect modification by hormone replacement therapy. The Danish nurse cohort study. Eur J Epidemiol 20: 871-877.
- Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312: 1254.
- Cho GJ, Shin JH, Yi KW, Park HT, Kim T, et al. (2012) Adolescent pregnancy is associated with osteoporosis in postmenopausal women. Menopause 19: 456-460.
- Schnatz PF, Barker KG, Marakovits KA, O'Sullivan DM (2010) Effects of age at first pregnancy and breast-feeding on the development of postmenopausal osteoporosis. Menopause 17: 1161-1166.
- Wang Q, Huang Q, Zeng Y, Liang JJ, Liu SY, et al. (2016) Parity and osteoporotic fracture risk in postmenopausal women: a dose-response meta-analysis of prospective studies. Osteoporos Int 27: 319-330.
- Carneiro RM, Prebehalla L, Tedesco MB, Sereika SM, Hugo M, et al. (2010) Lactation and bone turnover: A conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab 95: 1767-1776.
- VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, et al. (2003) Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest 112: 1429-1436.
- VanHouten JN, Wysolmerski JJ (2003) Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 144: 5521-5529.
- Chapman DJ (2012) Longer cumulative breastfeeding duration associated with improved bone strength. J Hum Lact 28: 18-19.
- Streeten EA, Ryan KA, McBride DJ, Pollin TI, Shuldiner AR, et al. (2005) The relationship between parity and bone mineral density in women characterized by a homogeneous lifestyle and high parity. J Clin Endocrinol Metab 90: 4536-4541.
- Wiklund PK, Xu L, Wang Q, Mikkola T, Lyytikäinen A, et al. (2012) Lactation is associated with greater maternal bone size and bone strength later in life. Osteoporosis Int 23: 1939-1945.
- Schnatz PF, Marakovits KA, O'Sullivan DM (2010) Assessment of postmenopausal women and significant risk factors for osteoporosis. Obstet Gynecol Surv 65: 591-596.
- Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet 359: 1841-1850.
- Henderson PH, Sowers M, Kutzko KE, Jannausch ML (2000) Bone mineral density in grand multiparous women with extended lactation. Am J Obstet Gynecol 182: 1371-1377.
Citation: Weryha G, Diédhiou D, Angelousi A, Agopiantz M, Diop SN, et al. (2017) Impact of Parity on Fracture Risk After Menopause: A Systematic Review. J Hum Endocrinol 2: 009.
Copyright: © 2017 Georges Weryha, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.