
In Vitro Evaluation of Anthelmintic Interaction of Plant Species Combinations Putatively Containing Different Bioactive Macromolecules in Sheep
*Corresponding Author(s):
Ignatius V NsahlaiDepartment Of Animal And Poultry Science, College Of Agriculture, Engineering And Sciences, 127 Rabie Saunders Building, SAEES, Private Bag X01, Scottsville 3209, PMB Campus Of UKZN, South Africa
Tel:+27 0332605067,
Fax:+27 0332605067
Email:fomslyw@gmail.com; nsahlaii@ukzn.ac.za
Abstract
Plant species containing identical putative macromolecules (1) alkaloids/condensed tannins, (2) flavonoids and proteases/nitrogen compounds (3) were used in pairs across groups to evaluate synergistic interactions in vitro in sheep. Intergroup combinations were 32 for condensed tannins/alkaloids and proteases/nitrogen compounds in sub experiment one (SEP 1); 13 combinations for flavonoids and alkaloids/tannin plant species (SEP 2); and 15 combinations for proteases/nitrogen compound and flavonoid containing plant species (SEP 3). Each SEP was run thrice. Four grams dry plant vegetative material was extracted in 70 % ethanol and made up to 100 ml. Sheep dung rectal grabs pooled to constitute test samples. Five grams samples were weighed into Petri dishes and cultured for 12 days at 27°C. On day 13, treatments dosed with combined crude extract at 2.5 ml with double dose concentration of each constituting pair, while some controls were moistened and others treated with 70 % ethanol to eliminate solvent killing effect. Surviving mixed L3 larvae counted on day fourteen. Corrected mortalities were adopted as indices of observed combined efficacies. Synergistic effects computed following Webb’s method and simple synergy from differences between observed and expected efficacies (a + b)/2. Any efficacy that is less than the expected is antagonistic; any that is higher than Web synergy is synergistic; and any that falls between the two is additive. There was no antagonism and all plant-to-plant combinations achieved additive effects. Efficacies of 14 (97.5 – 100%), nine (96.5 – 100%) and eight (96.6 – 98.5%) of plant-by-plant combinations achieved synergistic effects in SEP 1, SEP 2 and SEP 3, respectively. No association occurred between any of alkaloids, condensed tannins or flavonoids with observed efficacy. Multiple regression analysis as predictor of observed efficacy did not highlight any relationship among quantified macromolecules with efficacy for SEP1, 2 and 3. There was no correlation between any macromolecules with observed efficacy. Crude extracts of all combinations exhibited anthelmintic activity, which were not attributed to any specific macromolecule (s). Evidently, there is more to the active principles involved than has been examined in the current study.
INTRODUCTION
Plant species exerting anthelmintic activity are widely reported to have a variety of active photochemical [1-4], which collectively give them anthelmintic attributes [5]. Moreover, very closely related plant species or cultivars from same geographic location share some similar principles and others belonging to different biochemical classes; more often occurring in various concentrations [6,7]. This anthelmintic or antibiotic biochemical disposition among others is suggestive of different efficacies for each of them and the potential of some interaction if such plant cultivars were to be used in combination to treat livestock helminths. Constituting plant species combinations with similar or different anthelmintic principles would most likely increases the pool of photochemical, and concentration of those compounds shared by both plant species. These biochemical interactions in combination therapy have plenty of possible outcomes, some of which may result to improved activity (synergistic effect), parallel or independent activity, antagonistic or mutually inhibitory interaction and bioactive domination of some principles by others.
The failure of most chemical anthelmintics in current control programs has ignited research, development and implementation of other options that will potentially broaden and increase control strategies. Many more control strategies will provide the opportunity to employ various options interchangeably in order to sustain efficacy at economically beneficial levels [8]. One of such novel practices is the combine use of narrow and broad spectrum anthelmintics of different classes at carefully targeted and specific times to maximize efficacy, with attendant effectiveness of 99.9 % or more [8-11]. Adoption of such treatment regime has to be judiciously implemented to ensure that animals with unselected sub populations of parasites on the farm are retained untreated, in order to avoid development and propagation of resistant alleles [8,12]. This strategy has been predicted to conserve efficacy span of combinations for as long as 20 years, if adopted from inception of anthelmintic discovery that makes up the combination [13]. Similar combinations have been inadvertently applied in ethnoveterinary and ethnobotanical medicines, in the treatment of helminths and other microbial infections without profound understanding of pharmacological and biochemical bases of their function. Relative to efficacy of component members of a wide combination of plant species exerting anthelmintic activity, the combined anthelmintic efficacy was largely more potent at treatment of livestock nematodes [14]. Similarly, a combined ethnobotanical formulation for treatment of round worms in native chicken proved to be very efficient and was comparable to existing anthelmintics of choice [15]. Socio-economic implications of ethnobotanical treatment of livestock helminth are important considerations in the promotion and establishment of efficient and effective treatment regimes, beside pharmacological, consumer health and environmental benefits.
Efficacious plant combinations as treatment against helminths, will tremendously reduce the frequency of anthelmintic use, and in the process conserve efficacy span. Economically, cost of production will be reduced and enormous production gains realized as a result of better gut integrity, better feed conversion efficiency and reduced host morbidity [8,15,16]. Selection for anthelmintic resistance by helminth parasites and correspondingly selection for antibiotic resistance by microbes has been intimately linked to frequency of treatment among others [8]. Evolution of resistant alleles from recurrent use of anthelmintics will be briefly examined to explore some of the advantages plant combinations potentially confer on nematode control.
Constituting anthelmintic combinations from diverse anthelmintic classes usually renders the evolution of multi-alleles for resistance intricate and long drawn [17,13]. Single plant species exerting anthelmintic activity on their part, naturally harbour a variety of bioactive compounds, at which level, evolution of resistant anthelmintic alleles is complicated [3,4]. With the constitution of plant combinations, phytochemical anthelmintic principles increase tremendously; further complicating and rendering evolution of phytochemical anthelmintic resistant alleles far more remote or untenable, as huge and fundamental genetic changes will be required. This pharmacological and genetic interaction strongly motivates the use of plant-plant combinations in nematode control programs of livestock. Plants by their biological nature are supposedly non-resistible and better suited for sustainable helminth and other parasite control programs because of their inherent diverse biochemical disposition.
It is hypothesized that combinations of plant species possessing different anthelmintic macromolecular principles will interact to produce no synergistic effect and correspondingly that, plant species containing any or all of alkaloids, condensed tannins and flavonoids will not have any effect on observed efficacy and synergy. The objective of this study was to identify plant combinations possessing different macromolecules which would offer more effective remedy for livestock nematode control relative to component species in isolation.
