
Infertile Women with Diminished Ovarian Reserve have more Live Births Following Dehydroepiandrosterone Pre-Treatment
*Corresponding Author(s):
Siddhartha Chaterjee MBBS, DGO, DNB, FRCOG, FICOGDepartment Of Biochemistry And Endocrinology, Institute Of Post Graduate Medical Education & Research, Calcutta Fertility Mission, Kolkata, India
Tel:+91 9830387875,
Email:sidchat54@gmail.com
Abstract
Dehydroepiandrosterone (DHEA) has been proposed to improve ovulatory response in patients with Diminished Ovarian Reserve (DOR). The study was undertaken to find validity of the above fact, both for Timed Intercourse (TI) following OI and in IVF procedure.
Methods
596 women aged between 25 and 42 years with DOR were detected by Ovarian Reserve Test (ORT). 551 of them were subjected to DHEA pre-treatment for 90 days followed by OI with Clomiphene Citrate (CC) and Gonadotrophin (Gn). 223 patients with DOR were subjected to IVF program. 186 of them received DHEA pre-treatment and 37 of them did not accept it. The analysis was performed using the statistical software R.
Result
Clinical Pregnancy (CP) and Live Birth (LB) following IVF (33.3% & 25.7% respectively) is almost 3 times more than TI group (12.8% & 9% respectively), when all groups are taken together. However, in cases of advancing age, chance of getting TI pregnancy was much less than IVF pregnancy, as found from Odds Ratio (OR).
Conclusion
DHEA is found to be effective in achieving spontaneous or IVF pregnancy in patients with poor ovarian reserve. IVF offers more live births in elderly women.
Keywords
ABBREVIATIONS
DHEA: Dehydroepiandrosterone
DOR: Diminished Ovarian Reserve
TI: Timed Intercourse
CC: Clomiphene Citrate
Gn: Gonadotrophin
CP: Clinical Pregnancy
LB: Live Birth
AFC: Antral Follicular Count
TVS: Transvaginal Ultrasonography
GCs: Granulosa Cells
OI: Ovulation Induction
PR: Pregnancy Rate
ORT: Ovarian Reserve Test
FET: Frozen Embryo transfer
IM: Intramuscular
SC: Subcutaneous
COS: Controlled Ovarian Stimulation
OPU: Ovum Pick Up
LBR: Live Birth Rate
CPR: Clinical Pregnancy Rate
FOR: Functional Ovarian Reserve
TOR: Total Ovarian Reserve
INTRODUCTION
DHEA is one such molecule used in anti-aging treatment, has also proved to be beneficial to ovarian function. DHEA works in initial 60 days of folliculogenesis, starting from antral follicles which are non-responsive to Ovulation-Inducing (OI) agents. Although the mechanism of such benefits is not clearly understood till date, its promising pro-fertility action has been noted to improve both spontaneous and IVF Pregnancy Rates (PRs) clinically, in women having DOR [5]. Casson et al., [6] was the first to suggest that DHEA supplementation might improve some aspects of female ovarian functions, having DOR. The main idea came from a woman of advanced reproductive age, who underwent remarkable gains in her ovarian function, due to the effect of Insulin-like Growth Factor (IGF-1), after self-medication with DHEA [7]. It has also been observed that in IVF performed in patients with DOR, supplementation of DHEA improves response to ovarian stimulation with Gns, resulting in the increase of oocyte yield and embryo numbers [7,8]. The effect of DHEA picks at 3-4 months of treatment, a time span similar to complete follicular recruitment cycle, and this showed increase in follicular recruitment due to suppression of apoptosis [8,9]. Approximately 80% of spontaneous pregnancy losses result from chromosomal abnormalities [10], where aneuploidy elevates the rate of miscarriage [11,12]. Supplementation of DHEA reduces aneuploidy and miscarriage, thereby increasing the chances of LB in patients with DOR [13].
The primary end point of the study was to compare the CP and Live Birth Rate (LBR) between DHEA pre-treated and non-treated patients, after OI and after IVF treatment. The secondary end points were miscarriage rates, age-related PR and comparison between the success rates of IVF and conceiving spontaneously with OI at TI.
