
Infections in Relation to Peritoneal Dialysis: An 11-Year Single Center Experience in Vestfold County, Norway
*Corresponding Author(s):
Sadollah AbediniVestfold Hospital Trust, Medical Clinic, Department Of Nephrology, Toensberg, Norway
Tel:+47 33342000,
Email:sadollah.abedini@siv.no
Abstract
Introduction
The use of Peritoneal Dialysis (PD) in Norway has been limited compared to other western countries. PD is considered a good alternative to hemodialysis and the method is cost-effective. Infections related to PD are still the most frequent complication and often limits the extent of its use.
Methods
We identified all patients receiving PD-catheters in the district County Hospital of Vestfold during 11 years. All information was collected retrospectively through examination of patient charts and data from the local PD complication registry.
Results
65% were male (n=66) with median age 67 years (18-94). Cause of end-stage renal failure were hypertension 27%, diabetic nephropathy 16%, glomerulonephritis 23% and others 35%.
In total, 89 patients were initiated on PD. 2% of the patients developed postoperative infections. Exit site infections occurred in 43 (43%) patients. Staphylococcus aureus was the major cause of both exit site and peritoneal infections. The incidence of peritonitis was 0.65 episodes per patient year. Age older than 75 years was not associated with higher risk of infection. Female gender carries a higher peritonitis risk in multivariate analysis.
69 (69%) patients discontinued PD: 30 (30%) received renal to kidney transplants, 14 (14%) due to PD related infections, 8 due to non-infectious complications, 9 (9%) patients died during PD and 7 patients were discontinued PD due to terminal disease.
Conclusion
In our experience, the occurrence of PD related infections are low. Infections were however the reason for conversion to hemodialysis in some patients. In our population, PD is a safe bridge to kidney transplantation. We observed no excess risk of infection among the elderly and PD can be considered a safe treatment option. Female gender carries higher risk for peritonitis.
Keywords
Excite site infection; Peritoneal dialysis; Peritonitis; Renal replacement therapy; Tunnel infection
INTRODUCTION
The prevalence of Chronic Kidney Disease (CKD) in Norway is 10.2%. CKD is defined as GFR <90ml/min per 1,73m2 and albuminuria [1]. In Norway, the most common causes of End-Stage Renal Disease (ESRD) requiring Renal Replacement Therapy (RRT), is due to hypertension 27%, diabetes mellitus 17%, polycystic kidney disease 10%, immunological or systemic disease 10% and glomerulonephritis 8% [2]. By the end of 2018, 5256 patients received RRT (986.5 per million inhabitants) in Norway. Of these, 348 (6.6%) patients received Peritoneal Dialysis (PD), 1284 (24.4%) Hemodialysis (HD) and 3624 (68.9%) (Received Kidney Transplantation (RTx)). Median age was 62 years, mean 59.9 years ranging from 0.9-96.4 years. The RRT population were predominately male (64.5%) [2].
PD is proposed as an optimal treatment modality in about one third of all patients requiring dialysis [1]. However, the proportion of patients receiving PD or HD differs between countries and different regions in the same country. Variation in the provided dialysis modality are multifactorial and not fully understood [3-5].
Patients on RRT have increased morbidity and mortality, regardless of the type of RRT; comorbidity due to diabetes or hyper- or hypotension have a worse outcome. Residual renal function is associated with better outcome [6].
PD elaborated in the late 1800’s and was performed on human for the first time in 1923. The first indwelling Tenckhoff catheter was used in 1968 for PD access. Continuous Ambulatory PD (CAPD) was taken into clinical use in 1978 [6-8]. For successful PD the patient has to meet several requirements, however inflammatory bowel disease, active Clostridium difficile infection, unrepaired hernias and liver failure with ascites are the most important contraindications for PD [9].
PD utilization had its peak in the US between 1982 and 1985, but has decreased during the last decades [10,11]. There are several studies comparing HD to PD, some suggesting PD to be inferior to HD [1,6,10]. However, many of these studies are poorly designed. Several studies worldwide suggest better survival with PD compared to HD, and PD is considered a safe and patient friendly treatment [6].
The overall risk of death is highest during the first years of RRT [12]. More than 50% of deaths in both PD and HD are due to cardiovascular events [6]. Some studies suggest that RRT initiated with PD is more likely to have a better graft survival in patients undergoing Kidney Transplantation (KTx) later on [11,13]. Utilization of PD differs between countries. The worldwide prevalence of PD is 11% [3]. Countries such as Mexico, Canada, New Zealand, Australia and Hong Kong use PD at a wider extent [1,6]. As in other western countries with low PD utilization, PD is only used in 6.6% of patients on RRT in Norway [2,14].
