
In vitro Evaluation of the Antimicrobial Activity of a Topical Skin Preparation Containing 0.1% Polyhexanide vs a Topical Skin Preparation Containing 1% Silver Sulfadiazine
*Corresponding Author(s):
Barbara MaglioneFarmaceutici Damor S.p.A., Via E. Scaglione N.27 Napoli, Italy
Tel:+39 3343293357,
Email:bmagione@hotmail.com
Abstract
Aim: The effective in vitro antibacterial activity on Staphylococcus aureus (S.aureus), Pseudomonas aeruginosa (P.aeruginosa), Klebsiella pneumoniae (K.pneumoniae),Escherichia coli (E.Coli) and the combination of S.aureus and K. pneumonia of a topical cream based on 0.1% polyhexanidewas compared to a topical cream based on 1% silver sulfadiazine.A topical cream containing 0,1% gentamicin was used as a positive control and a white blank topical cream was used as negative control.
Materials and Methods: The in vitro antibacterial activities were determined by agar well-diffusion assay. Two-way Analysis of Variance (ANOVA) was used to test, by calculation of P-values, for significant antiseptic activity in bacteria treated with 0.1% polyhexanide topical cream compared to 1% silver sulfadiazine and to the negative and positive controls.
Results: Among the derivatives tested, all the active topical creams analyzed were able to reduce microbial strains. The topical cream based on 0.1% polyhexanide showed a significantly higher antibacterial efficacy in comparison to the topical cream based on 1% silver sulfadiazine on S. aureus and K. pneumonia and on the combination of S. aureus and K. pneumoniae,while no significant difference was detected between the antibacterial activity of the two topical creams against P. aeruginosa and E. coli.
Conclusion: These results provide a further insight into the antibacterial activity of polyhexanide and its non-inferiority compared to silver sulfadiazine towards certain bacterial strains (P. aeruginosa and E. coli) and superiority towards other (S. aureus and K. pneumoniae)and support the use of 0.1% Polyhexanide topical preparation for the treatment of wounds that are infected or at risk of infection.
Keywords
Antibacterial activity; E. coli; K. pneumonia; P. aeruginosa; S. aureus; 0.1% Polyhexanide; 1% silver sulfadiazine
INTRODUCTION
The skin is the largest organ of the body. Microbiologically, the primary functionof the skin is to control the microbial populations that live on its surface.In fact, the skin is an effective barrier against bacterial infections. Although many bacteria reside on the skin surface, they are normally unable to cause an infection.A loss of skin integrity (i.e., a wound) alters the defense functions of the skin and creates favorable conditions to microbial colonization and proliferation.
Since wound colonization involves numerous microorganisms that are potentially pathogenic, any wound is exposed to the risk of becoming infected.
Wound contaminants are most likely to originate from the environment (i.e. exogenous microorganisms in the air), from the surrounding skin, involving members of the normal skin microflora such as Staphylococcus epidermidis and micrococci, or from the mucosae membranes [1].
However, to date, Staphylococcus aureus, Pseudomonas Aeruginosa and Klebsiella Pneumoniae, are the most frequently isolated bacteria from skin lesions, causing delayed healing and infection in both chronic and acute wounds [2].
The effect of the microorganisms on wound healing has been widely studied, and even if the majority of wounds are polymicrobial, involving both aerobes and anaerobes, aerobic pathogens such as S. aureus, P. aeruginosa, and Klebsiella Pneumoniae have been most frequently cited as the cause of delayed wound healing and infection. S. aureus is considered to be the most problematic bacterium in traumatic, surgical, and burn infections [3]. Moreover, S. aureus and K. pneumoniae are known as the principal responsible for biofilm formation, a major virulence factor contributing to the chronicity of infections [4]. Furthermore, several reports associating the enterobacterium Escherichia coli (E. coli) with Skin and Soft Tissue Infections (SSTIs) have been published: E. coli was found to be the causative agent of neonatal omphalitis, cellulitis localized to lower or upper limbs, necrotizing fasciitis, surgical site infections, infections after burn injuries, and others [5].