MATERIALS AND METHODS
Selection of patients
Inclusion criteria
• FSH value >12 mIU/ml, AMH value <1.8 ng/ml and AFC <5
• Subjected to TI initially for 3 cycles only after OI or single attempt of IVF with COS and antagonist protocol
Exclusion criteria
• Normal FSH and normal AMH value, AFC >5 and with any other endocrine defect like hypothyroidism and hyperprolactinemia
Poor Ovarian Response (POR) can be obtained mostly in stimulated cycle like IVF irrespective of ovarian reserve. POR requires at least one cycle of stimulation to detect it and may not recur in next cycle.It is commonly observed in advanced maternal age, abnormal ovarian reserve test and in cases of previous POR [14,15]. In this case patients with DOR were only included in the study. DOR is commonly observed in women withany of the risk factors for POR and/or an abnormal ovarian reserve test (i.e., Antral Follicular Count (AFC) <5-7 follicles or AMH <0.5-1.1 ng/ml). But the hypothesis requires validation [16].
Study Design
Sample size
Consent
Ethical approval
Treatment protocol
Hormonal measurements
STATISTICAL ANALYSIS
RESULTS
|
Pre-treatment |
Cases |
CP/CPR |
LB/LBR |
Miscarr. |
Cases |
CP/CPR |
LB/LBR |
Miscarr. |
|
|
TI |
IVF |
||||||
|
NIL |
45 |
2 (4.44%) |
1 (2.2%) |
1 (2.2%) |
37 |
3 (8.01%) |
2 (5.3%) |
1 (2.8%) |
|
DHEA |
551 |
73 (12.9%) |
63 (9.8%) |
20 (3.4%) |
186 |
52 (33.3%) |
48 (25.7%) |
14 |
Table 2 presents age stratified difference in CPR and LBR in TI and IVF group. It shows that there was no statistically significant difference between CPR and LBR among age group 25-30 years and above 40 years (as per p-value). However, women of 30-40 years age group show statistically significant difference in both. Table 3shows comparison of Odds Ratio (OR) of CP and Live Born (LB) as a whole in TI and IVF group. It was found that the OR was about 3 times more in IVF as compared to TI group in both CPR and LBR. When this age stratified OR for CP and LB in both TI and IVF group (Table 4) were plotted in chart, a significant uniform decrease in both CPR and LBR (Figure1) was found with advancing age, and LBR decreased rapidly in TI group as compared to IVF group (Figure 2).The miscarriage rate was more in IVF group as compared to TI group (7.3 % in IVF group versus 3.4% of TI group), even after DHEA pre-treatment.
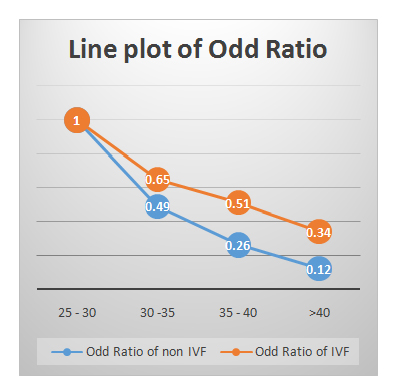 Figure 1: Line plot of OR of CP in TI &IVF groups (age-stratified).
Figure 1: Line plot of OR of CP in TI &IVF groups (age-stratified).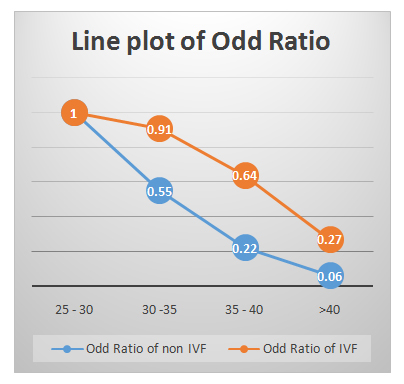 Figure 2: Line plot of OR of LB in TI &IVF groups (age-stratified).