There are several reasons for variation in PD utilization between countries. These relate to economical/ reimbursement issues and lack of expertise training and knowledge [10,11,14,15]. However PD-related infections, or rather the fear of them, is thought to be the main reason for low use of PD [1,10,14]. It is important to emphasize that infections are more common in HD, with septicemia as the most important condition, due to catheter-infection and increased mortality [14].
The most common complication of PD are infections limited to the peritoneal cavity [10,16]. Type of infection is defined in relation to the anatomical site; exit-site infection, tunnel-infection and peritonitis [17]. They can occur independently, or in combination.
An exit-site infection can occur as both an acute and a chronic infection and sometimes lasting several weeks despite sufficient treatment. Treatment is based on microbiological findings and consist of local or systemic antibiotics. A tunnel infection is treated with broad-specter Intravenous (IV) / Intraperitoneal (IP) antibiotic until microbiological findings. Peritonitis as the most important infectious complication, is suspected when the patient present with fever and abdominal pain, opalescent dialysate, or a combination of these. It requires IV and IP antimicrobial treatment for several weeks [17,18]. If the external cuff is extruded one should consider cuff-shaving to prevent recurrent infections [19].
There are specific indications for catheter removal, which lead to a pause or termination in PD [18]. The rare and serious complication of Encapsulating Peritoneal Sclerosis (EPS) is feared, but is not thought to be related to peritonitis [11,20]. After one episode of peritonitis there is an increased risk of death following the first month, then the risk is reduced during the next half year [6,11]. Peritonitis is the most common cause of terminating PD-treatment [14].
Risk factors for peritonitis in PD was assessed in a large Canadian study from 2009 including more than 4000 patients. Age, black race, conversion from HD to PD, diabetes in women and poor glycemic control is associated with increased risk and shorter time to first peritonitis [6,21]. Previous peritonitis is described as a risk factor for future peritonitis [17]. Different types of PD modalities Continuous Ambulatory Peritoneal Dialysis (CAPD) and Automated Peritoneal Dialysis (APD) carry the same risk of developing peritonitis [21].
The rate of peritonitis has decreased worldwide during the last 10-20 years, but the rates varies both between countries and between centers in the same country. This is largely due to different practices in training of staff, use of prophylactic antibiotics and varieties in guidelines and the different use of them [6].
We report our experience with PD related infections from a single center study during 11 years of follow-up in Vestfold County (Inhabitant aprox. 245.000) in Norway. Our primary objective was to assess the occurrence of PD related exit site infection and peritonitis. Secondary we wanted to evaluate how important individual patient characteristics influence the occurrence of PD related infections.
MATERIALS AND METHODS
All patients in Norway with ESRD are evaluated for RRT. KTx (RTx) is the preferred modality of RRT and dialysis is offered without limitations, if otherwise not contraindicated. Choosing the type of dialysis modality (PD or HD) is based on a shared decision between the patient and the care provider. Age per se is not decisive for providing RRT. The patient’s comorbidities, health related problems and personal values are the most important variables in choosing between active RRT and maximal care without RRT. The final decision is made after the patient has received information and education in all aspects of the different modalities in RRT.
This single center study was conducted through retrospective examination of patient charts and data from the local PD complication registry. All patients with ESRD receiving PD-catheters in the county hospital of Vestfold during an 11 year period was included. Standard Tenckhoff catheter was used in all patients. Preoperative antibiotic prophylaxis was not used routinely. The operating surgeon was not a designated PD-surgeon. None of the patients received catheters due to the acute need of PD.
In order to identify risk factors for peritonitis, a multivariate Cox regression was performed and the model was adjusted for clinically relevant factors such as age, gender, occurrence of exit site infection, diabetes mellitus, previous cardiovascular/cerebrovascular disease, body weight, smoking and if the PD was assisted by nurse at home or other institutions.
RESULTS
101 of the patients with ESRD received PD-catheters during the study period. There was a median delay of 1 month (1-38) before PD was started. Males were predominant 65 % (n=66), with median age of 67 years (18-94). Although fewer, the women tend to be younger, with a median age of 58 years (18-89). ESRD was most commonly caused by hypertension 27%, diabetic nephropathy 16% and glomerulonephritis 23%.
Few patients had complications associated with PD catheter insertion and only 2.0% (n=2) developed postoperative infections. In total, 9 of the patients (9%) did not start PD-treatment; 2 (2%) received kidney transplants pre-dialytic, 2% (n=) removed the catheter due to infection, 1(1%) required HD before PD could be initiated, 1 (1%) died and 3(3%) of the patients were not yet initiated in PD. 89% (n=89) patients were started on PD and the median time for active PD was 7.0 months (1-30 months).
