
Indications, Access and Techniques for Pericardiocentesis in South-South Nigeria
*Corresponding Author(s):
Emmanuel Auchi EdafeDepartment Of Medicine, University Of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria
Email:dremmanueledafe@gmail.com
Abstract
Introduction: Pericardiocentesis may be an emergency procedure to relieve pericardial effusion with Tamponade. The fluid from this procedure is examined and sent for further investigation which may guide the line of management and improve patient outcome. With the advancement of expertise in echocardiography assisted procedures, echocardiography-guided diagnostic and therapeutic pericardiocentesis is now considered standard clinical practice in the treatment of pericardial effusions.
Objectives: the aim to this study was to discuss the indications, access and techniques for pericardiocentesis in southern Nigeria.
Methods: This is a retrospective observational study done at the Emergency and Medical wards of the University of Port Harcourt Teaching Hospital, Emergency wards of Bayelsa Specialist Hospital, Yenagoa and Eternal heart hospital, Port Harcourt. We reviewed hospitals record files of 24 patients admitted from January 2017 to November 2021 who underwent pericardiocentesis.
Results: Results: Out of 24 patients, 13 (54.2%) were male. The mean age of the study population was 59.5±17.2 years. The subcostal/subxiphoid access was used in 24 patients. Apical and Parasternal accesses were not used in the 24 patients. Sanguineous fluid was found in 20 patients while 4 had serous fluid. Local anesthesia with 2% Lidocaine was used for 23 patients. Successful pericardiocentesis was performed in 24 patients with no complication noted.
Conclusion: The most common cause of pericardial effusion requiring pericardiocentesis wasTuberculosis. Dyspnea was the most common symptom of presentation with cardiac tamponade. Echocardiography was the most commonly used imaging modality for pericardiocentesis while the Bedside setting was the most common setting used for pericardiocentesis. Imaging guided pericardiocentesis has a very high success and very low complication rate.
Keywords
Indications; Management; Pericardiocentesis
Introduction
Pericardiocentesis is the most useful therapeutic procedure for the early management or diagnosis of large, symptomatic pericardial effusion and cardiac tamponade [1,2]. This procedure was first performed by Riolanus, et al. for cardiac tamponade [2]. He described the process of trephination of the sternum to remove the abnormally accumulated fluid from the pericardial space [2]. Blind pericardiocentesis without any imaging assistance was used in the past, but that failed to gain wide support due to high morbidity, life-threatening complications more than 20%, and mortality approaching 6% [3]. Echocardiography-guided pericardiocentesis both diagnostic and therapeutic is now considered the ideal clinical practice for the management of pericardial effusions [3].
Pericardiocentesis can be done without harm in hemodynamically stable patients in an outpatient setting. Pericardiocentesis can be performed quickly by an expert cardiologist in hemodynamically unstable patients to relieve symptoms of cardiac tamponade [4].
In medium to large-sized pericardial effusions, echocardiography-assisted pericardiocentesis has high success rates of >95% with very little risk. The morbidity rate is approximately 1-3% and the mortality rate associated directly with the procedure is less than 1% [4].
In this study, we discussed the indications, access and techniques for pericardiocentesis in southern Nigeria.
Methods
- Study population
This is a retrospective study done at Bayelsa Specialist Hospital and the Eternal heart Hospital, Port Harcourt. Bayelsa Specialist hospital is located in Yenagoa and is fully equipped with modern cardiac catherization laboratory. The Eternal heart hospital is located in Port Harcourt.
This study was approved by the ethical committees of the two hospitals. We reviewed hospital record files of consecutive 24 patients, admitted from 1st January 2017 to 30thNovember 2021 who underwent pericardiocentesis.
