
Injection of Enriched Hyaluronic Acid for Vaginal Dryness in a Tamoxifen User
*Corresponding Author(s):
Vivian De Carvalho AmaralDermatologic And Vulvar Academy, Brazil
Email:vivian.dermato@gmail.com
Abstract
Studies indicate that > 90% of breast cancer survivors taking adjuvant endocrine therapy as tamoxifen experience menopausal symptoms, including vaginal dryness and dyspareunia. Unfortunately, these sexual problems are closely related to depression and early discontinuation of treatment.
The injectable use of cross-linked Hyaluronic Acid (HA) gel in the vaginal walls has demonstrated encouraging results in improving the symptoms of vaginal dryness but it was associated with non-thrombotic pulmonar embolism.
We report the case of a young female patient, 39 years old, using tamoxifen for 4 years for preventing breast cancer recurrence, with vaginal dryness and dyspareunia, whose injection of a liquid formulation, containing non-cross linked hyaluronic acid, antioxidants and amino acids brought significant clinical improvement of sexual concerns.
Introduction
Approximately 80% of breast cancers amongst premenopausal women are hormone receptor-positive [1]. In these cases, adjuvant tamoxifen reduces 15-year breast cancer mortality by a third [2].
The recommended duration of treatment ranges from 5 to 10 years depending on risk of recurrence and the specific treatment regimen. However, the high rates of non-adherence due to the impact of side effects remains a barrier for obtaining the improved outcome benefits of long-term tamoxifen treatment [3-5].
The vaginal injection of cross-linked HA gel, has demonstrated improvement of the symptoms of vaginal atrophy but it was associated to non-thrombotic pulmonar embolism in small number of cases [6-11], in which an overdose of HA was injected into the deep layer of the anterior vaginal wall, an area of high vascular risk, by non-physicians [12-14].
To avoid this major complication, we did inject only 2ml of a liquid formulation containing HA, antioxidants and amino acids superficially, in the submucosal plane, in the lateral and posterior walls to treat vaginal dryness in a 39-year-old female, chronic user of tamoxifen for 4 years, with excellent results in improvement of sexual complaints.
Methods
A 39-year-old woman, who had been using tamoxifen for 4 years, with complaints of vaginal dryness and dyspareunia, received an injection of 2 ml of a liquid preparation with HA, antioxidants and amino acids, in the lateral and posterior walls of the vagina.
For the procedure, a 100mg/ml lidocaine topical solution was applied to the vaginal introitus, achieving satisfactory anesthesia after 10 minutes. Then the passage of the speculum and vaginal asepsis with an aqueous solution of chlorhexidine were performed. Next, the solution was applied with a 27G 1/2 needle, in retro injection, by fan technique, in the vaginal introits, in the area outside the hymenal caruncula, in three different points, on the lateral walls, at 3 and 9 o'clock, and on the posterior wall at 6 o’clock.
At the 3 and 9 o'clock positions, 0.6ml was injected, while at the 6 o'clock position, on the posterior wall, the area classically described as the most uncomfortable in terms of dryness, presence of fissures and dyspareunia, 0.8ml of the solution was injected.
The injection was organized into anatomical vectors, 3 on each lateral wall, each one receiving 0.2 ml of product, and 3 on the posterior wall, receiving the central vector 0.4 ml and the lateral vectors 0.2 ml each (Figures 1 & 2).
Patient does not have vaginitis, active genital herpes, urinary infection, bleeding or vaginal discharge to be clarified, autoimmune diseases or any alteration in the specular evaluation, conditions what would contraindicate the procedure.
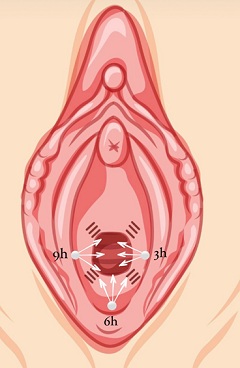 Figure 1: Enriched HA application scheme: 3 punctures are made (white circles at 3, 6 and 9 o'clock) from which the product will be injected in 3 vectors, by retroinjection.
Figure 1: Enriched HA application scheme: 3 punctures are made (white circles at 3, 6 and 9 o'clock) from which the product will be injected in 3 vectors, by retroinjection.
Each vector will receive 0.2ml of the solution, with the exception of the central vector of the posterior wall, which will receive 0.4ml, thus injecting a total volume of 2.0ml.