Although systemic antibiotic therapy is essential for advancing cutaneous infections, wounds that exhibit only localized signs of infection or are failing to heal but do not have clinical signs of infection (i.e., an heavy colonization) may initially be treated with topical agents. Topical agents against microbial contamination include antibiotics and antiseptics.
The topical antiseptic treatment is generally favored in chronic wounds that are heavily contaminated by a variety of microorganisms.The topical antiseptic agents most commonly used include iodine-releasing agents (e.g., povidone iodine and cadexomer iodine), chlorine-releasing solutions (e.g., Dakin’s solution and sodium hypochlorite solution), hydrogen peroxide, chlorhexidine, silver-releasing agents, polyhexanide and acetic acid [3].
The management of the microbial infection in wound healing is one of the most important components for an effective standard of care. In fact, the management of microbial infection is included in the TIMERSprotocol for the treatment of chronic lesions, which classifies the different conditions that occur in case of chronic injuries [6].
Microorganisms tend to interact with chronic wounds at four different levels: contamination, colonization, critical colonization and infection.
To accelerate wound healing, physicians use bioactive dressings which are capable of interacting with the microenvironment of the wound and of stimulating its healing. They represent the evolution of advanced dressings [7].
Many bioactive dressings contain also antiseptic compounds to reduce the risk of bacterial contamination; among the most used and most effective are silver sulfadiazine and polyhexanide.
Silver sulfadiazine is the silver salt of sulfadiazine, an antiseptic belonging to the class of sulfonamides.
Clinically, metallic silver is relatively inert, but its interaction with the moisture of the skin surface and with the wound fluids results in the release of silver ions with antibacterial properties [8].
The silver ions bind to the proteins present in the tissues causing structural changes of the cellular wall and of the nuclear membranes of the bacteria themselves. Silver binds to the DNA and RNA of bacteria inhibiting their replication [8].
Polyhexanide Biguanide (PHMB) is a synthetic polymer structurally similar to naturally occurring Antimicrobial Peptides (AMP).
The structural similarities between AMP and PHMB suggest that the latter can enter the membranes of bacterial cells and kill bacteria in a similar way to AMP [9].
PHMB is thought to adhere and destroy target cell membranes, causing potassium ions and other cytosolic components to leak, resulting in bacterial cell death [10].
This study aims to compare the antimicrobial activity of two preparations for topical use, one containing 0,1% PHMB, the other containing 1% silver sulfadiazine, against S. aureus, P. aeruginosa, K. pneumoniae, E. coli, and the combination of S. aureus and K. pneumoniae.
MATERIALS AND METHODS
Agar well diffusion assay - Cup-plate method
Each microorganism (S. aureus ATCC 6538 - E. coli ATCC 8739 - P.aeruginosa ATCC 9027 – Microbiologics K. pneumonia ATCC 13883 – Thermo scientifics) was streaked onto TSA 90 mm plates (Tryptic Soy Agar - Merck - prepared according to the manufacturer’s instructions) for 18/24h at 30-35°C. Subsequently, one isolated colony was picked from the plate and suspended in NaCl 0.9% and adjusted to equal the turbidity of 0,5 McFarland standard. This suspension was used to seed a molten Mueller-Hinton Agar medium (Sigma-Adrich - prepared according to the manufacturer’s instructions) stabilized at 45°C (0.1 ml microorganism test suspension/100 ml medium). The obtained cultured medium was poured into 90 mm petri dishes (20 ml of medium/dish) and allowed to solidify. Once the medium was solid, four wells of 9 mm diameter were created with a sterile cork borer and filled with: a topical cream containing 1% silver-sulfadiazine (0,05% of hyaluronic acid sodium salt and 1% of silver sulfadiazine,polyethylene glycol 400 monostearate, oleic acid decyl ester, emulsifying wax, glycerol, 70% sorbitol solution, purified water), a topical cream containing 0,1% PHMB(0,1 % PHMB; Rigenase®; polyethylene glycol 400; polyethylene glycol 1500; polyethylene glycol 3000; polyethylene glycol 4000; stearyl alcohol; cetyl alcohol; paraffin liquid, glycerol; purified water), a topical cream containing 0.1% gentamicin used as a positive control, and a white blank topical cream, without any active ingredients or preservatives, used as negative control. The plates were pre-incubated for 1 h at room temperature to ensure adequate diffusion and finally incubated at 30-35°C for 24 h. The experiments were run in triplicate and the areas of inhibition determined with a caliber and recorded as the mean ± SD (n=3) [11,12].
