
Journal of Alternative Complementary & Integrative Medicine Category: Medicine
Type: Case Report
In Vitro Evaluation of Anthelmintic Efficacy of Some Plant Species Possessing Proteinases and/or Other Nitrogenous Compounds in Small Ruminantsq
*Corresponding Author(s):
Ignatius V NsahlaiDepartment Of Animal And Poultry Science, College Of Agriculture, Engineering And Sciences, 127 Rabie Saunders Building, SAEES, Private Bag X01, Scottsville 3209, PMB Campus Of UKZN, South Africa
Tel:+27 0332605473,
Fax:+27 0332605067
Email:fomslyw@gmail.com; nsahlaii@ukzn.ac.za
Received Date: Jul 13, 2017
Accepted Date: Sep 18, 2017
Published Date: Sep 29, 2017
Abstract
This study was aimed at determining in vitro anthelmintic efficacy of five plant species (Allium cepa, Ananas comosus, Bidens pilosa, Carica papaya and Ricinus communis) possessing proteinases and/or nitrogenous compounds on mixed third stage (L3) nematode larvae of goats and sheep. Dried leaf samples (40 g dry matter (DM)) from plant species were extracted in 70 % ethanol and concentrated to 100 ml (4x concentrations). Half (20 g DM) crude extract (2x concentration) and one quarter (10 g DM) crude extract (1x concentration), were made up to 100 ml. Rectal faeces was collected from two animal species (Merino sheep, n = 10; Nguni goats, n = 25), pooled within species and hand-mixed. Samples of 5 g were cultured for 12 days at 27°C. On day 13, 4 plates were watered and four others treated with 70% ethanol to correct for solvent effect. The experiment was run 3 times and had 2 (animal) x 5 (plants) x 3 (concentration) factorial design. In each run, three plates were treated with each concentration of a plant extract. L3larvae were isolated on day 14, larval counts taken on day 15, and mean mortality adopted as indices of anthelmintic efficacy. Data were analysed using the general linear model of SAS to determine effects of animal species, plant species, concentration and various interactions of animal species, plant species and concentration on efficacy.
Animal species affected (P = 0.0004) anthelmintic efficacy. Similarly, concentration affected (P=0.0002) anthelmintic efficacy. Additionally, interaction between animal species and concentration also affected (P = 0.0015) anthelmintic efficacy. Animal species, concentration and their interaction are crucial to retaining consistent in vitro efficacy of selected plant species. None of these observations could be explained by alkaloid, flavonoid or tannin content.
Animal species affected (P = 0.0004) anthelmintic efficacy. Similarly, concentration affected (P=0.0002) anthelmintic efficacy. Additionally, interaction between animal species and concentration also affected (P = 0.0015) anthelmintic efficacy. Animal species, concentration and their interaction are crucial to retaining consistent in vitro efficacy of selected plant species. None of these observations could be explained by alkaloid, flavonoid or tannin content.
Keywords
Anthelmintic efficacy; Crude extract; Goats and sheep; L3 larvae; Nitrogen compounds; Plant species; Proteinases
INTRODUCTION
Medicinal plants have a long historical application in the treatment/control of various human and domestic livestock ailments [1]. The use of those possessing anthelmintic activity in most communities around the world is common; with most pastoral farmers having acquired ethno veterinary knowledge and resources from previous generations to treat their animals against nematodes and other related parasitic infections [2-4]. In South Africa, many local farmers depend on traditional remedies to treat themselves and their animals [5]. A typical example is that of the Eastern Cape, where seventy five percent (75 %) of rural stock owners use ethno veterinary medicinal plants [6].
Developed and developing regions of the worlds have both expressed renewed interest in this field as a result of intense selection for resistance by most pathogenic bacteria and gastrointestinal helminth parasites of livestock [7-11] against antibiotics and chemical anthelmintics. On a global scale, most of these communities are not sufficiently equipped with the technical and managerial capacity to deter re-infection and optimize the efficacy of these plant remedies.
Phytochemical control of gastrointestinal nematodes presents an important area of research, given its historical and traditional background, and incidental application in most communities around the world [4]. They have the potential of being sustainable in addition to their environmental friendly nature [12,13]. Plant-based remedies are also credited with being non-resistible compared to chemical ones, and do not lodge chemical residues in animal products and the environment [14-16]. They also possess fewer or no side effects and contraindications relative to chemical anthelmintics [17]. Additionally, they have benefits such as low cost, availability and acceptability, which are not prohibitive to their use relative to conventional chemical anthelmintics [18]. Consequently, they are suitable for organic farming of livestock. Authors acknowledged the paucity of work relating to number of plants analysed for their biological activities and toxicity in addition to other related studies in view of scientific global validation [5].
Plant species containing proteinases and other protein/nitrogen related compounds make an important contribution to helminth parasite management and control in livestock [19]. Five plant species; Allium cepa, Ananas comosus, Bidens pilosa, Carica papaya and Ricinus communis, some of which contain proteinases and others nitrogenous compounds were evaluated for their anthelmintic activity at three different concentrations in vitro on mixed nematode L3 larval species. Besides the study of A. comosus, these plants have been used locally without much evidence to relieve animals from gastrointestinal nematodes [20]. So it would be interesting to establish a link between plant metabolites and anthelmintic efficacy. Anthelmintic efficacy measures the degree to which each extract can kill gastrointestinal nematodes and larvae, thus reducing the chance of re-infection and disease incidence. The objective of study was to evaluate the anthelmintic efficacy of five plants species at different concentration for sheep and goats.