MATERIALS AND METHODS
Collection of vegetative plant material and processing of crude extracts
Selected plant species exerting anthelmintic activity, related families and common names. Sixteen plant species were collected, each washed, chopped for those with large/long leaves, and oven dried at 60°C to constant weight (Oven mark: LABCON, Model5SOEIB, Maraisburg 1700).
Primarily in the current study, intermacromolecular class combinations were constituted to explore how phytochemical interaction will affect anthelmintic efficacy and synergy. Correspondingly, quantitative contents of alkaloids, condensed tannins and flavonoids were also examined for their implication on observed efficacy, simple and Webb’s synergies.
Extraction and dosing of sheep dung
Four grams of each milled plant sample was weighed and extracted in 70% ethanol. When extraction was completed, the volume of crude extract was made up to 100 ml. Dung was collected from 18 sheep as per rectum, pooled and thoroughly mixed. Five gram sample of faecal material was weighed into Petri dishes and incubated at 27°C for 12 days. On day 13, each of the cultured samples was dosed with 5 ml of combined plant extract, or 2.5 ml each of the designated combined pair of crude extracts with double concentration (2 x 2.5 ml). Negative or untreated controls were processed thus: some control samples were watered at time of dosing treated samples, while others were treated with 5 ml of 70 % ethanol. Mean parasite count from ethanol treated controls was deducted from that of negative controls to eliminate any influence on killing by the solvent. All samples were further incubated for one day. L3 larvae that survived combined dosing were isolated on day 14, including those of controls following Baermann method. Fluid volume (10 ml) was drawn from the stem of each funnel into a labelled test tube and allowed to settle for 15 minutes. Fluid was again drawn from the supernatant of the test tube using a Pasteur pipette, filled into a McMaster slide and isolated L3 larvae counted. Corrected mortality for different samples was computed using Abott’s formula and served as indices of in vitro combined anthelmintic efficacy [18]. Synergistic activity from combination therapy was evaluated following two different methods. One of them was simple synergy, computed as difference between observed and expected efficacies, and the other, Webb’s synergy was computed following Webb’s fractional product method. Following this method, if the efficacies of two plant species “A” and “B” represented by “a” and “b” the proportion of worms killed, then expected efficacy of combinations assuming additive effect is computed thus: Efficacy (A + B) = 1 - ((1 - a) x (1 - b)). Synergistic effect is considered to have occurred when the response of combined administration is greater than additive.
Analysis of alkaloids, flavonoids and condensed tannins in plant samples
Alkaloids were quantified following [19]. Five grams of ground sample was weighed into a 250 ml beaker, into which 200 ml of 10 % acetic acid in ethanol was added and covered to extract for 4 hours. The solution was filtered using a fine sieve into a beaker of same capacity to the former and the extract concentrated in a water bath to ¼ its original volume at 100°C. Concentrated ammonium hydroxide was added drop wise to completely precipitate extract and solution allowed to settle. A precipitate was collected and washed with dilute ammonium hydroxide 50:50 volume for volume. The residue was filtered using WhatmanTM 42 filter paper (GE Healthcare UK Limited, Amersham Place Little Chalfont, Buckinghamshire HP7 9NA, UK and Made in China), oven dried at 60°C and weighed.
Flavonoids content of selected plant vegetative material was determined following [20]. Ten grams (10 g) of oven-dried plant material was milled to pass through a 1-mm sieve, weighed into a 250 ml sterile beaker, and 100 ml of 80 % aqueous methanol added to it. The content was allowed to stand for 10 hours at room temperature, while being stirred intermittently with a magnetic stirring bar over a magnetic rotor without heat. Each solution was filtered individually through WhatmanTM No 42 filter paper. The filtrate of each sample was transferred into pre-weighed 250 ml conical flask and evaporated to dryness in a water bath at constant temperature (80°C). Flasks and their contents were allowed to cool and subsequently placed in the desiccator for one hour to rid them of any moisture. Each of them was weighed, and the weight of the sterilized conical flask deducted from that of flask and flavonoid. The difference was computed as a percentage of flavonoid content of a plant species.
Correspondingly, condensed tannins were analysed following HCl-Butanol proanthocyanidin assay as leucocyanidin equivalent [21,22]. By which, one and a half grams (1.5 g) of this material was weighed into pre-weighed filter paper (WhatmanTM , number 1, diameter 110 mm, Cat number 1001 - 110, GE Healthcare UK limited, Amersham Place, Little Chalfont, Buckinghamshire. HP7, 9NA, UK, and Made in China) and Sohxlet extracted in 1% glacial in petroleum ether to rid them of pigments and fats that could interfere with quantitative determination of condensed tannins. It should be noted that added glacial to petroleum ether would serves as antioxidant, and prevents condensed tannins from being oxidized and bound to vegetative material. Weights of maximum 0.2001 g samples of the different plant species were measured into 100 ml plastic centrifuge tubes, and 10 ml of 70% aqueous acetone added to extract condensed tannins. Centrifuge tubes and their contents were Vortex mixed and placed in an ice bath. Samples were subjected to ultrasonic treatment for 3 minutes in ice cold water and vortex mixed intermittently for 12 minutes, resulting to 4 ultrasonic treatments in all. The content was centrifuged at 5000 rotations per minute (rpm) for 20 minutes at 4°C, and supernatant carefully collected in a glass test tube and stored on ice. Appropriate dilutions of tannin extracts with 70% aqueous acetone were made. Butanol reagent in a 6 ml volume (950 ml of butanol and 50 ml of HCl 37% ) and 0.2 ml of ferric reagent (16.6 ml of concentrated HCl 37 % diluted to 100 ml with water to make 2 M HCl and 2 g of ammonium ferric sulphate dissolved in it) was added to tubes and vortex mixed. Tubes were covered and placed in a heating bath adjusted to between 96 - 100°C for 60 minutes. At the end of the incubation, they were cooled and their absorbances measured using Beckman DU®640 Spectrophotometer at visible wavelength of light 550 nm. From each of these absorbances was deducted that of an unheated mixture (blank). The method allows for appropriate absorbances between 0.30 and less than or equal to 0.60 to be considered stable and most appropriate. Percentage condensed tannins in each of these plant samples was computed following the formula below:
Percentage condensed tannins in dry matter = A550nm x 78.26 x D/% dry matter, where: A550nm = Absorbance at 550 nm; 78.26 = Accumulative factor taking into account: extinction coefficient of leucocyanidin, mass of sample (200 g) and other factors except dilution; D = Dilution factor.
Experimental design and statistical analysis
Designated macromolecular classes included alkaloids/condensed tannins, flavonoids and proteases/nitrogen compounds. These groups consisted of: (1) plant species containing alkaloids/tannins and those containing proteases/nitrogen compounds (sub-experiment one (SEP 1)); (2) plant species containing flavonoids and others containing alkaloids/condensed tannins (SEP 2); and (3) plant species containing proteases/nitrogen compounds and others designated as containing flavonoids (SEP 3). All combinations of plant species were run three times each run being replicated thrice. Sub experiment one had 32 combinations, experiment two 13 and sub-experiment three, 15. Data was analysed using the General Linear Model (GLM), Pearson correlation and multiple regression of [23].