The patient information recorded included;
- Demographic information including age, gender, and
- Symptoms on presentation was recorded after reviewing written medical records
- Comorbidities eg tabulated including diabetes mellitus, hypertension
- Congestive heart failure, cardiomyopathy, and valvular heart disease
- Echocardiographic data were reviewed as follow
- Size of pericardial effusion
- Distribution of pericardial effusion
- Eechocardiographic signs of tamponade, and
- Left ventricular systolic function
The size of the pericardial effusion was graded as small (<10 mm), medium (10-20 mm), and large (more than 20 mm) on bases of largest echo-free space, when viewed from the transthoracic echocardiographic window.
Etiology of pericardial effusion was established based on clinical reasoning, lab reports, echocardiography report, and the results of the pericardial fluid analysis.
Procedure
After informed consent, pericardiocentesis was performed by a trained cardiologist under standard aseptic measures and local anesthesia with 2% Lidocaine. Details of procedure including access site, anesthesia, imaging modality, and complications were recorded. Procedure success was defined as successful drainage pericardial fluids with the placement of a pericardial drain if required.
- In-hospital monitoring
All patients were followed from the time of the procedure to the time of discharge. Hemodynamic parameters including heart rate and blood pressure were recorded at the time of procedure and post-procedure till the time of discharge. Complications related to pericardiocentesis were recorded.
Approaches
- Fluoroscopy-guided technique
The fluoroscopic approach was the first imaging system used for percutaneous pericardiocentesis. It is performed through the subxiphoid approach with a needle containing a contrast medium, directed towards the left shoulder at an angle of 30° to the skin. Needle position in the pericardial space is confirmed by the contrast agent medium injection: the inferior position appearance of a sluggish layering of the contrast medium indicates the correct position and that a soft J-tip guide wire can be introduced. It is essential to check the guidewire position in at least two angiographic projections (lateral view and anterior-posterior view) [4].
- Echo-guided technique
Echocardiography-guided pericardiocentesis is a safe and simple technique, introduced at the Mayo Clinic in 1979 and widely used nowadays [5]. The echocardiography-guided approach allows defining the position of the effusion, the ideal entry site and needle trajectory for pericardiocentesis. There are two different approaches to echo guidance: the first (described by the Mayo Clinic) is the echo-assisted method, in which the operator memorizes the optimal needle trajectory and advances the needle towards the pericardial space without a continuous ultrasound visualization [5]. The second approach is the echo-guided method with a continuous echocardiographic monitoring. It has also been proposed to use a needle carrier mounted on the ultrasound transducer to advance the needle to the pericardial space [6].
Echocardiography-guided pericardiocentesis can be performed in the following sequences.
Step 1: Check clinical indications and medical history, such as taking an antiplatelet or anti-coagulation drugs, and get a consent form.
Step 2: Positioning and echocardiographic imaging: the patient could be positioned in the semi-fowler position in bed. Then perform 2D echocardiographic imaging at the apical view, subxiphoid view, and left para-sternal view. These views help in gaining insite to the effusion size.
Step 3: Determination of puncture site: Choose sites with the biggest echo-free space for safe needle entry. This may be marked with pen.
Step 4: Preparation of puncture site: Gather all required tools e.g. Pericardiocentesis kit, on the table. Then perform the sterile skin preparation. Cover the patient with a surgical drape and screened the eye away from the operation site to reduce tension and anxiety. Perform sufficient local anesthesia at the puncture site along the site of needle entry.
Step 5: Pericardiocentesis: Perform a preliminary exploration with the local anesthetic needle (21 gauge) to confirm the direction of the needle approach and to feel the nature of the effusion. This will guide the entry of the puncture needle. Put the puncture needle (18 gauge) to the tip of the syringe and gently insert and advance the puncture needle from the skin of the puncture site toward the heart at an angle of 30 degree. The patient may be asked to hold breath if he can. The puncture needle must maintain the direction that was determined during the preliminary exploratory test. As the needle advances, feel the “pop” moment at the puncture and observe negatively drained fluid in the syringe as it enters the pericardial space filled with fluid.