In the first session, we performed the procedure through 9 punctures, 3 on each lateral wall and 3 on the posterior wall. However, this brought a lot of discomfort to the patient in the first 24 hours at the time of urination, in addition to believing that multiple punctures increase the possibility of hematomas and infections. The patient reported significant improvement in post procedure discomfort with the decrease in the number of punctures.
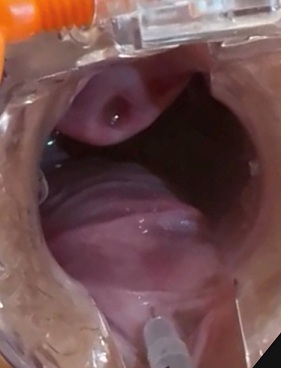 Figure 2: Image of the application of the enriched HA solution, in retro injection, with a 27G 1/2 needle, in the central vector, on the posterior wall, where we injected 0.4ml of the solution.
Figure 2: Image of the application of the enriched HA solution, in retro injection, with a 27G 1/2 needle, in the central vector, on the posterior wall, where we injected 0.4ml of the solution.
We guide her to not manipulate the vagina and remain sexually abstinent for 07 days. No local or systemic medication was prescribed.
Were performed 3 treatment sessions, with an interval of 30 days between them.
Results
For analysis of results, in addition to obtaining images before and 30 days after the third injection, we advise the patient to answer the Sabbatsberg Sexual Self-Assessment Scale (SSS) (Table 1) before the injection and 30 days after the final procedure (Table 2).
Images were obtained before and after HA injection, showing that the mucosa was less pale and more hydrated after the treatment (Figure 3). The functional sexual improvement however, evaluated by the SS, were more expressive than the images were able to demonstrate (Table 2).
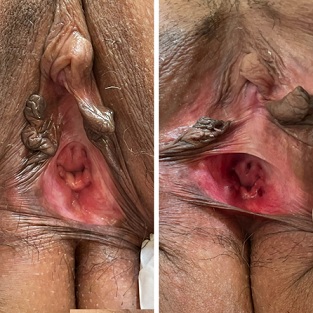 Figure 3: Images obtained before (left) and after (right) the HA injection, showing that the mucosa became less pale and more hydrated at the injected sites. Compare in the image on the right how the treated areas (posterior and lateral walls of the vagina) present a more hyperemic and hydrated mucosa in relation to the labia minora, still quite pale and atrophic.
Figure 3: Images obtained before (left) and after (right) the HA injection, showing that the mucosa became less pale and more hydrated at the injected sites. Compare in the image on the right how the treated areas (posterior and lateral walls of the vagina) present a more hyperemic and hydrated mucosa in relation to the labia minora, still quite pale and atrophic.
|
Sabbatsberg Sexual Self-Rating Scale
Please tick the appropriate answer (only one) for each section. Please do not respond to any question that does not currently apply to you. Just mark such questions cicarly NA. Sexual activity includes intercourse, masturbation, oral sex, and anal sex.
|
|
1. a. My sexual interest during the past month has been: very great ( ) great ( ) moderate ( ) little ( ) very little or nonexistent ( )
b. In comparison to prevlous years, my sexual interest is now: much greater ( ) greater ( ) unchanged ( ) less ( ) much less ( ) |
|
2. a. My sexual activity during the last month has been: very great ( ) great ( ) moderate ( ) little ( ) very little or nonexistent ( )
b. In comparison to previous years, my sexual activity is now: much greater ( ) greater ( ) unchanged ( ) less ( ) much less ( ) |
|
3. a. My sexual life during the last month has been: very satisfying ( ) satisfying ( ) rather satisfying ( ) less satisfying ( ) not satisfying ( )
b. In comparison to previous years, my sexual life is now: much more satistying ( ) more satisfying ( ) unchanged ( ) less satisfying ( ) much less satisfying ( ) |
|
4. a. Sex during the last month has given me: very great pleasure ( ) great pleasure ( ) moderate pleasure ( ) little pleasure ( ) no pleasure ( )
b. In comparison to previous years, sex now has given me: much greater pleasure ( ) greater pleasure ( ) the same pleasure ( ) less pleasure ( ) much less pleasure ( ) |
Table 1: Sabbatsberg Sexual Self-Assessment Scale (SSS).
