
Influence of Abiotic and Biotic Factors on the Population Dynamics of Diplonychus indicus Venk.&Rao (Hemiptera: Belostomatidae) a Potent Biocontrol Agent for Mosquito Larva
*Corresponding Author(s):
Samuel TennysonDepartment Of Zoology, Madras Christian College, Tamil Nadu, India
Tel:+91 9884116135,
Email:samtennyson@gmail.com
Abstract
Study of aquatic insect population has assumed importance in the context of pollution to water bodies and the potentialities of many aquatic bugs in controlling vector population especially Culex mosquitoes. The population dynamics of Diplonychus indicus was studied in a perennial pond in Kanyakumari district, Tamil Nadu, Southern India for a period of eleven months from February 2018 to December 2018. The study yielded eleven aquatic species, viz., Agriocnemis species, Anisops bouvieri, Culex species, Diplonychus indicus, Dytiscus marginalis, Gerris spinolae, Laccotrephes griseus, Lethocerus indicus, Limnogonus nitidus, Mesogomphus lineatus and Ranatra filiformis. Abiotic factors like temperature, pH, dissolved oxygen and rainfall were studied and the correlation between the abiotic factors and Diplonychus indicus nymphs and adults showed positive correlation except for temperature. Correlation between the nymphal population and adults of Diplonychus indicus and all the coexisting insects exhibited a perfect positive correlation and between Culex larvae and Diplonychus indicus it was +0.6797. Further, field value ratios of Diplonychusindicus and biotic factors especially with Culex during the south west monsoon indicated the fact that Culex larval population was under control.
Keywords
Abiotic, Biotic factors, Diplonychus indicus
INTRODUCTION
The study of aquatic insect population has assumed importance in the context of pollution to water bodies and the potentialities of many aquatic bugs in controlling the vector population especially Culex mosquitoes. The ecology and distribution of aquatic insects are highly governed by the type of habitat they inhabit. One of the most fascinating characteristics of the aquatic insect population is their diverse pattern of distribution coupled with their adaptability. Some naucorid bugs inhabit lakes and prefer streams [1], while belostomatid bugs remain in clusters clinging to the rootlets of floating vegetation such as Eichhornia [2]. The aquatic insects are sufficiently flexible to withstand often severe and sometimes unpredictable environments. Hence, the co-existence of nymphal stages and adults of aquatic insects with reference to dispersal pattern as well as spatial temporal changes in their abundance are very essential to assess the effect of comparable parameters on species growth and development. The rate of development of a species in a habitat is dependent on various environmental factors. Some aquatic insects spend their immature life in water bodies. One of the vital factors that govern the population dynamics of aquatic insects is the substratum upon which the drama of their ecology is acted out. The population dynamics of Diplonychus indicus is governed in time by the rate of association to substratum, development of immature stages, and the prevalence of cannibalistic behavior among the immature stages and the impact of hydrological and climatic factors [3]. The present work was undertaken to study the influence of abiotic and biotic factors on the population dynamics of Diplonychusindicus, a biocontrol agent for the management of mosquitoes which is of great importance and the need of the hour as they transmit vector-borne diseases to man especially Culex since these larvae forms the food for this bug.
MATERIALS AND METHODS
A permanent pond situated 25kms away from Kanyakumari district, Tamil Nadu, India was selected as the study area. The pond was rectangular with an area of 1.5 hectares and an average depth of 0.9 meters. It had only one inlet and depends on rain water besides the inlet from the river Pazhayar. The pond has rich vegetation of floating and submerged plants on the northern side and devoid of vegetation on the southern side, where there is a bathing ghat. Habitat sampling of insects were made during the early hours of the day since many aquatic insects migrate to deeper waters during late hours of the day. Weekly collections of aquatic insects were carried for 11months (February 2018-December 2018). The insects were collected by filtering 40 litres of water with the help of a pond net and were identified with taxonomic keys. Vegetation during the collection were quantified, and the immature and adult stages of aquatic insects were collected, categorized, recorded and released back into the pond. Insects clinging to the filtered vegetation were also carefully collected. The environmental parameters of the pond, viz., dissolved oxygen (mg/L), pH, air temperature (°C), water temperature(°C), relative humidity (%) and wind speed (km/h) were also recorded simultaneously on a weekly basis. Rainfall (mm) was chosen as the vital meteorological factor in the region of the study area. A correlation and regression analysis was established between Diplonychus indicus and the biotic factors in the pond.