RESULTS
Sub experiment one (alkaloids/tannins and proteases/nitrogen compounds)
Observed efficacies of plant species containing alkaloids/tannins and of those containing proteases/nitrogen compounds were not different (p= 0.276) but high with mean 95.6 ± 0.12 %. Efficacies of combined plant species ranged from 80.8 ± 0.12 % for Ricinus communis/Sarcostema viminale, to 100 ± 0.12 % for Allium cepa/Vernonia amygdalina, Allium cepa/Zizyphus mucronata, Ananas comosus/Crinum macowanii, and Ricinus communis/Zingiber officinale (Table 1). Differences between observed and expected efficacies (simple synergy) were similar (p= 0.47350) with a mean 2.4 ± 0.12 % (Figure 1). Webb’s synergy of these combinations were similar (p= 0.322), with mean -4.0 ± 0.12 %, and ranged from -18 ± 0.12 % for Ricinus communis/Sarcostema viminale to 0.9 ± 0.12 % for Ricinus communis/Zingiber officinale (Figure 1). All fourteen plant species interacted synergistically in combination, while the rest achieved additively. There was no correlation of either alkaloids or condensed tannins to combined anthelmintic efficacy, but for that of flavonoids (r = 0.204; p = 0.0459). Multiple-regression analysis of alkaloids, condensed tannins and flavonoids as predictors of combined anthelmintic efficacy yielded no positive results as none of them entered the model at the significant level(p = 0.15).
|
Plant species |
Family |
Common name |
|
Allium cepa |
Amaryllidaceae |
Common onion |
|
Aloe vanbalenii |
Aloeaceae |
Van Balen’s aloe |
|
Ananas comosus |
Bromeliaceae |
pineapple |
|
Bidens pilosa |
Asteraceae |
Black-jack |
|
Carica papaya |
Caricaceae |
Pawpaw |
|
Crinum macowanii |
Amaryllidaceae |
River lily |
|
Gunnera perpensa |
Gunneraceae |
River pumkin |
|
Nicotiana tabacum |
Solanaceae |
Tobacco |
|
Ricinus communis |
Euphorbiaceae |
Castor oil plant |
|
Sarcostemmaviminale |
Asclepiadaceae (L.) R.Br. subsp. viminale |
Caustic vine |
|
Trema orientalis |
Cannabaceae |
Pigeon wood |
|
Urtica dioica |
Urticaceae |
Stinging nettle |
|
Vernonia amygdalina |
Asteraceae |
Bitterleaf |
|
Zanthozylum capense |
Rutaceae |
Small knobwood |
|
Zingiber officinale |
Zingiberaceae |
Ginger |
|
Zizyphus mucronata |
Rhamnaceae |
Buffalo thorn |
Table 1: Selected plant species exerting anthelmintic activity, related families and common names.
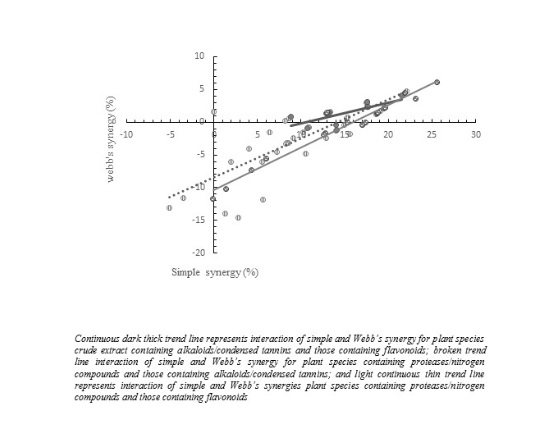
Figure 1: Scattered plot showing interaction between simple and Webb’s synergy in sub experiments 1, 2 and 3.
Sub experiment two (flavonoids and alkaloids/tannins)
Observed anthelmintic efficacies of these combined plant species were similar (p= 0.432) and had a high mean 95.2 ± 0.34 %. Observed efficacies ranged from 81.3 ± 0.34 % for Crinum marcownii/Urtica dioica to 100.0 ± 0.34 % for Gunnera perpensa/Urtica dioica, Nicotiana tabacum/Urtica dioica, Sarcostema viminale/Trema orientalis and Sarcostema viminale/Urtica dioica (Table 2). Simple synergies were not different (p = 0.3814) among combined plant species and had a mean of 2.9 ± 0.36 % (Figure 1). Webb’s synergy of anthelmintic effects were not different (p = 0.2685) among pairs, with a mean -5.4 ± 0.34 % and ranged from -17.7 ± 0.34 % for Crinum marcowanii/Urtica dioica to 1.0 ± 0.34 % for Gunnera perpensa/Urtica dioica (Figure 1). Nine combinations interacted synergistically and the rest achieved additively. There was no association of any of alkaloids, condensed tannins or flavonoids with combined anthelmintic efficacy of these plant species. Additionally, multiple regression analysis of alkaloids, condensed tannins and flavonoids as predictors or explanatory variables of combined anthelmintic efficacy did not qualify any to enter at (p = 0.15) significance level.