Developed and developing regions of the worlds have both expressed renewed interest in this field as a result of intense selection for resistance by most pathogenic bacteria and gastrointestinal helminth parasites of livestock [7-11] against antibiotics and chemical anthelmintics. On a global scale, most of these communities are not sufficiently equipped with the technical and managerial capacity to deter re-infection and optimize the efficacy of these plant remedies.
Phytochemical control of gastrointestinal nematodes presents an important area of research, given its historical and traditional background, and incidental application in most communities around the world [4]. They have the potential of being sustainable in addition to their environmental friendly nature [12,13]. Plant-based remedies are also credited with being non-resistible compared to chemical ones, and do not lodge chemical residues in animal products and the environment [14-16]. They also possess fewer or no side effects and contraindications relative to chemical anthelmintics [17]. Additionally, they have benefits such as low cost, availability and acceptability, which are not prohibitive to their use relative to conventional chemical anthelmintics [18]. Consequently, they are suitable for organic farming of livestock. Authors acknowledged the paucity of work relating to number of plants analysed for their biological activities and toxicity in addition to other related studies in view of scientific global validation [5].
Plant species containing proteinases and other protein/nitrogen related compounds make an important contribution to helminth parasite management and control in livestock [19]. Five plant species; Allium cepa, Ananas comosus, Bidens pilosa, Carica papaya and Ricinus communis, some of which contain proteinases and others nitrogenous compounds were evaluated for their anthelmintic activity at three different concentrations in vitro on mixed nematode L3 larval species. Besides the study of A. comosus, these plants have been used locally without much evidence to relieve animals from gastrointestinal nematodes [20]. So it would be interesting to establish a link between plant metabolites and anthelmintic efficacy. Anthelmintic efficacy measures the degree to which each extract can kill gastrointestinal nematodes and larvae, thus reducing the chance of re-infection and disease incidence. The objective of study was to evaluate the anthelmintic efficacy of five plants species at different concentration for sheep and goats.
MATERIALS AND METHODS
Plant collection and identification
Five plant species that are used traditionally to treat infectious nematodes of livestock were selected from available literature and included Allium cepa and Ananas comosus, both of which were bought/collected from a commercial food and vegetable store in Pietermaritzburg. Bidens pilosa and Carrica papaya were obtained from a private garden and Ricinus communis from around the university as an invasive plant [21-29]. All plant species were said to have proteinases and/or nitrogenous compounds. Voucher samples were deposited at the UKZN Herbarium, Pietermaritzburg for identification.
Extraction
Fresh vegetative material was collected, washed, chopped for those with large/long leaves, air dried and subsequently oven dried (Oven mark; LABCON, Model5SOEIB, Maraisburg 1700) to constant weight at 60°C. Each dried material was ground, using an electric centrifuge mill (RETSCH, GmbH and Co.KG, 5657 HAANI, West Germany), fine enough to pass through a 1-mm sieve. Milled samples were put into air-tight labeled plastic containers and stored in boxes, away from light and moisture at room temperature. Ten grams of milled sample was weighed into labeled thimbles, fitted into a distillation column and extracted with 70% ethanol over a heating unit (Gerhardt Bonn, App. Nr 450893). The extraction process was assumed complete when the solvent in the thimble carrying unit was apparently free of any coloration. Three other masses of 10 g dry matter were extracted following the same procedure for each of the plant species and later concentrated to 100 ml of crude extract. Half and one quarter of the original crude extract were both made to 100 ml with the same solvent; equivalent to 20 g and 10 g DM crude extract. The initial crude extracts were 4x concentrations and others 2x concentrations and 1x the initial concentration. These crude extracts were stored in bottles, sealed with parafilm® (American National Can TM/NEENAH, WI 54956), then packaged into paper boxes and conserved in the fridge at 10ºC for subsequent in vitro assay of mixed cultures of nematode L3 larvae of goats and sheep.
Phytochemical screening
Alkaloids were determined following method based on which, 5 g of milled sample was weighed into a 250 ml beaker, into which 200 ml of 10 % acetic acid in ethanol was added and covered to extract for 4 hours [30]. The solution was filtered using a fine sieve into a beaker of similar capacity to the first and the extract concentrated in a water bath to ¼ its original volume at 100ºC. Concentrated ammonium hydroxide was added drop wise to complete precipitation of extract and the solution was allowed to settle. The precipitate was collected and washed with dilute ammonium hydroxide (50:50 volumes for volume). The residue was filtered using Whatman® 42 filter paper, oven dried under low heat and weighed.
Tannin determination was done following HCl-Butanol proanthocyanidin assay as leucocyanidin equivalent and absorbance’s read using Beckman DU®640 Spectrophotometer at visible wavelength of light 550 nm [31,32].
Flavonoid content of each milled sample was determined following [33]. Into 100 ml of 80 % aqueous methanol was added to 10 g DM of milled material in 250 ml sterile beakers. The content was allowed to stand for 10 hours at room temperature, while being shaken intermittently after each hour with a magnetic stirring bar over a magnetic rotor without heat. The solution was filtered individually through Whatman No 42 filter paper. The filtrate of each milled sample was transferred into pre-weighed 250 ml conical flask and evaporated to dryness over a water bath set at 80ºC. Flasks and flavonoid contents were allowed to cool and subsequently placed in desiccators for one hour. Each of them was weighed and the weight of the sterilized conical flask deducted from that of flask and flavonoid. Flavonoid (the difference) was computed as a percentage of the initial sample weight.