RESULTS
The study of the population dynamics of the perennial pond yielded eleven aquatic species,viz., Agriocnemis species, Anisops bouvieri, Culex species, Diplonychus indicus, Dytiscus marginalis, Gerris spinolae, Laccotrephes griseus, Lethocerus indicus, Limnogonus nitidus, Mesogomphus lineatus and Ranatra filiformis. The environmental parameters of the study area are shown in figure1. Dissolved oxygen, pH, air temperature, water temperature, relative humidity and wind speed values ranged from 7.8 to 8.4mg/L, 7.5 to 8.2, 29.8 to 32.1°C, 25.3 to 26.0°C, 63.0 to 79.0% and 12.0 to 19.5km/h respectively. Rainfall was maximum during September (10.7mm) when the study area experienced heavy south west monsoon. The relationship between abiotic factors and the nymphs as well as adults of Diplonychus indicus are shown in figures 2 & 3. With regard to the biotic factors, there existed a correlation with the nymphal population and adults of Diplonychus indicus and all the coexisting species, and it was found that there was a perfect positive correlation between Diplonychus indicus and its prey Culex larvae (+0.6797) (Figure 4).The field value ratios of Diplonychus indicus nymphs and biotic factors are presented in figure 5 and for adults in figure 6. The results also revealed a relative abundance of Diplonychus indicus adults and Culex (Figure 7) and the regression analysis between Diplonychus indicus and Culex exhibited a positive relationship (Figure 8).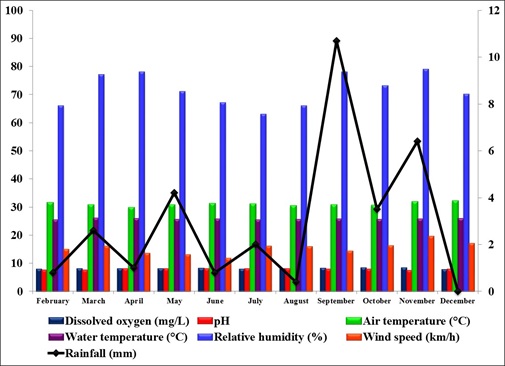
Figure 1: Environmental parameters recorded in the study area.
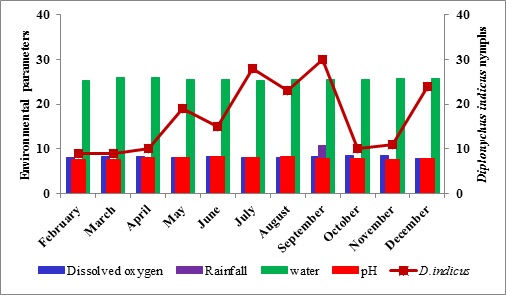 Figure 2: Relationship between abiotic factors and Diplonychus indicus nymphs.
Figure 2: Relationship between abiotic factors and Diplonychus indicus nymphs.
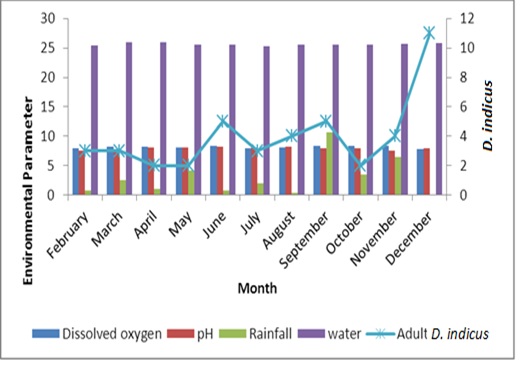 Figure 3: Relationship between abiotic factors and Diplonychus indicus adults.
Figure 3: Relationship between abiotic factors and Diplonychus indicus adults.
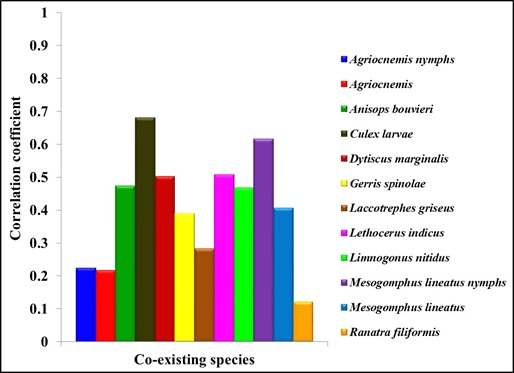 Figure 4: Correlation between biotic factors and Diplonychus indicus.