|
Combined treatments (A + B) |
Observed efficacy (A) % |
Observed efficacy (B) |
Expected comb. Efficacy (A + B)/2 % |
Observed combined efficacy % |
Webb combined interaction % |
Simple synergy |
Webb’s synergy % |
Synergistic (S) or Antagonistic (A) interaction |
|
All-Alo |
82.3 ± 0.13 |
91.2 ± 0.13 |
86.8 |
100.0 ± 0.04 |
98.4 |
13.3 ± 0.04 |
1.6 ± 0.04 |
S |
|
All-Crin |
82.3 ± 0.13 |
91.2 ± 0.13 |
86.8 |
95.2 ± 0.04 |
98.4 |
8.5 ± 0.04 |
-3.2 ± 0.04 |
A |
|
All-Gun |
82.3 ± 0.13 |
82.4 ± 0.13 |
82.4 |
97.5 ± 0.04 |
96.9 |
15.2 ± 0.04 |
0.6 ± 0.04 |
S |
|
All-Nic |
82.3 ± 0.13 |
91.2 ± 0.13 |
86.8 |
97.6 ± 0.04 |
98.4 |
10.9 ± 0.04 |
-0.8 ± 0.04 |
A |
|
All-Sarc |
82.3 ± 0.13 |
82.4 ± 0.13 |
82.4 |
99.9 ± 0.04 |
96.9 |
17.5 ± 0.04 |
3.0 ± 0.04 |
S |
|
All-Vern |
82.3 ± 0.13 |
82.5 ± 0.13 |
82.4 |
100.0 ± 0.04 |
96.9 |
17.6 ± 0.04 |
3.1 ± 0.04 |
S |
|
All-Zin |
82.3 ± 0.13 |
82.4 ± 0.13 |
82.4 |
97.6 ± 0.04 |
96.9 |
15.3 ± 0.04 |
0.7 ± 0.04 |
S |
|
All-Ziz |
82.3 ± 0.13 |
91.2 ± 0.13 |
86.8 |
100.0 ± 0.04 |
98.4 |
13.3 ± 0.04 |
1.6 ± 0.04 |
S |
|
Ana-Alo |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
97.6 ± 0.04 |
99.2 |
6.4 ± 0.04 |
-1.6 ± 0.04 |
A |
|
Ana-Crin |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
100.0 ± 0.04 |
99.2 |
8.8 ± 0.04 |
0.8 ± 0.04 |
S |
|
Ana-Gun |
91.2 ± 0.13 |
82.4 ± 0.13 |
86.8 |
99.8 ± 0.04 |
98.5 |
13.0 ± 0.04 |
1.3 ± 0.04 |
S |
|
Ana-Nic |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
99.4 ± 0.04 |
99.2 |
8.2 ± 0.04 |
0.2 ± 0.04 |
S |
|
Ana-Sarc |
91.2 ± 0.13 |
82.4 ± 0.13 |
86.8 |
95.2 ± 0.04 |
98.5 |
8.4 ± 0.04 |
-3.2 ± 0.04 |
A |
|
Ana-Vern |
91.2 ± 0.13 |
82.5 ± 0.13 |
86.9 |
96.0 ± 0.04 |
98.5 |
9.1 ± 0.04 |
-2.4 ± 0.04 |
A |
|
Ana-Zin |
91.2 ± 0.13 |
82.4 ± 0.13 |
86.8 |
97.6 ± 0.04 |
99.5 |
10.8 ± 0.04 |
-0.8 ± 0.04 |
A |
|
Ana-Ziz |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
95.2 ± 0.04 |
99.2 |
4.0 ± 0.04 |
-4.0 ± 0.04 |
A |
|
Bid-Alo |
73.5 ± 0.13 |
91.2 ± 0.13 |
82.4 |
83.7 ± 0.04 |
97.7 |
1.3 ± 0.04 |
-14.0 ± 0.04 |
A |
|
Bid-Crin |
73.5 ± 0.13 |
91,2 ± 0.13 |
82.4 |
92.9 ± 0.04 |
97.7 |
10.5 ± 0.04 |
-4.8 ± 0.04 |
A |
|
Bid-Gun |
73.5 ± 0.13 |
82.4 ± 0.13 |
78.0 |
93.6 ± 0.04 |
95.3 |
15.6 ± 0.04 |
-1.8 ± 0.04 |
A |
|
Bid-Nic |
73.5 ± 0.13 |
91.2 ± 0.13 |
82.4 |
97.2 ± 0.04 |
97.7 |
14.9 ± 0.04 |
-0.4 ± 0.04 |
A |
|
Bid-Sarc |
73.5 ± 0.13 |
82.4 ± 0.13 |
78.0 |
95.0 ± 0.04 |
95.3 |
17.1 ± 0.04 |
-0.3 ± 0.04 |
A |
|
Bid-Vern |
73.5 ± 0.13 |
82.5 ± 0.13 |
78.0 |
97.1 ± 0.04 |
95.4 |
19.1 ± 0.04 |
1.7 ± 0.04 |
S |
|
Bid-Zin |
73.5 ± 0.13 |
82.4 ± 0.13 |
78.0 |
83.6 ± 0.04 |
95.3 |
5.6 ± 0.04 |
-11.8 ± 0.04 |
A |
|
Bid-Ziz |
73.5 ± 0.13 |
91.2 ± 0.13 |
82.4 |
96.3 ± 0.04 |
97.7 |
14.0 ± 0.04 |
-1.3 ± 0.04 |
A |
|
Car-Alo |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
86.1 ± 0.04 |
99.2 |
-5.1 ± 0.04 |
-13.1 ± 0.04 |
A |
|
Car-Crin |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
87.7 ± 0.04 |
99.2 |
-3.5 ± 0.04 |
-11.6 ± 0.04 |
A |
|
Car-Gun |
91.2 ± 0.13 |
82.4 ± 0.13 |
86.8 |
97.0 ± 0.04 |
98.5 |
10.2 ± 0.04 |
-1.5 ± 0.04 |
A |
|
Car-Nic |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
99.8 ± 0.04 |
99.2 |
8.6 ± 0.04 |
0.6 ± 0.04 |
S |
|
Car-Sarc |
91.2 ± 0.13 |
82.4± 0.13 |
86.8 |
95.1 ± 0.04 |
98.5 |
8.3 ± 0.04 |
-3.3 ± 0.04 |
A |
|
Car-Vern |
91.2 ± 0.13 |
82.5± 0.13 |
86.9 |
94.0 ± 0.04 |
98.5 |
7.2 ± 0.04 |
-4.5 ± 0.04 |
A |
|
Car-Zin |
91.2 ± 0.13 |
82.4± 0.13 |
86.8 |
92.3 ± 0.04 |
98.5 |
5.5 ± 0.04 |
-6.1 ± 0.04 |
A |
|
Car-Ziz |
91.2 ± 0.13 |
91.2± 0.13 |
91.2 |
93.2 ± 0.04 |
99.2 |
2.0 ± 0.04 |
-6.0 ± 0.04 |
A |
|
Ric-Alo |
73.5 ± 0.13 |
91.2± 0.13 |
82.4 |
100.0 ± 0.04 |
97.7 |
17.7 ± 0.04 |
2.3 ± 0.04 |
S |
|
Ric-Sarc |
73.5 ± 0.13 |
82.4± 0.13 |
78.0 |
80.8 ± 0.04 |
95.3 |
2.8 ± 0.04 |
-14.6 ± 0.04 |
A |
|
Ric-Vern |
73.5 ± 0.13 |
82.5± 0.13 |
78.0 |
96.6 ± 0.04 |
95.4 |
18.6 ± 0.04 |
1.2 ± 0.04 |
S |
|
Ric-Zin |
73.5 ± 0.13 |
82.4± 0.13 |
78.0 |
100.0 ± 0.04 |
95.3 |
22.1 ± 0.04 |
4.7 ± 0.04 |
S |
|
Ric-Ziz |
73.5 ± 0.13 |
91.2± 0.13 |
82.4 |
95.2 ± 0.04 |
97.7 |
12.9 ± 0.04 |
-2.4 ± 0.04 |
A |
Table 2: Anthelmintic interaction (%) of combined plant species possessing alkaloids/tannins and those containing proteases/nitrogen compounds.