Stop the advancing puncture needle and hold the needle with your fingers to prevent further advancement. Insert the J-tip guidewire through the puncture needle. Into the pericardial space. Remove the puncture needle and advance the dilator, and the pigtail or double lumen catheter or sheath. Remove the guidewire, leaving the catheterinsitu and connect to drain. Suture the catheter on the skin. The figure 1a-1g showed pericardiocentesis.
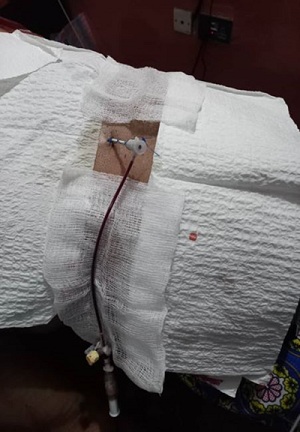 Figure 1a: Pericardial drain with a sheath.
Figure 1a: Pericardial drain with a sheath.
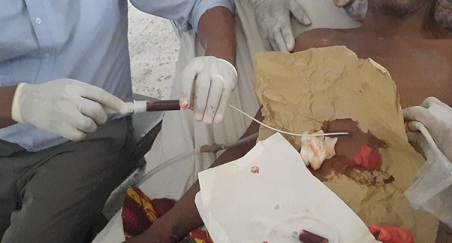 Figure 1b: Pericardiocentesis with pigtail catheter.
Figure 1b: Pericardiocentesis with pigtail catheter.
 Figure 1c: during the drainage procedure.
Figure 1c: during the drainage procedure.
 Figure d: drainage fluid.
Figure d: drainage fluid.
 Figure 1e: Fluid drained in a measuring cylinder after pericardiocentesis.
Figure 1e: Fluid drained in a measuring cylinder after pericardiocentesis.
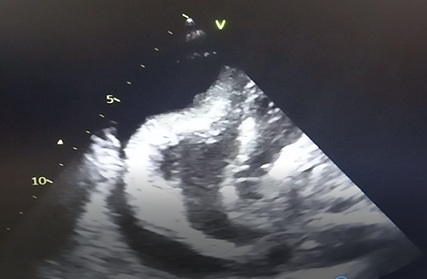 Figure 1f: pericardial fliud before drainage
Figure 1f: pericardial fliud before drainage
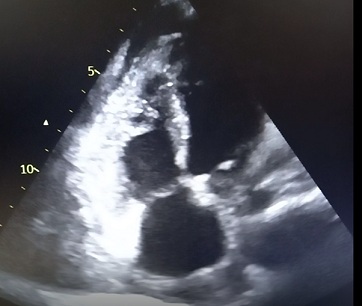 Figure 1g: Post pericardiocentesis.
Figure 1g: Post pericardiocentesis.
- Data analysis
Statistical analyses was performed using the Statistical Package for Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY). Continuous variables were expressed as mean value ± standard deviation and categorical variables were expressed as frequencies and percentages.
Results
Data was retrieved from the folders of the 24 patients. Thirteen (54.2%) were male, and the mean age of the study population was 59.5±17.2 years. Table 1 showed the demographic profile.
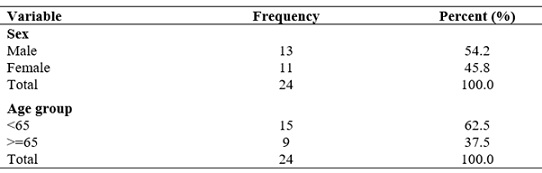 Table 1: Demographic Profile.
Table 1: Demographic Profile.
Note: with mean age at 59.5±17.2 years.
The patients age ranged from 9 to 90 years. The subcostal/subxiphoid access was used in 24 patients. Apical and Parasternal accesses were not used in the 24 patients. Sanguineous fluid was found in 20 patients while 4 had serous fluid. Local anesthesia with 2% Lidocaine was used for 23 patients. Echocardiography-guided account for 22 patients and fluoroscopy with echocardiography was used for 2 patients. Pigtail catheters were used for 20 patients and femoral sheaths were used for 4 patients. Table 2 showed the clinical profile of the variables and their frequencies.