Tannin determination was done following HCl-Butanol proanthocyanidin assay as leucocyanidin equivalent and absorbance’s read using Beckman DU®640 Spectrophotometer at visible wavelength of light 550 nm [31,32].
Flavonoid content of each milled sample was determined following [33]. Into 100 ml of 80 % aqueous methanol was added to 10 g DM of milled material in 250 ml sterile beakers. The content was allowed to stand for 10 hours at room temperature, while being shaken intermittently after each hour with a magnetic stirring bar over a magnetic rotor without heat. The solution was filtered individually through Whatman No 42 filter paper. The filtrate of each milled sample was transferred into pre-weighed 250 ml conical flask and evaporated to dryness over a water bath set at 80ºC. Flasks and flavonoid contents were allowed to cool and subsequently placed in desiccators for one hour. Each of them was weighed and the weight of the sterilized conical flask deducted from that of flask and flavonoid. Flavonoid (the difference) was computed as a percentage of the initial sample weight.
Bioassays
Faecal material was collected from two animal species, pooled within species and hand-mixed. Animals were Merino sheep (n = 10) and Nguni goats (n = 25) grazing on contaminated mixed pasture at Ukulinga Research Farm. Dung samples (each of 5 g) were weighed into plates and incubated (MEMMERT, 854 Schwabach, West-Germany) for 12 days at 27°C. Samples were watered at 12.00 noon every day during the 12 days of incubation to keep them moist; mindful of moisture consistency, in order not to drench and kill hatched developing larvae. On day 13, 4 plates were set aside, watered and left untreated and, 4 other plates treated with 5 ml of 70% ethanol to correct for solvent effect on mortality. Three plates were treated with each concentration of plant extract. All samples were further incubated for 24 hours. Nematodes larvae were isolated using the Baermann technique in the following 24 hours (day 15) [34]. Larvae identification was done for sheep in this farm.
According to Baermann method for isolating surviving nematode larvae, each faecal culture was placed in a double cheese cloth, a porch loosely formed around and tide with a rubber band. All faecal cultures in cheese cloth porches were immersed into lurkwarm water-filled funnels, placed into holes, drilled into a table structure to hold them in place. Sufficient attention was taken to avoid porches from blocking migrating L3 larvae falling down the funnel stem, from where they were collected in fluid. The apparatus was left for 24 hours at room temperature and 15 ml of fluid collected in test tubes from the funnel stem. Tubes containing fluid were left to stand for 30 minutes to enable L3 larvae to settle at the bottom and avoiding agitation during collection, supernatant was drawn with a Pasteur pipette, a McMaster slide filled and mounted on a microscope. Samples were examined and larvae counted using 10 x magnification.
The experiment had two types of animals, five plant species and three extract concentrations; hence it can be described as a 2 x 5 x 3 factorial design. In each run, three plates were treated with each crude extract concentration of each plant species. Surviving L3 larvae were isolated, larval counts taken and mortality (based on a mean of three plates) calculated during each run. The study was re-run three times. Nematode mortality was calculated using Abbott’s formula, as follows [35]:
According to Baermann method for isolating surviving nematode larvae, each faecal culture was placed in a double cheese cloth, a porch loosely formed around and tide with a rubber band. All faecal cultures in cheese cloth porches were immersed into lurkwarm water-filled funnels, placed into holes, drilled into a table structure to hold them in place. Sufficient attention was taken to avoid porches from blocking migrating L3 larvae falling down the funnel stem, from where they were collected in fluid. The apparatus was left for 24 hours at room temperature and 15 ml of fluid collected in test tubes from the funnel stem. Tubes containing fluid were left to stand for 30 minutes to enable L3 larvae to settle at the bottom and avoiding agitation during collection, supernatant was drawn with a Pasteur pipette, a McMaster slide filled and mounted on a microscope. Samples were examined and larvae counted using 10 x magnification.
The experiment had two types of animals, five plant species and three extract concentrations; hence it can be described as a 2 x 5 x 3 factorial design. In each run, three plates were treated with each crude extract concentration of each plant species. Surviving L3 larvae were isolated, larval counts taken and mortality (based on a mean of three plates) calculated during each run. The study was re-run three times. Nematode mortality was calculated using Abbott’s formula, as follows [35]:
Corrected % mortality = 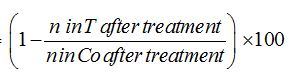
Where n = number of larvae, T = treated and Co = control. Results are expressed as least square means ± standard error of least square means to serve as indices of dosed anthelmintic efficacy for each plant species.
Where n = number of larvae, T = treated and Co = control. Results are expressed as least square means ± standard error of least square means to serve as indices of dosed anthelmintic efficacy for each plant species.
Statistical analysis
Data from nematode larval mortality were analysed using the General Linear Model to determine the effect of animal species, plant species and concentration of various levels of interaction on mortality [36]. L3 larvae mortalities were adoptd as indices of anthelmintic efficacy.
?ijkl = µ + Ai + Sj + Ck + (A × S)ij + (A × C)ik + ( S × C )jk + (A × S × C )ijk + eijkl
Where, ?ijkl = individual observation; µ = overall mean; Ai = effect of animal species; Sj = effect of plant species; Ck = effect of concentration; (A × S)ij = interaction between animal and plant species effects; (A × C)ik = interaction between animal species and concentration effects; ( S × C )jk = interaction between plant species and concentration effects; (A × S × C)ijk = interaction among animal species, plant species and concentration effect; eijkl = the error term.