All = Allium cepa, Ana = Ananascomosus, Bid = Bidenspilosa, Car = Carrica papaya, Crin = Crinum macowanii, Gun = Gunneraperpensa, Nic = Nicotianatabacum, Ric = Ricinuscommunis, Sarc = Sarcostemaviminale, Vern = Vernonia amygdalina, Zin = Zingiberofficinale, Ziz = Zizyphusmucronata, A= antagonistic interaction, S= synergistic interaction
Sub experiment three (proteases/nitrogen compounds and flavonoids)
Observed combined efficacies of plant species containing proteases/nitrogen compounds and flavonoids were similar (p = 0.597) but relatively high, with a mean of 95.8 ± 0.04 %. Observed efficacies ranged from 86.8 ± 0.04 % for Carica papaya/Zanthozylum capense to 100.0 ± 0.04 % for Carica papaya/Trema orientalis (Tables 3 and 4). Simple synergies were similar (p = 0.756), with mean of 2.0 ± 0.27 %. Webb’s synergy were also similar (p = 0.626), with a mean -3.8 ± 0.04 % and ranged from -12 ± 0.04 % for Carica papaya/Zanthozylum capense to 0.4 ± 0.04 % for Biden pilosa/Urtica dioica (Table 2). Eight combinations in this lot interacted synergistically and the rest achieved additively. There was no correlation between any of alkaloids, condensed tannins or flavonoids content of plant species and anthelmintic efficacy. Similarly, multiple regression analysis of alkaloids, condensed tannins and flavonoid content as predictors of combined anthelmintic efficacy was so poor that none could enter the model at P = 0.15 level of significance.
|
Combined treatments (A + B) |
Observed efficacy (A)% |
Observed efficacy (B) % |
Expected efficacy (A + B)/2 % |
Observed combined efficacy % |
Webb comb. interaction % |
Simple synergy % |
Webb’s synergy % |
Synergistic (S) or Antagonistic (A) interaction |
|
Flavonoids&Alkaloids &/or Condensed tannins |
||||||||
|
Trem-Alo |
73.5 ± 0.13 |
91.2 ± 0.13 |
82.4 |
96.5 ± 0.07 |
97.7 |
14.1 ± 0.07 |
-1.2 ± 0.07 |
A |
|
Trem-Vern |
73.5 ± 0.13 |
82.5 ± 0.13 |
78.0 |
97.5 ± 0.07 |
95.4 |
19.5 ± 0.07 |
2.1 ± 0.07 |
S |
|
Trem-Zin |
73.5 ± 0.13 |
82.4 ± 0.13 |
78.0 |
99.4 ± 0.07 |
95.3 |
21.5 ± 0.07 |
4.1 ± 0.07 |
S |
|
Trem-Ziz |
73.5 ± 0.13 |
91.2 ± 0.13 |
82.4 |
99.9 ± 0.07 |
97.7 |
17.6 ± 0.07 |
2.3 ± 0.07 |
S |
|
Urt-Alo |
82.5 ± 0.13 |
91.2 ± 0.13 |
86.9 |
100.0 ± 0.07 |
98.5 |
13.2 ± 0.07 |
1.5 ± 0.07 |
S |
|
Urt-Vern |
82.5 ± 0.13 |
82.5 ± 0.13 |
82.5 |
96.5 ± 0.07 |
96.9 |
14.0 ± 0.07 |
-0.4 ± 0.07 |
A |
|
Urt-Zin |
82.5 ± 0.13 |
82.4 ± 0.13 |
82.5 |
100.0 ± 0.07 |
96.9 |
17.6 ± 0.07 |
3.1± 0.07 |
S |
|
Urt-Ziz |
82.5 ± 0.13 |
91.2 ± 0.13 |
86.9 |
99.9 ± 0.07 |
98.5 |
13.1± 0.07 |
1.5 ± 0.07 |
S |
|
Zan-Alo |
82.5 ± 0.13 |
91.2 ± 0.13 |
86.9 |
99.7 ± 0.07 |
98.5 |
12.9 ± 0.07 |
1.3 ± 0.07 |
S |
|
Zan-Zin |
82.5 ± 0.13 |
82.4 ± 0.13 |
82.5 |
100.0 ± 0.07 |
96.9 |
17.6 ± 0.07 |
3.1± 0.07 |
S |
|
Zan-Ziz |
82.5 ± 0.13 |
91.2 ± 0.13 |
86.9 |
97.6 ± 0.07 |
98.5 |
10.7 ± 0.07 |
-0.9 ± 0.07 |
A |
|
Ziz-Alo |
91.2 ± 0.13 |
91.2 ± 0.13 |
91.2 |
100.0 ± 0.07 |
99.2 |
8.8 ± 0.07 |
0.8 ± 0.07 |
S |
|
Proteases/Flavonoids |
||||||||
|
All-Trem |
82.3 ± 0.13 |
73.5 ± 0.13 |
77.9 |
97.5 ± 0.08 |
95.3 |
19.6 ± 0.08 |
2.2 ± 0.08 |
S |
|
All-Urt |
82.3 ± 0.13 |
82.5 ± 0.13 |
82.4 |
95.2 ± 0.08 |
96.9 |
12.8 ± 0.08 |
-1.7 ± 0.08 |
A |
|
All-Zan |
82.3 ± 0.13 |
82.5 ± 0.13 |
82.4 |
95.0 ± 0.08 |
96.9 |
12.6 ± 0.08 |
-1.9 ± 0.08 |
A |
|
Ana-Trem |
91.2 ± 0.13 |
73.5 ± 0.13 |
82.4 |
97.6 ± 0.08 |
97.7 |
15.3 ± 0.08 |
-0.1 ± 0.08 |
A |
|
Ana-Urt |
91.2 ± 0.13 |
82.5 ± 0.13 |
86.9 |
92.9 ± 0.08 |
98.5 |
6.0 ± 0.08 |
-5.6 ± 0.08 |
A |
|
Ana-Zan |
91.2 ± 0.13 |
82.5 ± 0.13 |
86.9 |
99.9 ± 0.08 |
98.5 |
13.0 ± 0.08 |
1.4 ± 0.08 |
S |
|
Ana-Urt |
91.2 ± 0.13 |
82.5 ± 0.13 |
86.9 |
88.2 ± 0.08 |
98.5 |
1.4 ± 0.08 |
-10.2 ± 0.08 |
A |
|
Bid-Sarc |
73.5 ± 0.13 |
82.4 ± 0.13 |
78.0 |
95.3 ± 0.08 |
95.3 |
17.4 ± 0.08 |
-0.1 ± 0.08 |
A |
|
Bid-Trem |
73.5 ± 0.13 |
73.5 ± 0.13 |
73.5 |
99.1 ± 0.08 |
93.0 |
25.6 ± 0.08 |
6.1 ± 0.08 |
S |
|
Bid-Urt |
73.5 ± 0.13 |
82.5 ± 0.13 |
78.0 |
99.9 ± 0.08 |
95.4 |
21.9 ± 0.08 |
4.5 ± 0.08 |
S |
|
Bid-Zan |