Statistical analysis for agar well diffusion assay
Results from the agar well diffusion assay are expressed as the means ± the standard deviation of three independent experiments performed in triplicates. Two-way Analysis of Variance (ANOVA) was used to test, by calculation of P-values, for significant antiseptic activity in bacteria treated with 0,1% polyhexanide topical cream compared to 1% silver sulfadiazine and to the negative and positive controls.
RESULTS
In vitro antibacterial activity of 0.1% Polyhexanide vs 1% Silver Sulfadiazine
The in vitro antibacterial response to the creams was performed by measuring and comparing the diameter of the zones of inhibition (mm) against the tested microorganisms. The large zones of inhibition against the tested microorganisms for the 0.1% gentamicin cream, used as a positive control, confirms that the developed method is validated.
aureus
The in vitro agar well diffusion assay showed that the inhibition areas against S. aureus (Gram positive bacterial strain)evaluated for 0.1% PHMB cream, werelarger than the inhibition areas evaluated for 1% Silver Sulfadiazine cream (17 mm and 12.67 mm respectively, Figure 1 & Table 1). The inhibition areas of the positive control were of 28 mm and those of the negative control of 9 mm (Figure 1). The quantitative analysis indicated that the topical cream containing 0.1% PHMB and the positive control have a significantly higher antibacterial activity against S. aureus in respect to both 1% Silver Sulfadiazine cream and to the negative control (p<0.0001, Figure 2). However, Silver sulfadiazine antibacterial activity against S. aureus was significantly higher in respect to the negative control (p<0.0001, Figure 2).
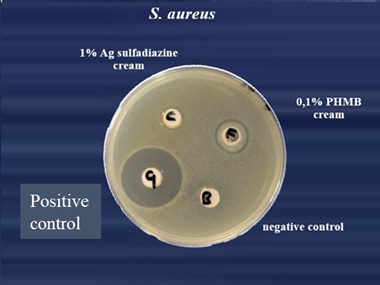 Figure 1: S.aureus.
Figure 1: S.aureus.
|
0,1%PHMB cream vs 1% Silver Sulfadiazine Cream |
||||
|
S.aureus |
||||
|
Product used |
Inhibition area 1 (mm) |
Inhibition area 2 (mm) |
Inhibition area 3 (mm) |
Average |
|
Negative Control |
9 |
9 |
9 |
9 |
|
Positive Control |
26 |
29 |
29 |
28,00 |
|
PHMB 0.1% |
17 |
17 |
17 |
17,00 |
|
Silver Sulfadiazine 1% |
13 |
13 |
12 |
12,67 |
Table 1: S. aureus agar well diffusion assayfor 0.1% PHMB, 1% Silver Sulfadiazine, positive control and negative control.
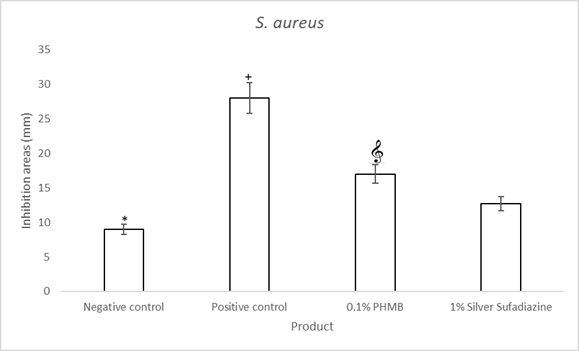 Figure 2: S. aureus agar well diffusion assay quantifications for 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*= p<0.0001 negative control vs each of the others; += p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine; = p<0.0001 0.1% PHMB vs 1% Silver Sulfadiazine).