Figure 2: Line plot of OR of LB in TI &IVF groups (age-stratified).|
|
TI |
IVF |
|
|
||||
|
Ages |
Cases |
CP |
LB |
Cases |
CP |
LB |
P value 1 |
P value 2 |
|
25-30 |
114 |
29 |
22 |
22 |
10 |
7 |
0.078 |
0.237 |
|
30-35 |
181 |
26 |
21 |
74 |
26 |
22 |
0.001 |
0.002 |
|
35-40 |
181 |
15 |
9 |
74 |
22 |
17 |
0.000 |
0.000 |
|
>40 |
75 |
3 |
1 |
18 |
4 |
2 |
0.070 |
0.194 |
|
|
Cases |
CP |
Odd Ratio |
Cases |
LB |
Odd Ratio |
|
TI |
551 |
73 |
1.00 |
551 |
53 |
1 |
|
IVF |
186 |
62 |
3.27 (2.21,4.84) |
186 |
48 |
3.27 (2.11,5.04) |
|
|
TI |
IVF |
TI |
IVF |
||||||
|
Ages |
Cases |
CP |
Odd Ratio 1 |
Cases |
CP |
Odd Ratio 1A |
LB |
Odd Ratio 2 |
LB |
Odd Ratio 2A |
|
25 - 30 |
114 |
29 |
1.00 |
22 |
10 |
1.00 |
22 |
1.00 |
7 |
1.00 |
|
30 -35 |
181 |
26 |
0.49 |
74 |
26 |
0.65 |
21 |
0.55 |
22 |
0.91 |
|
35 - 40 |
181 |
15 |
0.26 |
74 |
22 |
0.51 |
9 |
0.22 |
17 |
0.64 |
|
>40 |
75 |
3 |
0.12 |
18 |
4 |
0.34 |
1 |
0.06 |
2 |
0.27 |
DISCUSSION
Dehydroepiandrosterone (DHEA) supplementation is being used by many IVF centers around the world in poor ovarian responders despite the lack of convincing data. About 25% of IVF programs use DHEA currently but large randomized prospective trials are needed and hence the present study [5]. In a study by DE Ikhena et al., early follicular phase serum DHEAS levels were assessed in addition to markers of ovarian reserve (FSH, AMH, E2) in cycles of non-PCOS women (n=53) undergoing IVF. An inverse correlation was observed and this relationship was independent of age, BMI and smoking status (b coefficient -0.01, p=0.03). No relationship was seen between serum DHEAS levels and AMH nor with IVF cycle response or outcome [30]. In our study it has been found that FSH and AMH levels do not change significantly after DHEA pre-treatment; but the follicular development improves and more follicles are recruited after COS. This observation is similar to the study by Sonmezer M et al., which showed increased number of >17mm follicles and oocytes after DHEA supplementation [31].
It has been shown in the study that TI increases the CPR and LBR following OI following DHEA pre-treatment, as compared to no pre-treatment group. The CPR & LBR in IVF group were also found to be more after DHEA pre-treatment, as compared to TI group. More successful pregnancy occurred between 32-35 years and 35-40 years women both for TI and IVF group. But the PR (both CPR and LBR) was 3 times more in IVF group as compared to TI group after DHEA pre-treatment, as seen in statistical analysis (odds ratio). Poor PR in women less than 30 years and more than 40 years might be age related or other factors like genetic factors for DOR. When odds ratio was line plotted during statistical analysis, it was observed that with advancing age, chances of conception diminish both in TI and IVF group but this was profound in TI group, indicating that it is better to offer IVF treatment in patients with advanced age as soon as possible. High miscarriage rate may be due to disturbed endometrial receptivity following COS and can decrease following DHEA supplementation according to previous studies [32]. But in our study the miscarriage rate was more in IVF group as compared to TI group (7.3% in IVF group versus 3.4% of TI group), even after DHEA pre-treatment.
CONCLUSION
AUTHOR CONTRIBUTIONS
ACKNOWLEDGMENT
CONFLICT OF INTEREST
REFERENCES
- Liu K, Case A, Reproductive Endocrinology and Infertility Committee (2001) Advanced reproductive age and fertility. J Obstet Gynaecol Can 33: 1165-1175.
- Lukaszuk K, Ludwikowska B, Liss J, Kunicki M, Sawczak M, et al. (2014) Decreasing quality of the new generations of anti-Müllerian hormone assays. Biomed Res Int 2014: 165352.
- Themmen AP (2005) Anti-Müllerian hormone: Its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr: 18-21.
- Gleicher N, Weghofer A, Barad DH (2011) The role of androgens in follicle maturation and ovulation induction: Friend or foe of infertility treatment? Reprod Biol Endocrinol 17: 116.
- Fouany MR, Sharara FI (2013) Is there a role for DHEA supplementation in women with diminished ovarian reserve? J Assist Reprod Genet 30: 1239-1244.
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE (2000) Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: A case series. Hum Reprod 15: 2129-2132.
- Barad DH, Gleicher N (2005) Increased oocyte production after treatment with Dehydroepiandrosterone. Fertil Steril 84: 756.
- Barad D, Gleicher N (2006) Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod 21: 2845-2949.