73.5 ± 0.13 |
82.5 ± 0.13 |
78.0 |
96.7 ± 0.08 |
95.4 |
18.7 ± 0.08 |
1.3 ± 0.08 |
S |
|
Car-Trem |
91.2 ± 0.13 |
73.5 ± 0.13 |
82.4 |
100.0 ± 0.08 |
97.7 |
17.7 ± 0.08 |
2.3 ± 0.08 |
S |
|
Car-Urt |
91.2 ± 0.13 |
82.5 ± 0.13 |
86.9 |
91.1± 0.08 |
98.5 |
4.3 ± 0.08 |
-7.3± 0.08 |
A |
|
Car-Zan |
91.2 ± 0.13 |
82.5 ± 0.13 |
86.9 |
86.8 ± 0.08 |
98.5 |
-0.1 ± 0.08 |
-11.7 ± 0.08 |
A |
|
Ric-Trem |
73.5 ± 0.13 |
73.5 ± 0.13 |
73.5 |
96.6 ± 0.08 |
93.0 |
23.1± 0.08 |
3.6 ± 0.08 |
S |
|
Ric-Urt |
73.5 ± 0.13 |
82.5 ± 0.13 |
78.0 |
95.0 ± 0.08 |
95.4 |
17.0 ± 0.08 |
-0.4 ± 0.08 |
A |
|
Ric-Zan |
73.5 ± 0.13 |
82.5 ± 0.13 |
78.0 |
96.8 ± 0.08 |
95.4 |
18.8 ± 0.08 |
1.4 ± 0.08 |
S |
Table 3: Anthelmintic efficacies (%) of combined plant species possessing flavonoids and those containing alkaloids/tannins.
Crin = Crinum macowanii, Gun = Gunneraperpensa, Nic = Nicotianatabacum, Sarc = Sarcostemaviminale, Trem = Tremaorientalis, Urt = Urticadioica, Vern = Vernonia amygdalina, Zan = Zanthozylumcapense, Zin = Zingiberofficinale, Ziz = Zizyphusmucronata,All = Allium cepa, Ana = Ananascomosus, Bid = Bidenspilosas, Car = Carricapapya, Ric = Ricinuscommunis, Trem = Tremaorientalis, Urt = Urticadioica, Zan = Zanthozylumcapense.
|
|
Plant species |
n |
Alkaloids (gDM/Kg) |
n |
Cond. Tannins (gDM/Kg) |
n |
Flavonoids (gDM/Kg) |
|
Alkaloids and condensed tannins |
|
|
|
|
|||
|
|
Crinum m. |
2 |
20.9 ± 1.10A |
6 |
5.5 ± 1.28A |
2 |
117.9 ± 1.75B |
|
|
Gunnera p. |
2 |
44.4 ± 15.20A |
5 |
7.6 ± 1.30B |
2 |
26.0 ± 2.60A |
|
|
Nicotiana t. |
2 |
37.1 ± 3.20A |
5 |
6.4 ± 1.42B |
2 |
202.6 ± 0.75A |
|
|
Sarcostema v. |
2 |
46.7 ± 8.50A |
2 |
2.8 ± 0.01B |
2 |
117.0 ± 2.76B |
|
|
Vernonia a. |
2 |
42.4 ± 8.20A |
6 |
3.4 ± 0.63B |
2 |
125.0 ± 13.57B |
|
|
Zingiber o. |
2 |
48.3 ± 4.50A |
6 |
3.4 ± 0.55B |
2 |
172.1 ± 17.60A |
|
|
Zizyphus m. |
2 |
30.6 ± 0.68A |
6 |
13.7 ± 1.99B |
2 |
124.3 ± 10.67B |
|
Flavonoids |
|
|
|
|
|
|
|
|
|
Trema o. |
2 |
72.5 ± 13.80A |
3 |
11.5 ± 2.14A |
2 |
207.5 ± 1.66A |
|
|
Urtica d. |
2 |
23.6 ± 17.90A |
6 |
11.2 ± 1.61A |
2 |
138.6 ± 6.63B |
|
|
Zanthozylum c. |
2 |
16.3 ± 1.22A |
6 |
3.9 ± 1.47B |
2 |
129.1 ± 16.01B |
|
Proteases and or nitrogen compounds |
|
|
|
||||
|
|
Allium c. |
2 |
5.7 ± 0.30A |
6 |
4.7 ± 0.97A |
2 |
550.4 ± 25.42A |
|
|
Ananas c. |
2 |
47.5 ± 6.70A |
6 |
4.4 ± 0.75A |
2 |
133.5 ± 5.15B |
|
|
Bidens p. |
2 |
39.5 ± 6.10A |
6 |
5.9 ± 1.09A |
2 |
163.5 ± 1.92B |
|
|
Carica p. |
2 |
40.5 ± 6.10A |
4 |
2.6 ± 0.76A |
2 |
167.7 ± 12.38B |
|
|
Ricinus c. |
2 |
43.0 ± 4.80A |
6 |
4.4 ± 1.56A |
2 |
149.6 ± 10.27B |
Table 4: Alkaloids, condensed tannins and flavonoid c
DISCUSSION
Observed combined anthelmintic efficacies of plant combinations carrying different classes of bioactive compounds in all three sub experiments were high, but had various trends. These trends are suggestive of various interactions among different classes of anthelmintic principles involved and others that might have aided the process of combined anthelmintic efficacy [24-26]. Nonetheless, improved anthelmintic activity from plant combinations as exemplified by the trend of simple synergies is a potential indication of some positive biochemical interaction, which was not discernible at the level of putative macro-biochemical interaction including alkaloids, condensed tannins and flavonoids. Fundamentally, plant species in their nature and true to their species trait are suggested to be responsible for primary differences in combined anthelmintic activity; these same differences are pooled in combinations resulting to various interactions [27-32]. These species differences may therefore lead to many more interactions and further differences in anthelmintic activity exerted by various combinations. Basically, the nature, chemical structure, molecular size and variety of anthelmintic principle(s), and other related biochemical candidates in the pool of each pair of combination, most likely would contribute to explain trends of observed combined efficacies [33].