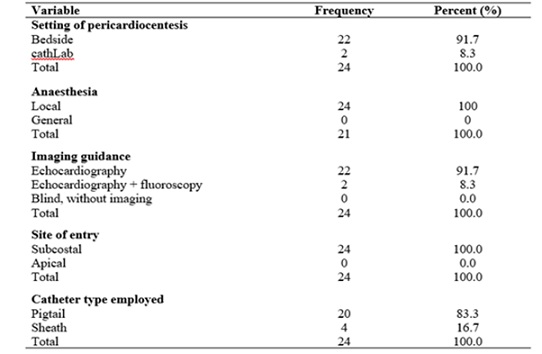 Table 2: Clinical profile.
Table 2: Clinical profile.
Tuberculosis was the commonest etiology from these patients. Twelve patients confirmed with tuberculosis were GeneXpert test positive. Two patients had connective tissue disease (Systemic Lupus Erythematosus). Figure 2 showed the distribution of results of laboratory analyses of pericardial fluid. Figure 3 showed the various etiologies of massive pericardial effusion. Successful pericardiocentesis was performed in the 24 patients with no complication noted.
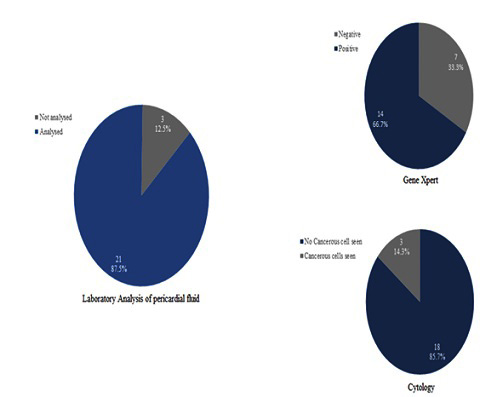 Figure 2: Distribution of results of laboratory analyses of pericardial fluid.
Figure 2: Distribution of results of laboratory analyses of pericardial fluid.
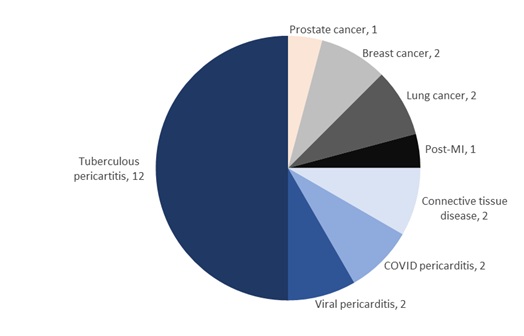 Figure 3: Etiology of massive pericardial effusion/cardiac tamponade (n = 24).
Figure 3: Etiology of massive pericardial effusion/cardiac tamponade (n = 24).
Discussion
Pericardiocentesis is a therapeutic procedure for management and diagnosis of severe, symptomatic pericardial effusion with impending cardiac tamponade [7]. Pericardiocentesis is the aspiration of pericardial fluid from the pericardial space [8]. Marfan in 1911,first described the subxiphoid approach, which had been used for the blind pericardiocentesis procedure for decades [2]. Thepericardiocentesis procedure is a lifesaving procedure though it may be associated with complications. In our study, most of the patients had cardiac tamponade and severe pericardial effusion with echocardiography measurement of pericardial space more 20 millimeters. Many different image approaches are now available with the aim of reducing complications [9].
Indications
The indication could be an emergency or non-emergency pericardiocentesis. Emergency pericardiocentesis refers to the presence of life-threatening hemodynamic changes in a patient with suspected cardiac tamponade [10]. Non-emergency pericardiocentesis refers to the aspiration of pericardial fluid in hemodynamically stable patients for the purpose of diagnostic, palliative, or prophylactic reasons, performed under ultrasonography, computerized tomography or fluoroscopic visualization [11,12]. In our study emergency pericardiocentesis predominant [13].