Figure 4: Correlation between biotic factors and Diplonychus indicus.
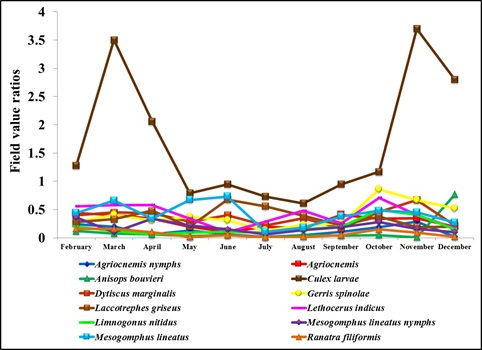 Figure 5: Field value ratios of Diplonychus indicus nymphs and biotic factors.
Figure 5: Field value ratios of Diplonychus indicus nymphs and biotic factors.
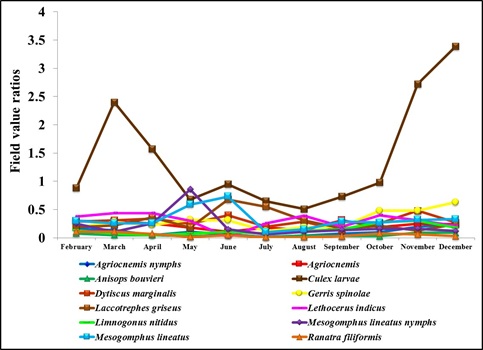 Figure 6: Field value ratios of Diplonychus indicus adults and biotic factors.
Figure 6: Field value ratios of Diplonychus indicus adults and biotic factors.
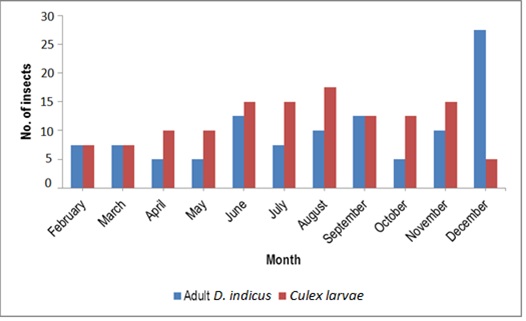 Figure 7: Relative abundance of Diplonychus indicus and Culex.
Figure 7: Relative abundance of Diplonychus indicus and Culex.
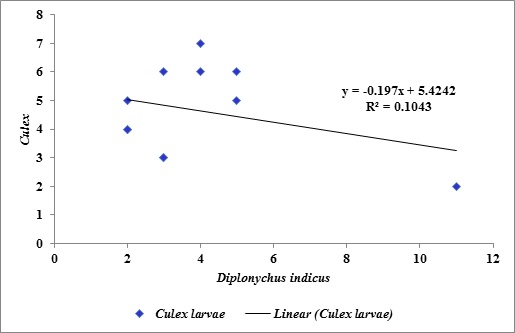 Figure 8: Regression analysis between Diplonychus indicus and Culex.
Figure 8: Regression analysis between Diplonychus indicus and Culex.
DISCUSSION
The results of the present study revealed that the population dynamics of aquatic insects especially Diplonychus indicus were regulated and governed by abiotic as well as biotic factors. Environmental parameters governs the population level of aquatic insects in the pond. From the high population density of Diplonychus indicus during June 2018 to September 2018 it was apparent that the hydrological parameters governed the population levels and this might be due to south west monsoon. According to Rao in nepids, the abiotic factors such as rainfall and temperature together with abundance of food have an augmentative effect on their population [4]. Further, Nebeker and Lemke have observed in stone fly Pteronarcys dorsata that emergence occurs in January in permanent waters probably due to higher water levels due to heavy rainfall [5]. Such an influence was also noticed in Nysiusvisitor by Kehat and Wnydham. and the present work showed that increase or decrease in rainfall promotes or suppresses the growth rate of Diplonychus indicus [6].