Mean combined anthelmintic efficacy of combinations involving alkaloids/tannins and proteases/nitrogenous compounds (95.6 ± 0.12 %), and that of proteases/nitrogenous compound and flavonoids (95.8 ± 0.04 %) were proportionally different, while that of combinations involving plant species carrying flavonoids and alkaloids/tannin containing plant species was relatively higher (98.9 ± 0.34 %) in accordance with unique biochemical nature of component plant species [29,30]. These observed differences most likely would have arisen from phytochemical interactions within various combinations. Combinations of different plant species would have produced a rich pool of biochemical compounds, some of which potentially interacted and improved on anthelmintic effect (synergy) [5,34]. The net effect from these interactions being the largely positive additive effects.
Improved anthelmintic activity in combination phytotherapy was measured first, by simple synergy (additive effect) and alternatively, by any further response above additive effect qualified as synergistic following Webb’s fractional product method [35]. However, both approaches interacted linearly though simple synergy was largely positive, whereas Webb’s synergy was negative (Figure 1). Combined dose adopted in the current study yielded high observed efficacies and were mostly close to 100%, leaving very little room to adequately evaluate synergistic effects for most combinations in the process. Lower combined doses, relative to the current one, will potentially better address determination of synergistic effect and any extensive improvement that will arise from combined phytoanthelmintic therapy. As hypothesized, there is neither rejection nor acceptance following the dose adopted in the current study.
In Sub Experiment one (SE1) flavonoids associated with observed efficacy, whereas alkaloids and condensed tannins did not. Additionally, in Sub Experiments two (SE 2) and three (SE 3) there was no association of any of flavonoids, condensed tannins and alkaloids with observed efficacy and other related parameters including simple and Webb’s synergies. Plant species containing or carrying some of these bioactive molecular classes, usually have a mixture of different molecular types, some of which may lack bioactive attributes for the relevant trait [36]. In this context, if the molecular constitution of the biochemical class were more of the inactive portion, then, there is likelihood that the activity will be obscured and rendered insignificant [36]. It is likely to occur when molecular classes of alkaloids, condensed tannins and flavonoids are considered holistically. On the other hand, if combinations containing different biochemical principles that exert anthelmintic and other related activities are overwhelmingly of the active type, additive and/or synergistic effects will occur (Tables 1 and 2). Improved observed efficacy of some combinations in the current study is in accord with the objective of adopting combination therapy in helminth treatment and other therapeutic processes. Anthelmintic efficacy of combinations involving alkaloids/tannins and proteases/nitrogen, flavonoids and alkaloids/tannin), and proteases/nitrogen compounds and flavonoids, all produced positive simple synergic (additive) effects. This is suggestive of a collective anthelmintic activity in combinations that might have arisen from a variety of active principles including alkaloids, condensed tannins and flavonoids, with no outstanding strong evidence of individual contributions.
Following Webb’s formula for computing additive and synergistic effects from anthelmintic drug combinations, additive effect of two acting independent drugs, is the product of the surviving or unaffected fractions after treatment with either agents alone [35]. The basis of adoption of combined anthelmintic therapy has been because, generally, the resulting combined efficacy is usually higher than that of any of the component anthelmintics [37]. It is expected that plant species combinations possessing different anthelmintic principles will yield similar results to those of drug combinations. Though no combination yielded antagonistic effects, it is important to select combinations with favourable responses to evade any negative effect (Tables 1 and 2). Phytochemical interactions in phytotherapeutic remedies is usually complex with synergistic and antagonistic processes taking place concurrently to enable healing [5]. Alternative evaluation focussing on simple synergy (additive effects) yielded overall positive mean in contrast to largely negative values following Webb’s fractional product method. While results of simple synergy (additive effects) paint a picture of positive synergism, Webb’s method yielded selective antagonistic and synergistic interaction. Our general observation showed that there was some marginal increase in anthelmintic potency as a result of plant species combinations possessing different principles.
This tool has been useful in regulating and mitigating selection for resistance, given that higher anthelmintic efficacy from combination therapy and synergistic interaction will eliminate almost all nematode parasites at drug/parasite interphase [17]. Anthelmintic efficacy that is close to 100% leaves virtually only parasites and their intermediate developmental stages that have no contact or interaction with anthelmintic remedy so, conserve their susceptibility for subsequent treatment or dosing [38]. In this scenario, non-interaction of sub parasite populations with potent combination can be likened to selective treatment of affected animals that leaves untreated ones with parasites that are highly vulnerable to killing effect [8,12]. Drug combination apart, augmentation of protein content of livestock diets resulted to much more resilient animals [39]. Proteins of nutritional benefit administered as supplement concurrently with copper wire particles to lambs and goats, reduced nematode egg count significantly and was suggested to have acted synergistically [39,40].
Better control of nematodes and other economically important parasites of grazing livestock are therefore not limited to application of combination anthelmintic therapy and those of plants possessing different bioactive principles. This opens ample space for multifaceted research on combination therapy and a huge potential of diverse approaches to this novel method of control. Protein supplementation and its interaction with plant secondary metabolites, especially condensed tannins is suggested to be another method of combination therapy and can also serve a prophylactic role. It exerts an indirect role similar to that of supplementation in diets of infected livestock by being temporally bound to tannins and evading ruminal microbial degradation, thus enabling their supply to the abomasum and small intestine for efficient enzymatic digestion, absorption and assimilation [41,42]. This reinforces protein availability for vital metabolic processes and immune defence activities against nematode parasites and other pathogens. However, this among other methods may reduce nematode burden considerably. Anthelmintic combination therapy and prophylaxis represent a more effective and efficient method, while anthelmintic bioactive plant species combinations have budding potentials to be implemented and improved upon.
CONCLUSION
Combinations of plant species exerting anthelmintic activity and possessing different anthelmintic bioactive classes are an important method of livestock nematode parasite control, in addition to other pathogens. Intergroup combinations apparently showed some marginal positive synergistic effects, while others were additive because of the wide range of bio-chemicals involved. Phytotherapy naturally involves these processes, but with a net positive or synergistic effect that engenders healing. Those combinations that have proven useful can be adopted for subsequent studies. The dose used in the current study has to be reduced to sufficiently address the element of synergy.
REFERENCES
- Sajid M, Mckerrow JH (2002) Cysteine proteases of parasitic organisms. Mol Biochem Parasitol 120: 1-21.
- Choi S, Chung M-H (2003) A review of the relationship between Aloe vera components and their biological effects. Seminars in Integrative Medicine 1: 53-62.
- Sandoval-Castro CA, DeB Hovell FD, Torres-Acosta JFJ, Ayala-Burgos (2006) Herbivores: assessment of intake, digestibility and the roles of secondary compounds. Nottingham University Press, Nottingham, UK.
- Hoste H, Torres-Acosta JF, Alonso-diaz MÁ, Brunet S, Sandoval-Castro C, et al. (2008) Identification and validation of bioactive plants for the control of gastrointestinal nematodes in small ruminants. Trop Biomed 25: 56-72.
- Efferth T, Koch E (2011) Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets 12: 122-132.