Pericardiocentesis is the treatment of choice in patients with cardiac tamponade. A scoring index has been recently proposed in patients with a suspicion of tamponade for deciding whether to perform urgent pericardiocentesis ordrainage later. It comprises of three components obtained at initial presentation: etiology, clinical presentation, and echocardiographic findings. Cardiac Tamponade is the most important indication, representing over 80-90% of the procedures [6,17,14].In cases of pericardial effusion without hemodynamic compromise, pericardiocentesis may be indicated if it is a large pericardial effusion (>20 mm one chocardiography), persistent(more than 3 months) and nonresponsive to medical therapy [14].
Contraindications
There are no absolute contraindications to pericardial drainage when cardiac tamponade and shock occur [14]. Aortic dissection and post infarction rupture of the free wall are contraindications to needle pericardiocentesis and indications for urgent surgical drainage. Relative contraindications include uncorrected coagulopathy, anticoagulant therapy, and thrombocytopenia with platelets less than < 50 000/mm [3,14].
Complications of pericardiocentesis.
Pericardiocentesis is an invasive procedure that carries potential risks. Major complications reported in the largest series (0.3-3.9% cases), include death, laceration of cardiac chambers, and coronary arteries or intercostals vessels, puncture of abdominal viscera or peritoneal cavity, pneumothorax requiring chest tube placement, pneumopericardium, severe bacteremia, ventricular arrhythmias, and pericardial decompression syndrome [15]. Our study had none of these complications probably because the procedure was echocardiography guided.Minor complications reported in 0.4-20% cases includetransient vasovagal hypotension and bradycardia, supraventricular arrhythmias, pneumothorax without hemodynamic instability, and pleuro-pericardial fistulas [15].
The most serious complications of pericardiocentesis are lacerations of the myocardium and the coronary vessels. These are life-threatening conditions that are difficult to address, especially in hospitals without cardiac surgery backup [16-18]. The choice of different imaging assistance is focused particularly on the reduction of these life-threatening complications [19]. Many other complications reported which may include rhythm disturbance or vasovagal reaction are the result of the ‘pericardiocentesis procedure’ and donot depend on the technique used [15-17].
The entry site must be chosen very carefully as well, as some iatrogenic injury might occur to surrounding structures. The subxiphoid approach may have a higher risk of injury to the diaphragm, phrenic nerve, liver, colon, stomach, and superior vena cava, whereas with the apical or parasternal approach the injury might occur in the lung, in the mammary internal artery, or in the vessels at the inferior margin of the ribs [15]. Pericardiocentesis window selection could be based on imaging guidance: the optimal and safest entry site is the point where the largest collection of fluid lies in closest proximity to the transducer and where echocardiography confirms that no structure is interposing in the path that the needle will take [20]. Puncture of the cardiac chambers with the needle can be solved in most cases with the needle retraction and the insertion of the catheter into the pericardium [21-23].
If the perforation is carried out while introducing the catheter, it must beleft in place and a new pericardiocentesis must be carried out by placing another catheter in the pericardium before removing the previous one, then the perforating catheter can be withdrawn [23]. If the controlled drainage resolves the tamponade, also with eventual autotrans fusion of pericardial blood, surgery can be avoided [24]. This is possible in the case of puncture of ventricular chambers. The puncture of right atrium is more troubling due to athin and poorly contractible myocardium and poses a greater risk for persistent bleeding after needle injury when compared with the ventricles. In the case of laceration of an arterial vessel, recourse to cardiac surgery is more probable [18,19,25].