Nebekar and Lemke reported that artificial increase in stream temperature would cause Ephermerella subvariae to develop more rapidly and to emerge earlier [5]. It was interesting to note that in the present work there was a negative correlation between the nymphs of Diplonychusindicus and temperature. Tonapi and Julka have observed based on their studies on five families of aquatic bugs including notonectids that temperature and rainfall are the governing factors that regulate the population of aquatic insects [7,8]. Okada and Nakasuji compared the population density and nymphal development of Diplonychus japonicus and Diplonychus major and found that water temperature had a profound influence on the population density and nymphal development [9]. In the present study, pH of water did not show much seasonal fluctuation in the pond. Further, Diplonychus indicus may be more adaptable to variations in salinity with the possession of hydrophilic cuticle as reported in Nepa cinerea by Staddon [10]. However, Diplonychusindicus generally maintained a good population even at higher pH. Dissolved oxygen too had a positive correlation with Diplonychus indicus. Interestingly, Thirumalai and Raghunathan found that dissolved oxygen apparently had no effect on the population of Diplonychus indicus [11]. Popham and Lansbury has indicated that deficiency in oxygen was a strong stimulus for the migration of corixids [12]. The capacity of aquatic bugs to migrate appears to be yet another important factor causing fluctuation in their population [8,13,14]. However, Stout found that the two species of predacious aquatic creeping water bugs of the family Naucordiae were not affected by reductions in dissolved oxygenas they are plastron breathing insects [15].
With regard to the biotic factors, there was a positive correlation between Diplonychus indicus and all the other co-existing insects. Culex larval population also showed a positive correlation with the nymphal and adult population of Diplonychus indicus. It is important to correlate predator and prey seasonality and habitats if mosquito control is to be considered fora particular predator that has proven to be effective in experimental conditions [16]. During the south west monsoon seasons there was an increase in Culex larval population followed by increase in Diplonychus indicus population in the present study. The availability of prey was due to high breeding of mosquitoes during the rainy season. Jeyanthi and Venkatesan in their study on the population dynamics of Diplonychus indicus in Cooumriver, Chennai, Tamil Nadu, India found that among the post embryonic stages, the fourth and fifth nymphal stages occurred throughout the year [17]. But the first and second nymphal instars were poorly represented and they attributed this to the vulnerability of early nymphal stages to predators. However in the present study, all the nymphal instars of Diplonychus indicus were well represented in the pond. The uniform population density of all the nymphal instars might be due to the rich vegetation which provides refuge to the nymphal instars to escape from the predators. The presence of vegetation significantly influenced predation activity in both nymph and adult Diplonychus indicus. The influence was highly significant and more pronounced due to Culex immature having an affinity for vegetation in larval habitats [16].
Laboratory research into aquatic insect predation is fairly common and studies of aquatic bugs have shown that they are quite effective predators of mosquito larvae [16]. Aquatic bugs are voracious feeders on dipteran larvae and their habitats are devoid of mosquitoes which indicates their potential in biological control [18]. The prey availability increased due to high breeding of mosquitoes during the monsoon season which enhanced the density of these insects. Among them Diplonychus indicus is found to be very effective in view of its life span, unique behavior of encumbrance and its predatory nature. The species indicus is one of the most common species of Diplonychus present in the southernmost part of our country and it belongs to the subfamily Belostomatidae and order Hemiptera. It is a predacious water bug even in its nymphal stages and is widely distributed in lentic as well as lotic habitats of Kanyakumari district. They are usually found clinging to the aquatic plants like Eichhornia and Hydrilla in large masses and exhibit varied behavioral patterns in the fresh water medium to fulfil their food requirements, respiration and other metabolic activities. These water bugs emerge continually throughout the year in permanent fresh water system in tropics and are adaptable to difference in salinity and temperature conditions [19]. This water bug is a highly versatile predator that forages both actively and from ambush and grasps prey items with their fore legs and consume them by sucking. It expends more energy for prey capture and prey utilization and the predation is governed by many attributes such as prey density, prey type, prey distribution, predatory periodicity, prey choice, and vegetation [[20-25]. The present findings on the population dynamics of aquatic insects in the study area established the fact that there was seasonal fluctuation in the population of aquatic bugs. The biotic factors especially Culex larvae that formed the main prey had a positive correlation with Diplonychus indicus which indicated the fact that Culex larval population was under control.
REFERENCES
- Polhemus JT (1979) Family Naucoridae: The semiaquatic and aquatic Hemiptera of California (Heteroptera: Hemiptera). Menke AS (ed.). Bulletin of the California Insect Survey, University of California Press, California, USA 21: 131-138.
- Venkatesan P (1981) Influence of temperature and salinity variations on an aquatic bug population in a tropical pond. Hydrobiologia 79: 33-50.
- Shaalan EAS, Canyon DV (2009) Aquatic insect predators and mosquito control. Trop Biomed 26: 223-261.