Figure 2: S. aureus agar well diffusion assay quantifications for 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*= p<0.0001 negative control vs each of the others; += p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine; = p<0.0001 0.1% PHMB vs 1% Silver Sulfadiazine).
coli
In vitro agar well diffusion assay against E. coli(Gram negative bacterial strain) showed that the inhibition areas of 1% silver sulfadiazine wascomparable with those of 0.1% PHMB (Figure 3 & Table 2). The positive control showed an inhibition area of 20.33 mm and the negative control of 9 mm. The quantitative analysis indicated that the topical cream containing 0.1% PHMB and the topical cream containing 1% Silver Sulfadiazine have a significant antibacterial activity against E. coli in respect to the negative control (p<0.0001) but the antibacterial activity against E. coli was lower as compared to that of the positive control (bothp<0,0001, Figure 4).0.1%
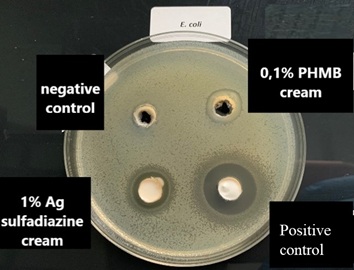 Figure 3: E. coli
Figure 3: E. coli
|
0,1%PHMB cream vs 1% Silver Sulfadiazine Cream |
||||
|
E. coli |
||||
|
Product Used |
Inhibition Area 1 (mm) |
Inhibition Area 2 (mm) |
Inhibition Area 3 (mm) |
Average |
|
Negative Control |
9 |
9 |
9 |
9 |
|
Positive Control |
20 |
20 |
21 |
20,33 |
|
PHMB 0.1% |
14 |
14 |
15 |
14,33 |
|
Silver Sulfadiazine 1% |
13 |
14 |
14 |
13,67 |
Table 2: E. coli agar well diffusion assay for 0.1% PHMB cream, 1% Silver Sulfadiazine cream, Positive control and negative control
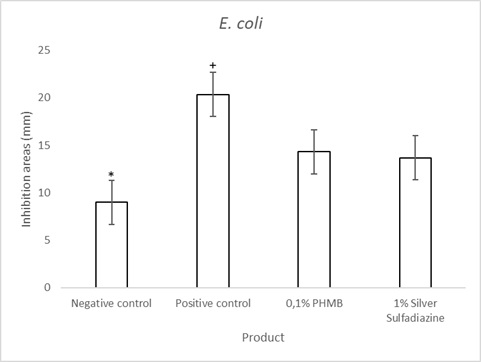 Figure 4: E. coli agar well diffusion assay quantifications of 0.1% PHMB cream, 1% Silver Sulfadiazine cream, Positive control (0.1% Gentamicin cream) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*=p<0.0001negative control vs each of the others; + = p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine).
Figure 4: E. coli agar well diffusion assay quantifications of 0.1% PHMB cream, 1% Silver Sulfadiazine cream, Positive control (0.1% Gentamicin cream) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*=p<0.0001negative control vs each of the others; + = p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine).
aeruginosa
The in vitro agar well diffusion assay for P. aeruginosa(Gram negative bacterial strain) showed that the inhibition areas of 0.1% Polyhexanide (PHMB) topical cream was similar to that of 1% silver sulfadiazine cream (Figure 5 & Table 3). The positive control showed an inhibition area of 30 mm and the negative control of 9 mm (Figure 5). The quantitative analysis of these data indicated that the topical cream containing 0.1% PHMB and that containing 1% of Silver Sulfadiazine have a significant antibacterial activity against P. aeruginosa in respect to the negative control (Figure 6). The positive control has a significantly higher antibacterial activity against P. aeruginosa in respect to both 1% Silver Sulfadiazine and to 1% PHMB (Figure 6).
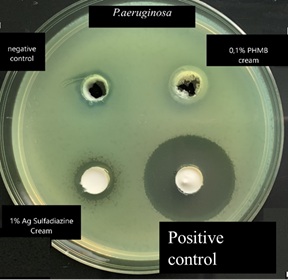 Figure 5: P. aeruginosa.