Pericardiocentesis accesses and Techniques
Pericardial fluid drainage could be carried using either surgical drainage or percutaneous techniques. Surgical drainage was the first approach to deal with pericardial effusion and it remains the gold standard for pericardial drainage if biopsy is required [6,15]. Two common surgical techniques exist for pericardial window surgery (the subxiphoid and thoracotomy approach) [15]. The subxiphoidaccess involves an incision under the sternal xiphoid process, whereas for the thoracotomy approach the chest is entered through a fifth intercostal space anterior access. pericardial window surgery via subxiphoid incision is associated with less postoperative pain and faster time to extubating, whereas the thoracotomy approach may be more effective in preventing effusion recurrence and may reduce the need to repeat surgery [26]. These techniques are equivalent in terms of postoperative and long-termmortality rate, with survival more dependent on preexisting conditions rather than on the type of incision used [27]. Surgical drainage is associated with a higher complication rate and greater length of hospitalization, while the rate of recurrences seems to remains higher with percutaneous techniques [28,29]. The disadvantages of the open surgical procedure are longer hospital stay, higher rate of complications, need for general anesthesia with the risk of sudden hypotension in patients with tamponade or pretamponade [15]. Such disadvantages make this method secondary, to be performed only when the pericardial effusion cannot be reached with a needle, when pericardial effusion recurs despite previous effective pericardiocentesis when a biopsy is required [6,15].
The introduction of the extended drainage, the recurrences after percutaneous pericardiocentesis have been significantly reduced, especially in neoplastic and connective tissue disease effusions [8,15-17].
Techniques for pericardiocentesis
There are many techniques to drain the pericardial fluid. These include
- Fluoroscopic technique: Fluoroscopic pericardiocentesis is performed only through the subxiphoid approach. The pericardium is punctured with a needle containing a contrast medium, which is slowly advanced toward the heart shadow and the epicardial halo phenomenon (the epicardial halodelineates the heart shadow in fluoroscopy and seems to be a highly sensitive sign for the detection of apericardial effusion) [15].
The needle passes through the diaphragm and enters the pericardial sac. This position is confirmed by the contrast agent medium injection [16]. The appearance of a sluggish layer of the contrast medium inferiorly indicates that the needle is positioned correctly and that a soft J-tip guide wire can be introduced. It is essential to check the position of the guide wire in at least two angiographic projections. The disadvantages of this technique is that it can only be performed in heart catheterization laboratories and it has significant exposure to radiation for both the patient and the physician [28]. This technique is very useful for iatrogenic cardiac tamponade during percutaneous procedures. It should be noted that an echocardiographic examination to assess the distribution and amount of pericardial effusion should always precede the procedure, if possible.
- Echo-guided technique: This technique was introduced by Mayo clinic and is widely in use till today. The echocardiography guided approach allows defining the position of the effusion, the ideal entry site and needle trajectory for pericardiocentesis. The proper site for needle insertion corresponds to the area where the pericardial space is closest to the probe and the fluid accumulation is maximal. This site is para-apical more often than subcostal, which requires a longer path to reach the fluid. Echo-guided was the predominant technique is the commonest method used in our patients [17,27].
There are two different approaches to the echo-guidance and include echo-assisted method and echo-guided method [6]. In the echo-assisted method, an operator memorizes the optimal needle trajectory and advances the needle toward the pericardial space without a continuous ultrasound visualization; this is the approach commonly used in clinical practice. In the echo-guided method with a continuous echocardiographic monitoring, a needle carrier mounted on the ultrasound transducer to advance the needle under continuous visualization [6].
- Computer tomography-guided technique: Through the planning CT scan, the full extension of the pericardial effusion is evaluated, and the optimal entrance point is defined and marked on the skin. Then the needle is advanced into the pericardial effusion and a single CT scan is used for verification of the needle position. After the pericardium is punctured, a guide wire is introduced through the needle, and then as heath is slightly advanced [29].
This method could be used in patients with poor ultrasound window. The CT-guided approach has the theoretical advantage that the density of the intrapericardial effusion can be measured through CT. Highly viscous effusions, such as purulent ones and intrapericardial hematoma, an approach with intrapericardial application of fibrinolytic therapy to dissolve the effusion can be planned before the beginning of the procedure.