?ijkl = µ + Ai + Sj + Ck + (A × S)ij + (A × C)ik + ( S × C )jk + (A × S × C )ijk + eijkl
Where, ?ijkl = individual observation; µ = overall mean; Ai = effect of animal species; Sj = effect of plant species; Ck = effect of concentration; (A × S)ij = interaction between animal and plant species effects; (A × C)ik = interaction between animal species and concentration effects; ( S × C )jk = interaction between plant species and concentration effects; (A × S × C)ijk = interaction among animal species, plant species and concentration effect; eijkl = the error term.
RESULTS
In this farm sheep larval count showed that Haemonchus constituted 87.3%, Trichostrongylus 7.3% and Oesophagostomum 5.4%; so both sheep and goats are said to be parasitized by mixed infection. Alliumcontained a slight amount of alkaloid as compared to other four plant materials (P < 0.001) which had similar alkaloid content (Table 1). All plants had similar (P > 0.05) condense tannin content. A. cepa had a very high flavonoid content which differed (P < 0.01) from the other four plants which were similar (P > 0.05).
Anthelmintic efficacy measures the degree to which each extract can kill gastrointestinal nematodes. Both animal species differed (P = 0.0004) in anthelmintic efficacy, with relatively more radical changes in efficacy for goats than sheep (Figure 1). At parity, efficacies for goats were generally lower than those of sheep (Table 2; Figure 1). Concentration affected (P < 0.0001) anthelmintic efficacy, with various trends of increases in efficacy following increasing concentration (Figure 1). Interaction between animal species and concentration (P = 0.0015) also affected anthelmintic efficacy (Table 2; Figure 1). At the lowest concentration, there was a far lower efficacy for goats relative to sheep, which subsequently increased radically through the intermediate to the highest concentration for goats. Generally, mean efficacy for both animal species were between 66.4 ± 3.13 and 97.5 ± 1.95 % (Figure 2).
Anthelmintic efficacy measures the degree to which each extract can kill gastrointestinal nematodes. Both animal species differed (P = 0.0004) in anthelmintic efficacy, with relatively more radical changes in efficacy for goats than sheep (Figure 1). At parity, efficacies for goats were generally lower than those of sheep (Table 2; Figure 1). Concentration affected (P < 0.0001) anthelmintic efficacy, with various trends of increases in efficacy following increasing concentration (Figure 1). Interaction between animal species and concentration (P = 0.0015) also affected anthelmintic efficacy (Table 2; Figure 1). At the lowest concentration, there was a far lower efficacy for goats relative to sheep, which subsequently increased radically through the intermediate to the highest concentration for goats. Generally, mean efficacy for both animal species were between 66.4 ± 3.13 and 97.5 ± 1.95 % (Figure 2).
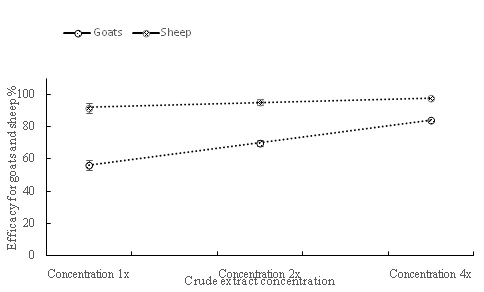
Figure 1: General trend of anthelmintic efficacy (%) for goats and sheep at 1x, 2x and 4x concentrations for plant species possessing proteinases and/or nitrogenous compounds.
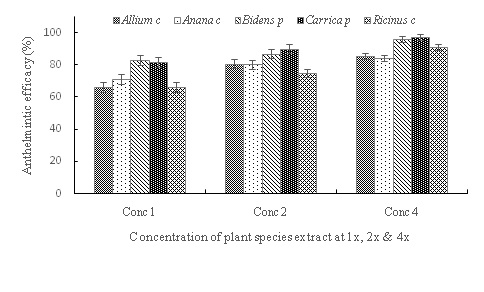
Figure 2: Interaction between concentration and plant species on anthelmintic efficacy (%) for plant species possessing proteases and other nitrogenous compounds for both goats and sheep.
| Plant species | n | Alkaloid (gKg-1) | n | Tannin (gKg-1) | n | Flavonoids (gKg-1) |
| Allium cepa | 2 | 5.7 ± 0.30B | 6 | 4.7 ± 0.97A | 2 | 550.4 ± 25.42A |
| Ananas comosus | 2 | 47.5 ± 6.70A | 6 | 4.4 ± 0.75A | 2 | 133.5 ± 5.15B |
| Biden pilosa | 2 | 39.5 ± 6.10A | 6 | 5.9 ± 1.09A | 2 | 163.5 ± 1.92B |
| Carica papaya | 2 | 40.5 ± 6.10A | 4 | 2.6 ± 0.76A | 2 | 167.7 ± 12.38B |
| Ricinus communis | 2 | 40.5 ± 6.10A | 6 | 4.4 ± 1.56A | 2 | 149.6 ± 10.27B |
Table 1: Alkaloid, condensed tannin and flavonoid content ± standard error of means (gKg-1 dry matter) of selected plant species possessing protease and/or nitrogenous anthelmintic activity.