Figure 5: P. aeruginosa.
|
0,1%PHMB cream vs 1% Silver Sulfadiazine Cream |
||||
|
P. aeruginosa |
||||
|
Product used |
Inhibition Area 1 (mm) |
Inhibition Area 2 (mm) |
Inhibition Area 3 (mm) |
Average |
|
Negative Control |
9 |
9 |
9 |
9 |
|
Positive Control |
29 |
29 |
30 |
29,33 |
|
PHMB 0.1% |
13 |
13 |
14 |
13,33 |
|
Silver Sulfadiazine 1% |
13 |
13 |
13 |
13,00 |
Table 3: P.aeruginosa agar well diffusion assayfor 0.1% PHMB cream, 1% Silver Sulfadiazine cream, Positive control and negative control
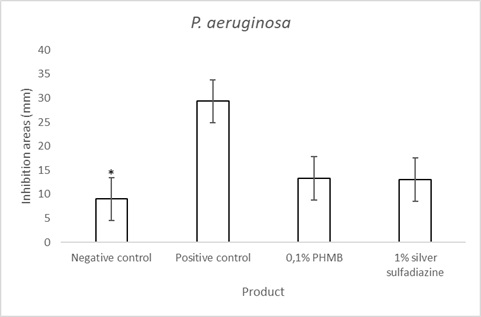 Figure 6: P. aeruginosa agar well diffusion assay quantifications of 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test (* = p<0.0001 negative control vs each of the others; + =p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine).
Figure 6: P. aeruginosa agar well diffusion assay quantifications of 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test (* = p<0.0001 negative control vs each of the others; + =p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine).
pneumonia
The in vitro agar well diffusion assay showed that the inhibition areas against K. pneumoniae(Gram negative bacterial strain)evaluated for 0.1% PHMB cream, were larger than the inhibition areas evaluated for 1% Silver Sulfadiazine cream (16,67 mm and 11,67 mm respectively, Figure 7 & Table 4). The inhibition areas of the positive control were of 25,33 mm and those of the negative control of 9 mm (Figure 7). The quantitative analysis indicated that the topical cream containing 0.1% PHMB and the positive control have a significantly higher antibacterial activity against K. pneumoniae in respect to both 1% Silver Sulfadiazine cream and to the negative control (p<0.0001, Figure 8). However, Silver sulfadiazine antibacterial activity against K. pneumoniae was significantly higher in respect to the negative control (p<0.0001, Figure 8).
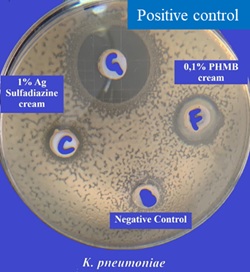 Figure 7: K. pneumoniae
Figure 7: K. pneumoniae
|
0,1%PHMB cream vs 1% Silver Sulfadiazine Cream |
||||
|
K. pneumoniae |
||||
|
Product used |
Inhibition area 1 (mm) |
Inhibition area 2 (mm) |
Inhibition area 3 (mm) |
Average |
|
Negative Control |
9 |
9 |
9 |
9 |
|
Positive Control |
26 |
25 |
25 |
25,33 |
|
0.1%PHMB |
17 |
16 |
17 |
16,67 |
|
1%Silver Sulfadiazine |
11 |
12 |
12 |
11,67 |
Table 4: K. pneumonia agar well diffusion assay for 0.1% PHMB cream, 1% Silver Sulfadiazine cream, Positive control and negative control
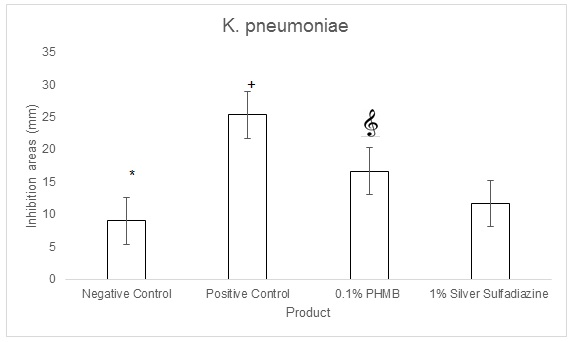 Figure 8: K. pneumoniae agar well diffusion assay quantifications for 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*= p<0.0001 negative control vs each of theothers; += p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine; = p<0.0001 0.1% PHMB vs 1% Silver Sulfadiazine).