Access for pericardiocentesis
There are mainly three needle entry accesses for draining pericardial fluid. These include the left parasternal, subxiphoid, and left apical. Traditionally, a subcostal approach has been preferred, largely because it was considered the safest route without image guidance. However, pericardial effusion is not always circumferential and equally distributed. Hence an ultrasound evaluation of the ideal entry site for drainage is fundamental forthe procedural success.
- Subxhiphoid/subcostal access: Here the needle insertion site is between the xiphisternum and left costal margin. Once beneath the cartilage cage, the needle should be lowered to a 15-30 degree angle with the abdominal wall and directed toward the left shoulder. A steeper angle may enter the peritoneal cavity and a medial direction increases the risk of right atrial puncture. This approach carries a lower risk of pneumothorax.
- Apical access site: The apical insertion site is 1 cm lateral to the apex beat within the fifth, sixth, or seventh inter costal space. This approach is at risk of ventricular puncture, due to the proximity to the left ventricle. However, as the wall is thicker than that of the right atrium and ventricle, it ismore likely to self-seal.15 The disadvantages include ventricular and pneumothorax. Using echo cardiographic guidance, because ultrasound does not penetrate air, avoidance of the lung is normally ensured.
- Parasternal access site: The needle insertion site is in the fifth left intercostals space, close to the sternal margin. The needle should bead vanced perpendicular to the skin (at the level of the cardiac notch of the left lung) [14-17]. This approach carries a high risk of pneumothorax and puncture of the internal thoracic vessels if the needle isinserted >1 cm laterally. Echocardiographic guidance also with phase array probe provides a good visualization of pericardial structure.
Conclusion
Tuberculosis was the most common cause of pericardial effusion requiring pericardiocentesis while dyspnea was the most common symptom of presentation with cardiac tamponade. Bedside echocardiography is beneficial imaging modality for pericardiocentesis. Imaging guided pericardiocentesis has a very high success and very low complication rate.
References
- Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, et al. (2015) European Society of Cardiology (ESC). 2015 ESC guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36: 2921-2964.
- Krikorian JG, Mancock EW (1978) Pericardiocentesis. Am J Med 65: 808-814.
- Ainsworth CD, Salehian O (2011) Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 123: 0-1.
- Maisch B, Ristic AD, Seferovic PM, Tsang TS (2011) Interventional Pericardiology: Pericardiocentesis, Pericardioscopy, Pericardial Biopsy, Balloon Pericardiotomy and Intrapericardial Therapy. Heidelberg: Springer Medizin Verlag.
- Tsang TS, Freeman WK, Sinak LJ, Seward JB (1998) Echocardiographically-guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc 73: 647-52.
- Maggiolini S, Gentile G, Farina A, De Carlini C, Lenatti L, et al. (2016) Safety, Efficacy, and Complications of Pericardiocentesis by Real-Time Echo-Monitored Procedure. Am J Cardiol 117: 1369-1374.
- Loukas M, Walters A, Boon JM, Welch TP, Meiring JH, et al. (2012) Pericardiocentesis: a clinical anatomy review. Clin Anat 25: 872-881.
- Cruz I, Stuart B, Caldeira D, Morgado G, Gomes AC, et al. (2015) Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: Experience of a centre without cardiothoracic surgery. Eur Heart J Acute Cardiovasc Care 4: 124-128.
- Krikorian JG, Mancock EW (1978) Pericardiocentesis. Am J Med 65: 808-814
- Reichman E, Simon R (2004) Pericardiocentesis. Emergency Medicine Procedures. McGraw Hill; USA Page no: 204-216.
- Klein SV, Afridi H, Agarwal D, Coughlin BF, Schielke LH (2005) CT directed diagnostic and therapeutic pericardiocentesis: 8-year experience at a single institution. Emerg Radiol 11: 353-363.