Means in the same column with different superscript are significantly different (P <0.05); Means in the same column with the same superscript are not (P > 0.05) statistically different.
| Goats | Sheep | |||||
| Concentration | Concentration | |||||
| Plant species | 1x | 2x | 4x | 1x | 2x | 4x |
| Allium c | 53.7 ± 3.13 | 70.3 ± 2.69 | 77.7 ± 1.95 | 79.0 ± 3.13 | 91.4 ± 2.69 | 93.4 ± 1.95 |
| Anana c | 46.7 ± 3.13 | 62.6 ± 2.69 | 69.5 ± 1.95 | 96.2 ± 3.13 | 98.1 ± 2.69 | 99.4 ± 1.95 |
| Bidens p | 75.3 ± 3.13 | 83.3 ± 2.69 | 94.8 ± 1.95 | 90.8 ± 3.13 | 90.8 ± 2.69 | 97.2 ± 1.95 |
| Carrica p | 67.5 ± 3.13 | 82.2 ± 2.69 | 96.2 ± 1.95 | 96.1 ± 3.13 | 97.9 ± 2.69 | 98.7 ± 1.95 |
| Ricinus c | 37.3 ± 3.13 | 51.3 ± 2.69 | 83.2 ± 1.95 | 95.4 ± 3.13 | 98.4 ± 2.69 | 99.0 ± 1.95 |
Allium c- Allium cepa; Ananas c- Ananas comosus; Bidens p - Bidens pilosa; Carica p- Carica papya; Ricinus c - Ricinus communis
DISCUSSION
Animal species had different in vitro anthelmintic efficacies regardless of the plant species. The trend in anthelmintic activity in relation to animal species is in agreement with work done by, who suggested consideration of animal species differences at dosing with anthelmintics in order to ensure high efficacy [37]. Similarly, differences in anthelmintic efficacy of plant remedies thought to associate with differences in animal species were highlighted to be taken care of at dosing, in accordance with the observations in this study [8]. In the process of developing anthelmintic, it is perhaps better to use the animal (goats) with a lower efficacy than sheep, given that whichever plant species exerts anthelmintic activity for the former, does exceedingly well for the later. Cysteine proteinases from pawpaw that were found to be very effective against parasitic helminths showed variable efficacy in vivo with different strains of rat [38]. Similarly, the present in vitro study highlights differences between goats and sheep in anthelmintic efficacy.
Given the same concentration, the anthelmintic efficacy for sheep was far higher than that of goats (Figure 1). This trend was suggestive of prior exposure to the same or similar active anthelmintic phytochemical by goats relative to sheep, in effect, creating some level of tolerance at low concentration. The most probable route of prior encounter is likely to have been through animal species diet, in which plant species or plant varieties possessing similar phytochemicals constituted some portion of their diet [39]. Goats browse and graze, whereas untrained sheep graze, rendering tree parts such as shoots, leaves, growing parts of shrubs and forbes main constituents of goats’ diet [40]. These forages contain plenty of plant secondary metabolites, some of which are implicated in control of parasitic nematodes and other microbial organisms [41,42]. Sheep on their part seldom encounter these plant secondary metabolites because their diet is predominantly grass in nature [39]. Differences in anthelmintic efficacy were also observed in the treatment of infected lambs and mice with extracts of Albizia anthelmintica Brong, wherein, there was no anthelmintic activity observed against mice parasites [43]. Though the outcome contrasted with that of the current study in which plant species possessing proteases and protein compounds exhibited anthelmintic activity against both goats and sheep in vitro, it highlights animal species differences vis a vis anthelmintic efficacy of the same plant species. How proteinases and related phytochemicals exercise their activity in helminth parasite control is worth brief exploration.
Concentration effect of plant crude extract was also observed to influence differences in anthelmintic efficacy in this study. Increase in concentration of plant species crude extract from lowest through the intermediate to the highest concentration for both animal species resulted to various changes in anthelmintic efficacy in conformation with dose-dependent activity of Ficus racemosa (Linn.) bark crude extract on adult earth worms [44]. The primary anthelmintic bioactive phytochemical in the above crude extract is proteases in nature, and similar to that of some plant species in the current study [44]. Cysteine proteinases from papaya latex administered to sheep infected with Haemonchus contortus and Trichostrongylus colubriformis, also exhibited dose dependent anthelmintic activity [45]. Additionally, aqueous extracts of pineapple skin and bromelain exhibited dose-dependent in vitro inhibition on Haemonchus contortus egg-hatch and larval development in sheep [46]. All of the former examples are corroborative of dose dependent anthelmintic activity of the same plant species and others in the current study. Variable anthelmintic efficacy emanating from different plant species possessing proteases such as pineapple, Kiwi fruits and pawpaw at the same and different concentration are in accord with results of the current study [38]. Concentration or dose effect may also vary between in vitro and in vivo activity in consonance with with Luoga [38], as a result of animal host internal environment. There were differences between animal species and concentration.