Figure 8: K. pneumoniae agar well diffusion assay quantifications for 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*= p<0.0001 negative control vs each of theothers; += p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine; = p<0.0001 0.1% PHMB vs 1% Silver Sulfadiazine).
pneumoniae and S. aureus
The in vitro agar well diffusion assay showed that the inhibition areas against the combination of K. pneumoniae and S. aureus evaluated for 0.1% PHMB cream, were larger than the inhibition areas evaluated for 1% Silver Sulfadiazine cream (17 mm and 13 mm respectively, Table 4). The inhibition areas of the positive control were of 29,67 mm and those of the negative control of 9 mm (Table 4). The quantitative analysis indicated that the topical cream containing 0.1% PHMB and the positive control have a significantly higher antibacterial activity against the combination of K. pneumoniae and S. aureus in respect to both 1% Silver Sulfadiazine cream and to the negative control (p<0.001, Figure 9). However, Silver sulfadiazine antibacterial activity against K. pneumoniae and S. aureus was significantly higher in respect to the negative control (p<0.0001, Figure 9).
|
0,1%PHMB cream vs 1% Silver Sulfadiazine Cream |
||||
|
K. pneumoniae + S. aureus |
||||
|
Product used |
Inhibition area 1 (mm) |
Inhibition area 2 (mm) |
Inhibition area 3 (mm) |
Average |
|
Negative Control |
9 |
9 |
9 |
9 |
|
Positive Control |
30 |
29 |
30 |
29,67 |
|
PHMB 0.1% |
18 |
17 |
16 |
17 |
|
Silver Sulfadiazine 1% |
13 |
12 |
14 |
13 |
Table 5: K. pneumoniae and S. aureus agar well diffusion assay for 0.1% PHMB cream, 1% Silver Sulfadiazine cream, Positive control and negative control
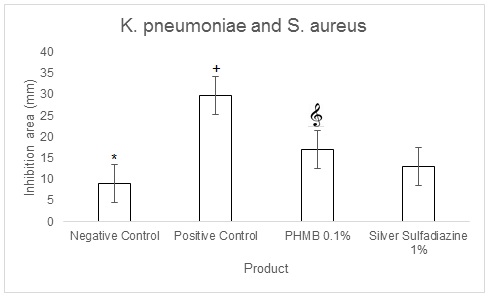
Figure 9: K. pneumoniae and S. aureus agar well diffusion assay quantifications for 0.1% PHMB, 1% Silver Sulfadiazine, Positive control (0.1% Gentamicin) and negative control. Results are the mean ± the standard deviation of three independent experiments. P values were calculated by the two-way ANOVA test. (*= p<0.0001 negative control vs each of the others; += p<0.0001 positive control vs 0.1% PHMB and 1% Silver Sulfadiazine; = p<0.0001 0.1% PHMB vs 1% Silver Sulfadiazine).
DISCUSSION
Bacterial superinfection of skin lesions, especially in case ofdystrophic ulcers, is a complication of no marginal relevancein the natural history of this pathology since it determines a slowdown in the wound healing process and marked discomfort for the patient [13-14].
Topical skin preparations containing 0.1% Polyhexanide and 1% Silver Sulfadiazine are used for the treatment of infected wounds or for wounds that are at risk of infection [1,2]. Even if these preparations are known since many years [15], some aspects related to their specific antimicrobial activity need to be further understood both in vitro and in vivo.
Agar diffusion assay were used for evaluation of antibacterial activity of these two topical skin preparations. This method had already been used for antibacterial activity of gentamicin [11,12].