- Callahan JA, Seward JB (1997) Pericardiocentesis Guided by Two-Dimensional Echocardiography. Echocardiography 14: 497-504.
- Balmain S, Hawkins NM, MacDonald MR, Dunn FG, Petrie MC (2008) Pericardiocentesis practice in the United Kingdom. Int J Clin Pract 62: 1515-1519.
- Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, et al. (2015) 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36: 2921-2964.
- Maggiolinia S, De Carlinia CC, Imazio M (2018) Evolution of the pericardiocentesis technique. J Cardiovasc Med 19: 267-273.
- Eichler K, Zangos S, Thalhammer A, Jacobi V, Walcher F, et al. (2010) CT-guided pericardiocenteses: clinical profile, practice patterns and clinical outcome. Eur J Radiol 75: 28-31.
- Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, et al. (2002) Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo ClinProc 77:429-436.
- Tsang TS, El-Najdawi EK, Seward JB, Hagler DJ, Freeman WK, et al. (1998) Percutaneous echocardiographically guided pericardiocentesis in pediatric patients: evaluation of safety and efficacy. J Am Soc Echocardiogr 11: 1072-1077.
- Vayre F, Lardoux H, Pezzano M, Bourdarias JP, Dubourg O (2000) Subxiphoid pericardiocentesis guided by contrast two-dimensional echocardiography in cardiac tamponade: experience of 110 consecutive patients. Eur J Echocardiogr 1: 66-71.
- Akyuz S, Zengin A, Arugaslan E, Yazici S, Onuk T, et al. (2015) Echo-guided pericardiocentesis in patients with clinically significant pericardial effusion. Outcomes over a 10-year period. Herz 40:153-159.
- Lindenberg M, Kjellberg M, Karlsson E, Wranne B (2003) Pericardiocentesis guided by 2-D echocardiography: the method of choice for treatment of pericardial effusion. J Intern Med 253: 411-417.
- Duvernoy O, Borowiec J, Helmius G, Erikson U (1992) Complications of percutaneous pericardiocentesis under fluoroscopic guidance. Acta Radiol 33: 309-313.
- Maisch B, Ristic´ AD, Seferovic´ PM, Pankuweit S (2011) Pericardioscopy and epi- and pericardial biopsy - a new window to the heart improving etiological diagnoses and permitting targeted intrapericardial therapy. Heart Fail Rev 18: 317-328.
- Seferovic´ PM, Ristic´ AD, Imazio M, Maksimovic R, Simeunovic D, et al. (2006) Management strategies in pericardial emergencies. Herz 31: 891-900.
- Kumar R, Sinha A, Lin MJ, Uchino R, Butryn T, et al. (2015) Complications of pericardiocentesis: A clinical synopsis. Int J Crit Ill Inj Sci 5: 206-212.
- Langdon SE, Seery K, Kulik A (2016) Contemporary outcomes after pericardial window surgery: impact of operative technique. J Cardiothorac Surg 11: 73.
- Naunheim KS, Kesler KA, Fiore AC, Turrentine M, Hammell LM, et al. (1991) Pericardial drainage: subxiphoid vs. transthoracic approach. Eur J Cardiothorac Surg 5: 99-103.
- Saltzman AJ, Paz YE, Garvey Rene A, Green P, Hassanin A, et al. (2012) Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J Invasive Cardiol 24: 590-593.
- Horr S, Mentias A, Houghtaling P, Toth AJ, Blackstone EH, et al. (2017) Comparison of Outcomes of Pericardiocentesis Versus Surgical Pericardial Window in Patients Requiring Drainage of Pericardial Effusions. Am J Cardiol 120: 883-890.
Citation: Edafe EA, Ocheli E, Dodiyi-Manuel ST (2023) Indications, Access and Techniques for Pericardiocentesis in South-South Nigeria. J Angiol Vasc Surg 8: 102.
Copyright: © 2023 Emmanuel Auchi Edafe, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