Proteinases have a wide range of characteristics, some of which include tissue dissolution and remodelling [47]. The same biochemical mode of action is used by parasites to penetrate host tissues in order to obtain nutrients, be it plug tissue feeding Strongyloides or other parasitic species that burrow and lodge in the subcutaneous walls of the gastrointestinal tract among others [48]. These parasites protect themselves from host protease analogues which aid protein digestion and other biochemical processes by secreting proteases inhibitors [47]. Proteases exert their activity in context by binding to protein or peptide substrate in their active site, in the process cleaving them, and retaining site specificity by amino acids on both sides of the cleavage [49]. It is implicit that several active sites are therefore involved in dissolution of parasite protein or peptide, for them to exert anthelmintic activity. Extensive in vitro and in vivo cuticular damage was done to rodent intestinal nematode Heligmosomoid bakeri by cysteine proteases from Kiwi fruit (Actinidia deliciosa), latex of Carrica papya and stem and fruit bromelain of Ananas comosus, reaffirming tissue digesting trait of proteases [20]. So a combination of proteases could even become more devastating on helminths.
cepa differed from the other four plants in alkaloids and flavonoids contents (Table 1), but A. cepa exerted similar anthelmintic efficacy (Figure 2) compared to A. comosus and R. communis at low and intermediate concentrations; delineation being obvious only for B. pilosa and C. papaya on the one hand and A. cepa. This lack of alignment between anthelmintic efficacy and these plant chemicals leaves proteases and/or other nitrogenous compounds, which were unable to separate and analyze needing verification, though some unknown biochemical constituents may be responsible.
Given the same concentration, the anthelmintic efficacy for sheep was far higher than that of goats (Figure 1). This trend was suggestive of prior exposure to the same or similar active anthelmintic phytochemical by goats relative to sheep, in effect, creating some level of tolerance at low concentration. The most probable route of prior encounter is likely to have been through animal species diet, in which plant species or plant varieties possessing similar phytochemicals constituted some portion of their diet [39]. Goats browse and graze, whereas untrained sheep graze, rendering tree parts such as shoots, leaves, growing parts of shrubs and forbes main constituents of goats’ diet [40]. These forages contain plenty of plant secondary metabolites, some of which are implicated in control of parasitic nematodes and other microbial organisms [41,42]. Sheep on their part seldom encounter these plant secondary metabolites because their diet is predominantly grass in nature [39]. Differences in anthelmintic efficacy were also observed in the treatment of infected lambs and mice with extracts of Albizia anthelmintica Brong, wherein, there was no anthelmintic activity observed against mice parasites [43]. Though the outcome contrasted with that of the current study in which plant species possessing proteases and protein compounds exhibited anthelmintic activity against both goats and sheep in vitro, it highlights animal species differences vis a vis anthelmintic efficacy of the same plant species. How proteinases and related phytochemicals exercise their activity in helminth parasite control is worth brief exploration.
Concentration effect of plant crude extract was also observed to influence differences in anthelmintic efficacy in this study. Increase in concentration of plant species crude extract from lowest through the intermediate to the highest concentration for both animal species resulted to various changes in anthelmintic efficacy in conformation with dose-dependent activity of Ficus racemosa (Linn.) bark crude extract on adult earth worms [44]. The primary anthelmintic bioactive phytochemical in the above crude extract is proteases in nature, and similar to that of some plant species in the current study [44]. Cysteine proteinases from papaya latex administered to sheep infected with Haemonchus contortus and Trichostrongylus colubriformis, also exhibited dose dependent anthelmintic activity [45]. Additionally, aqueous extracts of pineapple skin and bromelain exhibited dose-dependent in vitro inhibition on Haemonchus contortus egg-hatch and larval development in sheep [46]. All of the former examples are corroborative of dose dependent anthelmintic activity of the same plant species and others in the current study. Variable anthelmintic efficacy emanating from different plant species possessing proteases such as pineapple, Kiwi fruits and pawpaw at the same and different concentration are in accord with results of the current study [38]. Concentration or dose effect may also vary between in vitro and in vivo activity in consonance with with Luoga [38], as a result of animal host internal environment. There were differences between animal species and concentration.
Proteinases have a wide range of characteristics, some of which include tissue dissolution and remodelling [47]. The same biochemical mode of action is used by parasites to penetrate host tissues in order to obtain nutrients, be it plug tissue feeding Strongyloides or other parasitic species that burrow and lodge in the subcutaneous walls of the gastrointestinal tract among others [48]. These parasites protect themselves from host protease analogues which aid protein digestion and other biochemical processes by secreting proteases inhibitors [47]. Proteases exert their activity in context by binding to protein or peptide substrate in their active site, in the process cleaving them, and retaining site specificity by amino acids on both sides of the cleavage [49]. It is implicit that several active sites are therefore involved in dissolution of parasite protein or peptide, for them to exert anthelmintic activity. Extensive in vitro and in vivo cuticular damage was done to rodent intestinal nematode Heligmosomoid bakeri by cysteine proteases from Kiwi fruit (Actinidia deliciosa), latex of Carrica papya and stem and fruit bromelain of Ananas comosus, reaffirming tissue digesting trait of proteases [20]. So a combination of proteases could even become more devastating on helminths.
cepa differed from the other four plants in alkaloids and flavonoids contents (Table 1), but A. cepa exerted similar anthelmintic efficacy (Figure 2) compared to A. comosus and R. communis at low and intermediate concentrations; delineation being obvious only for B. pilosa and C. papaya on the one hand and A. cepa. This lack of alignment between anthelmintic efficacy and these plant chemicals leaves proteases and/or other nitrogenous compounds, which were unable to separate and analyze needing verification, though some unknown biochemical constituents may be responsible.
CONCLUSION
Important insight has been gained from key drivers of anthelmintic efficacy of plants possessing proteinases and nitrogen compounds in the present study. Primary drivers included animal species, plant species, dose/concentration of crude extract and the interaction between concentration and animal species. This, therefore, provides some bearing or guide to subsequent studies. Moreover, the potential of enhanced anthelmintic efficacy by exploration of other modes of dosing will be recommended for further studies.