The antibacterial activity of 0.1% Polyhexanide and of 1% Silver Sulfadiazine was evaluated against four bacterial strains (S. aureus, E. coli, P. aeruginosa and K. pneumoniae) and against the combination of K.pneumoniae a P.aeruginosa. 0.1% Polyhexanide was found to be significantly more effective than 1% Silver Sulfadiazine in its antimicrobial activity against S. aureus and K. pneumoniae. Furthermore, the topical preparation containing 0.1% Polyhexanide was found to be significantly more effective than 1% Silver Sulfadiazine against the combination of S. aureus and K. pneumoniae. Instead, the relative effectiveness of both 0.1% Polyhexanide and 1% Silver Sulfadiazine against E. coli and P. aeruginosa was comparable.
CONCLUSION
The results of the present study indicate that 0.1% Polyhexanide topical preparation is at least as efficacious as 1% Silver Sulfadiazine in the treatment of microbial infections and support the use of 0.1% Polyhexanide topical preparation for the treatment of wounds that are infected or at risk of infection.
REFERENCES
- Duerden BI (1994) Virulence factors in anaerobes. Clin Infect Dis 18: 253-259.
- Brandon M, Van Laar TA, You T, Clegg S, Leung KP (2013) MrkD1P from Klebsiella pneumoniae Strain IA565 Allows for Coexistence with Pseudomonas aeruginosa and Protection from Protease-Mediated Biofilm Detachment. Infect Immun 81: 4112-4120.
- Bowler PG, Duerden BI, Armstrong DG (2001) Wound Microbiology and Associated Approaches to Wound Management, Clinical Microbiology Reviews 14: 244-269.
- Murray K, Mende K, Beckius ML, Akers KS, Romano DR, et al. (2013) Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis 13: 47.
- Petkovšek Z, Eleršic K, Gubina M, Žgur-Bertok D, Erjavec MS (2009) Virulence Potential of Escherichia coli Isolates from Skin and Soft Tissue Infections. J Clin Microbiol 47: 6.
- Atkin L, Bucko Z, Montero EC, Cutting K, Moffatt C, et al. (2019) Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care 23: 1-50.
- Tsala DE, Amadou D, Habtemariam S (2013) Natural wound healing and bioactive natural products. Phytopharmacology 4: 532-560.
- Adhya A, Bain J, Ray O, Hazra A, Adhikari S, et al. (2015) Healing of burn wounds by topical treatment: A randomized controlled comparison between silver sulfadiazine and nano-crystalline silver, J Basic Clin Pharm 6: 29-34.
- Butcher M (2012) PHMB: An effective antimicrobial in wound bioburden management. ritish journal of nursing (Mark Allen Publishing) 21: 18-21.
- Gilliver S. (2009) PHMB: A well-tolerated antiseptic with no reported toxic effects. Jounal of Wound care / Activa health care supplement.
- Rose SB, Miller RE (1939) Studies with the Agar Cup-Plate Method I. A Standardized Agar Cup-Plate Technique. J Bacteriol 38: 525-537.
- Johnson T, Case C (1995) Chemical Methods of Control," adapted from Laboratory Experiments in Microbiology, Brief Edition, 4th ed. Redwood City, CA, USA.
- Swartz MN (1999) Cellulitis and superficial infections. In: Mandel GL, Douglas RG, Bennet JE (eds.). Principles and practice of infectious diseases. John Wiley & Sons Inc., New York, USA. Page no: 598-608.
- Rode HRA et al. (1993) Surgical skin and soft tissue infections. Curr Opin Infect 6: 683-690.
- Gray D, Barrett S, Battacharyya M, Butcher M, Enoch S, et al. (2010) PHMB and its potential contribution to wound management. Wounds uk 6: 2.
Citation: Schiavo G, Falciglia D, Maurelli S, Riccio S, Maglione B (2020) In vitro Evaluation of the Antimicrobial Activity of a Topical Skin Preparation Containing 0.1% Polyhexanide vs a Topical Skin Prepa-ration Containing 1% Silver Sulfadiazine. J Clin Dermatol Ther 6: 055.
Copyright: © 2020 Giulia Schiavo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