- Rao TKR (1981) Ecology of the population of some species of aquatic Hemiptera. Proceedings of the Symposium on Ecology and Animal Population. Zoological Survey of India 2: 91-94.
- Nebekar AV, Lemke AE (1968) Preliminary studies on the tolerance of aquatic insects to heated waters. Journal of the Kansas Entomological Society 41: 413-418.
- Kehat M, Wyndham M (1973)The influence of temperatureon development, longevity and fecundity in the Rutherglen bug Nysiusvisitor (Hemiptera:Lygaeidae). Australian Journal of Ecology 20: 67-78.
- Tonapi GT (1959) Studies on the aquatic insect fauna of Poona (Aquatic Heteroptera). Proceedings of the National Institute of Science India. 25: 321-332.
- Julka JM (1977) On possible seasonal fluctuations in the population of aquatic bugs in a fish pond. Oriental Insects 11: 139-149.
- Okado H, Nakasuji F (1993) Patterns of local distribution and coexistence of two giant water bugs, Diplonychusjaponicus and major (Hemiptera: Belostomatidae) in Okayama, Western Japan. The Entomological Society of Japan 61: 79-84.
- Staddon BW (1973) Water uptake through the cuticle of the water scorpion, Nepacinerea L. (Heteroptera: Nepidae). Journal of Entomology 48: 91-93.
- Thirumalai G, Raghunathan MB (1988) Population fluctuations of three families of aquatic Heteroptera in a perennial pond. Records of the Zoological Survey of India 85: 381-389.
- Popham E, Lansbury I (1960) The uses and limitation of light traps in the study of the ecology of Corscidae. Entomologist 288-327.
- Hutchinson CH (1933) The zero geography of the African aquatic Hemiptera in relation to past climatic change. Internationale Revue der gesamten Hydrobiologie und Hydrographie 28: 436-468.
- Fernando CH (1961) Colonization of fresh water habitat with special reference to aquatic insects. Records of Central Bicentennial Congress. Singapore,182-186.
- Stout JR (1981) How abiotic factors affect the distribution of two species of tropical predaceous aquatic bugs (Family Naucoridae). Ecology 62: 1170-1178.
- Shaalan EAS, Canyon DV, Muller R, Younes MWF, Wahab HA, Mansour AH (2007) A mosquito predator survey in Townsville, Australia, and an assessment of Diplonychus sp. and Anisops sp. predatorial capacity against Culex annulirostris mosquito immatures. Journal of Vector Ecology 32: 16-21.
- Jeyanthi M, Venkatesan P (1997) Population dynamics of the water bug, Diplonychusindicus & Rao, a bioagent in mosquito breeding pond. Journal of Entomological Research 21: 17-23.
- Venkatesan P, Jeyachandra CM(1985) Estimation of mosquito predation by the water bug. Diplonychyusindicus & Rao. Indian Journal of Experimental Biology 23: 227-229.
- Corbett PS (1964) Temporal patterns of emergence in aquatic insect. Canadian Entomologist 96: 264-279.
- Cloarec A (1989) Variation of foraging tactics in a water bug, Diplonychus Journal of Ethology 7: 27-34.
- Venkatesan P, Sivaraman S (1984) Changes in the functional response of instars of Diplonychusindicus & Rao (Belostomatidae) in its predation of two species of mosquito larvae of varied size. Entomon 9: 191-196.
- Venkatesan P, Gullaume GC, Halberg F(1986) Modelling prey-predator cycles using hemipteran predators of mosquito larvae for reading worldwide mosquito-borne disease incidence. Chronobiology, 13: 351-354.
- Venkatesan P, D’Sylva T (1990) Influence of prey size on choice by the water bug, Diplonychusindicus &Rao (Hemiptera: Belostamidae). Journal of Entomological Research 14: 130-138.
- Savage AA (2000) Community structure during a 27 year study of the macroinvertebrate fauna of chemically unstable lake. Hydrobiologia 421: 115-127.
- Warfe DM, Barmuta LA (2004) Habit structural complexity mediates the foraging success of multiple predator species. Oecologia 141: 171-178.
Citation: Marin G, Vincent S, Arivoli S, Tennyson S (2019) Influence of Abiotic and Biotic Factors on the Population Dynamics of Diplonychus indicus Venk. & Rao (Hemiptera: Belostomatidae) a Potent Biocontrol Agent for Mosquito Larva. Archiv Zool Stud 2: 010.
Copyright: © 2019 Grace Marin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