ACKNOWLEDGEMENT
This work was supported by financial contribution from the University of KwaZulu-Natal Pcode: p029. The services of Mrs Young Alison were key to sourcing and identification of some of the plant species used in the trial. Support was also received from the National Botanical Garden Pietermaritzburg for plant material. Gratitude is also profoundly accorded to Dr. KS Yobo and Mr. Richard Burgdorf of the Discipline of Plant Pathology for laboratory space and material assistance. Additionally, Dr. Christina Potgieter of University Herbarium Pietermaritzburg was very helpful and instrumental at sourcing and preparation of referral samples. Mr. Samuel Khumalo helped in collecting rectal faecal material from both goats and sheep.
REFERENCES
- Barata S, Centeno M, Marques JP, Clode N, da Graça LM (2009) Hemorragia feto-materna grave Massive feto-maternal haemorrhage. Acta Obstet Ginecol Port 3: 169-172.
- Kliegman RM (2011) Nelson Textbook of Pediatrics, (19thedn). Elsevier/Saunders, USA.
- Kim YA, Makar RS (2012) Detection of fetomaternal hemorrhage. Am J Hematol 87: 417-423.
- Lobo AL, Tomás E, da Silva FP, Barbot J, Raposo T (2001) Anemia Neonatal Grave por Hemorragia Feto-Materna Caso Clínico. Acta Pediatr Port 32: 395-397.
- Zuppa AA, Scorrano A, Cota F, D’Andrea V, Francciolla A, et al. (2008) Massive fetomaternal hemorrhage and late-onset neutropenia: description of two cases. Turk J Pediatr 50: 400-404.
- Solomonia N, Playforth K, Reynolds EW (2012) Fetal-Maternal Hemorrhage: A Case and Literature Review. AJP Rep 2: 7-14.
- Dziegiel MH, Nielsen LK, Berkowicz A (2006) Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol 13: 490-495.
- Kecskes Z (2003) Large fetomaternal hemorrhage: clinical presentation and outcome. J Matern Fetal Neonatal Med 13:128-132.
- Macedo IT, Bevilaqua CM, de Oliveira LM, Camurça-Vasconcelos AL, Vieira Lda S, et al. (2010) Anthelmintic effect of Eucalyptus staigeriana essential oil against goat gastrointestinal nematodes. Vet Parasitol 173: 93?98.
- Brandt HD, Osuch E (1995) Plants associated with accidentally poisoned patients presenting at Ga-Rankuwa Hospital, Pretoria. South African Journal of Science 91: 57.
- McCorkle MC, Mathias E, Schillhorn van Veen TW (1996) Ethnoveterinary research & development: IT studies in indigenous knowledge and development. Intermediate Technology Publications, London, UK.
- Tandon V, Yadav AK, Roy B, Das B (2011) Phytochemical as cure of worm infections in traditional medicine systems. Emerg Trends Zool 16: 351-378.
- McGaw LJ, Eloff JN (2008) Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. J Ethnopharmacol 119: 559?574.
- Masika PJ, van Averbeke W, Sonandi A (2000) Use of herbal remedies by small-scale farmers to treat livestock diseases in Central Eastern Cape Province, South Africa. J S Afr Vet Assoc 71: 87-91.
- Dorman HJ, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88: 308-316.
- Makkar HP, Francis G, Becker K (2007) Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 1: 1371-1391.
- Geerts S, Gryseels B (2000) Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev 13: 207-222.
- Mortensen LL, Williamson LH, Terrill TH, Kircher RA, Larsen M, et al. (2003) Evaluation of prevalence and clinical implications of anthelmintic resistance in gastrointestinal nematodes in goats. J Am Vet Med Assoc 223: 495-500.
- Wang GX, Zhou Z, Jiang DX, Han J, Wang JF, et al. (2010) In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim) Fedde against Dactylogyrus intermedius in Carassius auratus. Vet Parasitol 171: 305-313.
- Hammond JA, Fielding D, Bishop SC (1997) Prospects for plant anthelmintics in tropical veterinary medicine. Vet Res Commun 21: 213-228.
- Yeap SK, Ho WY, Beh BK, Liang WS, Ky H, et al. (2010) Vernonia amygdalina, an ethnoveterinary and ethnomedical used green vegetable with multiple bio-activities. J Med Plant Res 4: 2787-2812.
- Osbourn AE, Lanzotti V (2009) Plant-derived Natural Products: Synthesis, Function, and Application. Springer Science & Business Media, Berlin, Germany.
- Kaemmerer K, Butenkotter S (1973) The problem of residues in meat of edible domestic animals after application or intake of organophosphate esters. Residue Rev 46: 1-240.
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS (2009) Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet Parasitol 160: 83-88.
- Saxena M, Saxena J, Nema R, Singh D, Gupta A (2013) Phytochemistry of medicinal plants. J Pharmacogn Phytochem 6: 168-182.
- Hoste H, Torres-Acosta JF (2011) Non chemical control of helminths in ruminants: adapting solutions for changing worms in a changing world. Vet Parasitol 180: 144-154.
- Stepek G, Behnke JM, Buttle DJ, Duce IR (2004) Natural plant cysteine proteinases as anthelmintics? Trends Parasitol 20: 322-327.
- Luoga W, Mansur F, Buttle DJ, Duce IR, Garnett MC, et al. (2015) The relative anthelmintic efficacy of plant-derived cysteine proteinases on intestinal nematodes. J Helminthol 89: 165-174.