- Ress SB, Harborne JB (1985) The role of sesquiterpene lactones and phenolics in the chemical defence of the chicory plant. Phytochem 24: 2225-2231.
- Foster JG, Cassida KA, Turner KE (2011) In vitro analysis of the anthelmintic activity of forage chicory (Cichorium intybus ) sesquiterpene lactones against a predominantly Haemonchus contortus egg population. Vet Parasitol 180: 298-306.
- Shalaby HA (2013) Anthelmintic Resistance; How to overcome it? Iran J Parasitol 8: 18-32.
- Dobson RJ, Besier RB, Barnes EH, Love SC, Vizard A et al. (2001) Principles for the use of macrocyclic lactones to minimise selection for resistance. Aust Vet J 79: 756-761.
- Leathwick DM, Pomroy WE, Heath AC (2001) Anthelmintic resistance in New Zealand. N Z Vet J 49: 227-235.
- Krecek RC, Waller PJ (2006) Towards the implementation of the “basket of options” approach to helminth parasite control of livestock: Emphasis on the tropics/subtropics. Vet Parasitol 139: 270-282.
- van Wyk JA, Hoste H, Kaplan RM, Besier RB (2006) Targeted selective treatment for worm management--how do we sell rational programs to farmers? Vet Parasitol 139: 336-346.
- Leathwick DM, Waghorn TS, Miller CM, Candy PM, Oliver AM (2012) Managing anthelmintic resistance--use of a combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin. Vet Parasitol 187: 285-294.
- Dwivedi A, Dwivedi S, Sitoke AK, Patel R, Jhade D (2009) Anthelmintic Activity of a Polyherbal Preparation. Ethnobotanical Leaflets 13: 259-62.
- Ozaraga BP, Ozaraga MSI, Barrios MB (2015) Ethnobotanical Dewormer Composition for Free Range Native Chickens. Mindanao Journal of Science and Technology 13: 12-19.
- Ketzis JK, Vercruysse J, Stromberg B, Larsen M, Athanasiadou S, et al.(2006) Evaluation of efficacy expectations for novel and non-chemical helminth control strategies in ruminants. Vet Parasitol 139: 321-335.
- Bartram DJ, Leathwick DM, GeurdenT, Taylor MA, Maeder SJ (2012) The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet Parasitol 186: 151-158.
- Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18: 265-267.
- Harborne JB (1973) Phytochemical methods. Chapman Hall. London, UK.
- Bohan DA, Kocipal-Abyazan R (1974) Flavonoids and condensed tannins from leaves of Vaccinium vaticulatum and calycinium. Pac Sci 48: 458-163.
- Porter LJ, Hrstich LN, Chan BG (1985) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25: 223-230.
- Makkar HPS (1995) Quantification of tannins: A laboratory manual. International Centre for Agricultural Research in the Dry Areas, Aleppo, Syria.
- SAS (2000) Statistical Analysis System user guide (Version 8). SAS Institute Inc., SAS Campus Drive, Cary, NC, USA.
- Kubola J, Siriamornpun S (2008) Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia ) leaf, stem and fruit fraction extracts in vitro. Food Chem 110: 881-890.
- Rochfort S, Parker AJ, Dunshea FR (2008) Plant bioactives for ruminant health and productivity. Phytochemistry 69: 299-322.
- Madalaa NE, Piater L, Duberya I, Steenkamp P (2016) Distribution patterns of flavonoids from three Momordica species by ultra-high performance liquid chromatography quadrupole time of flight mass spectrometry: a metabolomic profiling approach. Rev Bras Farmacogn 26: 507-513.
- Hammond JA, Fielding D, Bishop SC (1997) Prospects for plant anthelmintics in tropical veterinary medicine. Veterinary research communications 21: 213-228.
- Aherne SA, O’Brien NM (2002) Dietary flavonols: chemistry, food content, and metabolism. Nutrition 18: 75-81.
- Makkar HPS, Francis G, Becker K (2007) Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 1: 1371-1391.
- Cala AC, Chagas ACS, Oliveira MCS, Matos AP, Borges LMF, et al. (2012) In vitro Anthelmintic effect of Melia azedarach L. and Trichilia claussenii C. against sheep gastrointestinal nematodes. Exp Parasitol 130: 98-102.
- Wenk C (2003) Herbs and Botanicals as Feed Additives in Monogastric Animals. Asian-Australian Journal of Animal Science 16: 282-289.
- Shaik SA, Terrill TH, Miller JE, Kouakou B, Kannan G, et al. (2006) Sericea lespedeza hay as a natural deworming agent against gastrointestinal nematode infection in goats. Vet Parasitol 139: 150-157.
- Cody V, Middleton E, Harborne JB (1986) Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological, and Structure Activity Relationships, John Wiley & Sons, Canada.
- Che CT, Wang ZJ, Chow MS, Lam CW (2013) Herb-herb combination for therapeutic enhancement and advancement: theory, practice and future perspectives. Molecules 18: 5125-5141.
- Webb JL (1963) Effect of more than one inhibitor. Enzyme and Metabolic inhibitors 1: 487-512.
- Klongsiriwet C, Quijada J, Williams AR, Mueller-Harvey I, Williamson EM, et al. (2015) Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int J Parasitol Drugs Drug Resist 5: 127-134.
- Leathwick DM, Besier RB (2014) The management of anthelmintic resistance in grazing ruminants in Australasia--strategies and experiences. Vet Parasitol 204: 44-54.
- Geary TG, Hosking BC, Skuce PJ, Samson-Himmelstjerna G, Maeder S, et al. (2012) World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) Guideline: Anthelmintic combination products targeting nematode infections of ruminants and horses. Vet Parasitol 190: 306-316.
- Burke JM, Morrical D, Miller JE (2007) Control of gastrointestinal nematodes with copper oxide wire particles in a flock of lactating Polypay ewes and offspring in Iowa, USA. Vet Parasitol 146: 372-375.
- Burke JM, Miller JE, Olcott DD, Olcott BM, Terrill TH (2004) Effect of copper oxide wire particles dosage and feed supplement level on Haemonchus contortus infection in lambs. Vet Parasitol 123: 2335-2243.
- Athanasiadou S, Houdijk J, Kyriazakis I (2008) Exploiting synergisms and interactions in the nutritional approaches to parasite control in sheep production systems. Small Ruminant Research 76: 2-11.
- Barry TN, McNabb WC (1999) The implication of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br J Nutr 81: 263-272.
Citation: Fomum SW, Nsahlai IV (2019) In Vitro Evaluation of Anthelmintic Interaction of Plant Species Combinations Putatively Containing Different Bioactive Macromolecules in Sheep. J Altern Complement Integr Med 6: 087.
Copyright: © 2020 Sylvester W Fomum, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