Exit site infections
Infections occurred in 43% (n=43) of the patients. Of these, 23% (n=23) had one episode, 12% (n=12) had two episodes and 9% (n=9) had three or >3 episodes. The incidence of exit site infections for the entire cohort is 0.75 episodes per patient year (Figure 1).
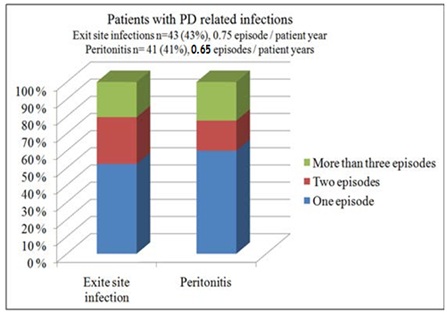 Figure 1: Rate of PD-related infections.
Figure 1: Rate of PD-related infections.
Peritonitis
41 patients (41%) developed peritonitis; 24 (24%) experienced one, 7 (7%) had two and the rest of the patients 28 (28%) had 3 or >3 episodes. The incidence of peritonitis was 0.65 episodes per patient year (Figure 1).
PD discontinuation
69% (n=69) of the patients discontinued PD. 30% (n=30) received kidney transplants, 14% (n=14) due to PD related infections, 8% (n=8) due to non-infectious complications, 9% (n=9) patients died during PD and 7% (n=7) discontinued PD due to terminal disease (Figure 2).
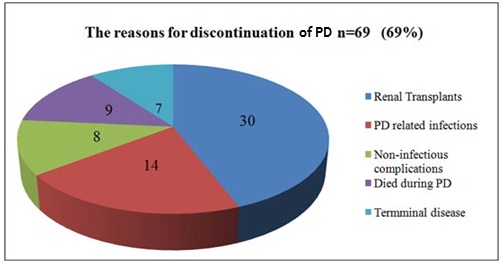 Figure 2: Reasons for discontinuation of PD.
Figure 2: Reasons for discontinuation of PD.
Cause of infections
Staphylococcus aureus was the cause of both exit site and peritonitis in the majority of the patients.
Patients older than 75 years of age had no significant higher rate of infection compared with younger age groups (log rank test p=NS). Occurrence of both exit site infection and peritonitis was higher in female (Figure 3). Female gender was associated with significantly higher risk of peritonitis in both univariate (Figure 4) and multivariate analysis (HR 0.25, CI: 0.70-0.87) (p<0.03).
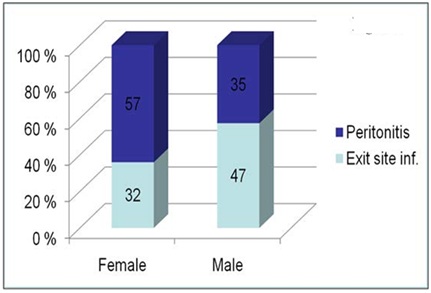 Figure 3: Rate of PD related infections with regard to gender.
Figure 3: Rate of PD related infections with regard to gender.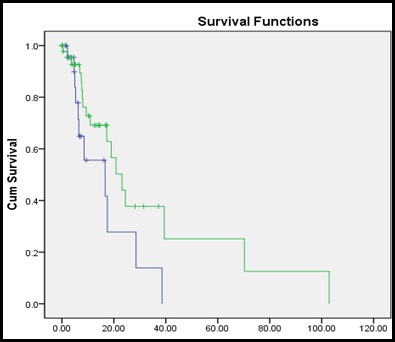
Time
Figure 4: Univariate analysis, Kaplan Mayer curve. Female gender was associated with significantly higher risk of peritonitis in both univariate.
Note: ------- Female; -------- Male.
DISCUSSION
The population of ESRD patients is large and growing [22]. With an increase in metabolic diseases, including DM and HT, an increasing number of patients will converge towards ESRD. Also, a larger part of the population is getting older with increased risk of developing CKD and some may need RRT [23]. In our experience, PD is a good treatment option as RRT and the occurrence of PD-related infections are low and similar to that of HD, even with self-care PD and luckily the general rate is decreasing. This is seen in many recent cohorts despite the fact that medical outcome data would seem to favor more utilization of PD [6,14]. For instance, data from the United States Renal Data Systems suggests that the relative risk of death for patients on PD versus center hemodialysis (CHD).