- Vieira S, Cavalcante ACR, Pereira MF, Dantas LB, Ximenes LJF (1999) Evaluation of the anthelmintic efficacy of plants in northeastern Brazil in experimentally infected goats by Haemonchus contortus. Rev Med Vet 5: 447-452.
- Marwat SK, Rehman F, Khan MA, Ahmad M (2011) Medicinal folk recipes used as traditional phyto therapies in district Dera Ismail Khan, KPK, Pakistan. J Bot 43: 1453-1462.
- Bidkar A, Ghadiali M, Patel C, Aswar M, Sanghai D, et al. (2012) Anthelmintic Activities of the Crude Extracts of Allium cepa Bulbs and Elletatria cardomomum Res J Pharm Biol Chem Sci 3: 50-58.
- Stepek G, Buttle DJ, Duce IR, Lowe A, Behnke JM (2005) Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology 130: 203-211.
- Graham K, Graham EA, Towers GHN (1980) Cercaricidal activity of phenylheptatriyne and α-terthienyl, naturally occurring compounds in species of Asteraceae (Compositae). Can J Zool 58: 1955-1958.
- Hoffmann B, Hölzl J (1988) New chalcones from Bidens pilosa. Planta Med 54: 52-54.
- Adongo OS (2013) Medicinal plants of Chhuka community in Tharaka Nithi County, Kenya and some of their selected essential elements. Master thesis in applied and analytical chemistry, Kenyatta University, Nairobi, Kenya.
- Wafa G, Amadou D, Larbi KM, Héla EFO (2014) Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis L. Ind Crop Prod 56: 43-51.
- Rampadarath S, Puchooa D, Ranghoo-Sanmukhiya VM (2014) A comparison of polyphenolic content, antioxidant activity and insecticidal properties of Jatropha species and wild Ricinus communis found in Mauritius. Asian Pac J Trop Med 7: 384-390.
- Harborne JB (1973) Phytochemical Methods: a Guide Plant to Modern Techniques of Plant Analysis. Chapman and Hall, Ltd., London, UK.
- Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25: 223-230.
- Makkar HPS (1995) Quantification of tannins: a laboratory manual. Aleppo: International Centre for Agricultural Research in the Dry Areas.
- Bohan BA, Kocipai-Abyazan MR (1994) Flavonoids and Condensed Tannins from Leaves of Hawaiian Vaccinium reticulatum and calycinum (Ericaceae). Pac Sci 48: 458-463.
- Hansen J, Perry B (1994) The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants. International Laboratory for Research on Animal Diseases, Nairobi, Kenya.
- Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265-267.
- SAS (2000) Statistical Analysis System User’s Guide. Version 8, SAS Institute, Cary, North Carolina, USA.
- Vercruysse J, Holdsworth P, Letonja T, Barth D, Conder G, et al. (2001) International harmonisation of Anthelmintic Efficacy Guidelines. Vet Parasitol 96: 171-193.
- Luoga W (2013) Factors affecting the anthelmintic ef?cacy of cysteine proteinases against GI nematodes and their formulation for use in ruminants. PhD Thesis, University of Nottingham, England, UK.
- Gordon IJ (1989) Vegetation Community Selection by Ungulates on the Isle of Rhum. III. Determinants of Vegetation Community Selection. J App Ecol 26: 65-79.
- Solaiman SG (2010) Feeds and feeding management. In: Solaiman SG (ed.). Goat science and production. Wiley-Blackwell, Hoboken, New Jersey, USA.
- McAllister TA, Martinez T, Bae HD, Muir AD, Yanke LJ, et al. (2005) Characterization of condensed tannins purified from legume forages: chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J Chem Ecol 31: 2049-2068.
- Rogosic J, Estell RE, Ivankovic S, Kezi J, Razov J (2008) Potential mechanisms to increase shrub intake and performance of small ruminants in Mediterranean shrubby ecosystems. Small Ruminant Res 74: 1-15.
- Githiori JB, Höglund J, Waller PJ, Baker RL (2003) The anthelmintic efficacy of the plant, Albizia anthelmintica, against the nematode parasites Haemonchus contortus of sheep and Heligmosomoides polygyrus of mice. Vet Parasitol 116: 23-34.
- Chandrashekhar CH, Latha KP, Vagdevi HM, Vaidya VP (2008) Anthelmintic activity of the crude extracts of Ficus racemosa. Int J Green Pharm 2: 100-103.
- Buttle DJ, Behnke JM, Bartley Y, Elsheikha HM, Bartley DJ, et al. (2011) Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus. Parasit Vectors 4: 36.
- Domingues LF, Giglioti R, Feitosa KA, Fantatto RR, Rabelo MD, et al. (2013) In vitro and in vivoevaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet Parasitol 197: 263-270.
- Knox DP (2007) Proteinase inhibitors and helminth parasite infection. Parasite Immunol 29: 57-71.
- Sutherland I, Scott I (2009) Gastrointestinal Nematodes of Sheep and Cattle: Biology and Control. John Wiley & Sons, Hoboken, New Jersey, USA.
- Sajid M, McKerrow JH (2002) Cysteine proteases of parasitic organisms. Mol Biochem Parasit 120: 1-21.
Citation: Fomum SW, Nsahlai IV (2017) in vitro Evaluation of Anthelmintic Efficacy of Some Plant Species Possessing Proteinases and/or Other Nitrogenous Compounds in Small Ruminants. J Altern Complement Integr Med 3: 038.
Copyright: © 2017 Sylvester W Fomum, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2024, Copyrights Herald Scholarly Open Access. All Rights Reserved!