- Barad D, Brill H, Gleicher N (2007) Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet 24: 629-634.
- Morales C, Sánchez A, Bruguera J, Margarit E, Borrell A, et al. (2008) Cytogenetic study of spontaneous abortions using semi-direct analysis of chorionic villi samples detects the broadest spectrum of chromosome abnormalities. Am J Med Genet A 146A: 66-70.
- te Velde ER, Pearson PL (2002) The variability of female reproductive ageing. Hum Reprod Update 8: 141-154.
- Pal L, Santoro N (2003) Age-related decline in fertility.Endocrinol Metab Clin North Am 32: 669-688.
- Gleicher N, Weghofer A, Barad DH (2010) Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: Direct evidence from Preimplantation Genetic Screening (PGS). Reprod Biol Endocrinol 8: 140.
- Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, et al. (2011) ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum Reprod 26: 1616-1624.
- Frattarelli JL, Hill MJ, McWilliams GD, Miller KA, Bergh PA, et al. (2008) A luteal estradiol protocol for expected poor-responders improves embryo number and quality. Fertil Steril 89: 1118-1122.
- Cohen J, Chabbert-Buffet N, Darai E (2015) Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J Assist Reprod Genet 32: 1709-1712.
- de Carvalho BR, Sobrinho DBG, Vieira ADD, Resende MPS, Barbosa ACP, et al. (2012) Ovarian Reserve Assessment for Infertility Investigation. ISRN Obstet Gynecol 2012: 576385.
- Practice Committee of the American Society for Reproductive Medicine (2015) Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil Steril 103: 9-17.
- Ubaldi F, Vaiarelli A, D’Anna R, Rienzi L (2014) Management of Poor Responders in IVF: Is There Anything New? BioMed Research International 352098: 10.
- Badawy A, Wageah A, El Gharib M, Osman EE (2011) Prediction and Diagnosis of Poor Ovarian Response: The Dilemma. J Reprod Infertil 12: 241-248.
- Oehninger S (2011) Poor responders in In Vitro Fertilization (IVF) therapy: The challenge continues. Facts Views Vis Obgyn 3: 101-108.
- Yan Y, Gong Z, Zhang L, Li Y, Li X, et al. (2013) Association of follicle-stimulating hormone receptor polymorphisms with ovarian response in Chinese women: a prospective clinical study. PLoS ONE 8: 78138.
- Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, et al. (2009) Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online 18: 509-515.
- Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L (2011) Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci 18: 892-899.
- Stadtmauer L, Vidali A, Lindheim Sr (1998) Follicular Fluid Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-Binding Protein-1 and -3 Vary as a Function of Ovarian Reserve and Ovarian Stimulation. J Assist Reprod Genet 15: 587-593.
- Paul S, Pramanick K, Kundu S, Kumar D, Mukherjee D (2010) Regulation of ovarian steroidogenesis in vitro by IGF-I and insulin in common carp, Cyprinus carpio: Stimulation of aromatase activity and P450arom gene expression. Mol Cell Endocrinol 315: 95-103.
- Martinez F, Barri PN, Coroleu B, Tur R, Sorsa-Leslie T, et al. (2002) Women with poor response to IVF have lowered circulating Gonadotrophin Surge-Attenuating Factor (GnSAF) bioactivity during spontaneous and stimulated cycles. Hum Reprod 17: 634-640.
- Messinis IE, Messini CI, Dafopoulos K (2014) Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online 28: 714-722.
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, et al. (2010) Addition of Dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: A randomized prospective study. Human Reprod 25: 2496-2500.
- Ikhena DE, Kallen A, Kodaman PH, Pal L (2013) Day 3 FSH is inversely correlated with serum DHEAS level in patients undergoing IVF. Fertil Steril 100: 508.
- Sönmezer M, Ozmen B, Cil AP, Ozkavukçu S, Ta?çi T, et al. (2009) Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reprod Biomed Online 19: 508-513.
- Gleicher N, Ryan E, Weghofer A, Blanco-Mejia S, Barad DH, et al. (2009) Miscarriage rates after Dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: A case control study. Reprod Biol Endocrinol 7: 108.
Citation: Chatterjee S, Chaudhuri R, Chowdhury RG, Datta A, Bishista B (2019) Infertile Women with Diminished Ovarian Reserve have more Live Births Following Dehydroepiandrosterone Pre-Treatment. J Reprod Med Gynecol Obstet 4: 020.
Copyright: © 2019 Rajib Gon Chowdhury, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