HD carries a higher risk of infections in some studies [6]. However, infections were the reason for conversion to HD in a few patients in this study. In our population, PD is a safe treatment option in both individuals awaiting kidney transplantation and in elderly individuals, with no excess infection rate. The economical aspect that receives great attention in the USA [24], is less important in Norway, where the funding of health care services is covered by the health authorities and all treatments are free of charge for the patient including RRT disregarding the modality [25].
In this cohort, female gender was associated with higher rate of peritonitis and may need more attention. The results from our study is comparable to the large multicenter study done in Canada in 2009 [21].
CONCLUSION
In our experience PD is a safe treatment option regardless of age. Infections related to PD occurred in acceptable rate in our population. Infections were however the reason for conversion to haemodialysis in few patients. PD is a safe bridge to kidney transplantation and PD is safe treatment option in elderly individuals with no excess infection rate. Female patients might carry a higher risk of infection.
REFERENCES
- Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, et al. (2006) International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275-2284.
- NRR (2018) The Norwegian Renal Registry. Annual report.
- Machowska A, Rutherford P (2016) Peritoneal dialysis use within the context of the population and healthcare systems of Europe-differences, trends and future challenges. Int J Artif Organs 39: 211-219.
- Perl J, Davies SJ, Lambie M, Pisoni RL, McCullough K, et al. (2016) The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): Unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int 36: 297-307.
- van de Luijtgaarden MW, Jager KJ, Segelmark M, Pascual J, Collart F, et al. (2016) Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant 31: 120-128.
- Mehrotra R, Devuyst O, Davies SJ, Johnson DW (2016) The current state of peritoneal dialysis. J Am Soc Nephrol 27: 3238-3252.
- Tenckhoff H, Schechter H (1968) A bacteriologically safe peritoneal access device. Trans Am Soc Artif Intern Organs 14: 181-187.
- Oreopoulos DG, Robson M, Izatt S, Clayton S, deVeber GA (1978) A simple and safe technique for Continuous Ambulatory Peritoneal Dialysis (CAPD). Trans Am Soc Artif Intern Organs 24: 484-489.
- Al-Natour M, Thompson D (2016) Peritoneal Dialysis. Semin Intervent Radiol 33: 3-5.
- Novak MJ, Sheth H, Bender FH, Fried L, Piraino B (2008) Improvement in Pittsburgh symptom score index after initiation of peritoneal dialysis. Adv Perit Dial 24: 46-50.
- Chaudhary K, Sangha H, Khanna R (2011) Peritoneal dialysis first: Rationale. Clin J Am Soc Nephrol 6: 447-456.
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, et al. (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725-1730.
- Burkart JM (2001) Peritoneal dialysis should be considered as the first line of renal replacement therapy for most ESRD patients. Blood Purif 19: 179-184.
- Burkart J (2009) The future of peritoneal dialysis in the United States: Optimizing its use. Clin J Am Soc Nephrol 1: 125-131.
- Klarenbach S, Manns B (2009) Economic evaluation of dialysis therapies. Semin Nephrol 29: 524-532.
- Davenport A (2009) Peritonitis remains the major clinical complication of peritoneal dialysis: The London, UK, peritonitis audit 2002-2003. Perit Dial Int 29: 297-302.
- Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, et al. (2016) ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int 36: 481-508.
- Szeto CC, Li PK, Johnson DW, Bernardini J, Dong J, et al. (2017) ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int 37: 141-154.
- Debowski JA, Waerp C, Kjellevold SA, Abedini S (2017) Cuff extrusion in peritoneal dialysis: Single-centre experience with the cuff-shaving procedure in five patients over a 4-year period. Clin Kidney J 10: 131-134.
- Kawanishi H, Moriishi M (2005) Epidemiology of encapsulating peritoneal sclerosis in Japan. Perit Dial Int 4: 14-18.
- Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV (2009) Predictors of peritonitis in patients on peritoneal dialysis: Results of a large, prospective Canadian database. Clin J Am Soc Nephrol 4: 1195-1200.
- Sennfalt K, Magnusson M, Carlsson P (2002) Comparison of hemodialysis and peritoneal dialysis--a cost-utility analysis. Perit Dial Int 22: 39-47.
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, et al. (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382: 260-272.
- Takemoto Y, Naganuma T (2019) Economic issues of chronic kidney disease and end-stage renal disease. Contrib Nephrol 198: 87-93.
- The Norwegian Health Care System by Anne Karin Lindahl, Norwegian Knowledge Centre for the Health Services.
Citation: Bjune T, Kjellevold SA, Abedini S (2020) Infections in Relation to Peritoneal Dialysis: An 11-Year Single Center Experience in Vestfold County, Norway. J Nephrol Renal Ther 6: 029.
Copyright: © 2020 Thea Bjune, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