|
Sabbatsberg Sexual Self-Rating Scale
Please tick the appropriate answer (only one) for cach section. Please do not respond to any question that does not currently apply to you. Just mark such questions cicarly NA. Sexual activity includes intercourse, masturbation, oral sex, and anal sex. |
|
1. a. My sexual interest during the past month has been: very great ( ) great ( 0 ) moderate ( ) little ( ) very little or nonexistent ( x )
b. In comparison to prevlous years, my sexual interest is now: much greater ( ) greater ( 0 ) unchanged ( ) less ( ) much less ( x ) |
|
2. a. My sexual activity during the last month has been: very great ( ) great ( 0 ) moderate ( ) little ( ) very little or nonexistent ( x )
b. In comparison to previous years, my sexual activity is now: much greater ( 0 ) greater ( ) unchanged ( ) less ( ) much less ( x ) |
|
3. a. My sexual life during the last month has been: very satisfying ( ) satisfying ( 0 ) rather satisfying ( ) less satisfying ( ) not satisfying ( x )
b. In comparison to previous years, my sexual life is now: much more satistying ( ) more satisfying ( 0 ) unchanged ( ) less satisfying ( ) much less satisfying ( x ) |
|
4. a. Sex during the last month has given me: very great pleasure ( ) great pleasure ( 0 ) moderate pleasure ( ) little pleasure ( ) no pleasure ( x )
b. In comparison to previous years, sex now has given me: much greater pleasure ( ) greater pleasure ( 0 ) the same pleasure ( ) less pleasure ( ) much less pleasure ( x ) |
Table 2: patients’ answer before ( x ) and 30 days after ( 0 ) the third treatment.
Notice how all sexual assessment parameters improve after the 3 sessions of enriched HA.
After 30 days, the patient reported an important improvement in symptoms of dryness and dyspaurenia, with increased sexual interest, improved quality of intercourse and increased pleasure during sex (Table 2).
In the immediate post procedure period, the most common complaint was burning sensation when urinating, with spontaneous resolution within 24 hours.
Discussion
Tamoxifen is used to prevent recurrence and reduce mortality for women with hormone receptor positive breast cancer. Poor adherence to therapy, closely related to the sexual side effects, is a significant problem and contributes to increased breast cancer mortality [1]. Because of that, the treatment of vaginal symptoms related to the use of tamoxifen is essential.
Currently, alternatives for the treatment of vaginal dryness in tamoxifen users are based on the local use of vaginal lubricants and moisturizers and the application of vaginal technologies such as lasers and radiofrequency. Creams for vaginal use are considered uncomfortable for most women, and the devices, despite the promising results, are not approved by the FDA for this kind of treatment and their use has been associated with serious adverse events, such as chronic vaginal pain [15].
HA is an essential polysaccharide in the maintenance of water balance, in the regulation of inflammation, in the immune response, in wound healing and in angiogenesis of skin and mucous membranes [16]. Histological analyzes demonstrated that the vaginal mucosa in the absence of estrogen, is thick, with reduced vascularization, quiescent fibroblasts, reduced and dehydrated extracellular matrix and inversion of the proportion of collagen I-III, with altered trabecular architecture and elasticity [17].
The injection of HA into the vaginal mucosa demonstrated the expression of the CoL1A1 and CoL3A1 genes, suggesting the stimulation of local collagen formation [6]. Indeed, studies have shown that vaginal injection of HA considerably improves the symptoms of mucosal atrophy due to the lack of estrogen [6].
Pulmonary complications of non-thrombotic embolism due to HA are uncommon, with only six reported cases of isolated injection of HA leading to such a complication. Of these, only two cases were associated with vaginal injection [12-14]. It is believed that in these cases the embolism occurred due to the injection of cross linked HA gel, in the anterior wall of the vagina [11], where there is an extensive venous plexus [18]. In addition, injections were performed with high amounts of HA, by non-medical professionals [12].
Note that, in order to keep the application safe, although we are using a liquid preparation, which alone would already bring safety in relation to the risks of pulmonary embolism, we also chose to keep our injection in the anatomical sites considered safer, thus avoiding the injection in deep regions of the anterior vaginal wall. We only injected superficially, gently, in the vaginal-vestibular lateral and posterior walls, areas of lower vascular risk and where most patients report greater discomfort in relation to pain, dryness and fissures [7].
In addition to HA, the injectable preparation also contains vitamins A, B, C and E, glutathione and amino acids. The use of enriched HA appears to be beneficial in soft tissue healing. In fact, compared to the isolated use of HA, studies have shown, through histological and immunohistochemical analyses, greater micro vascular density and better organized collagen fibers in compact and well-oriented bundles in tissues treated with HA enriched with vitamins and amino acids [19-22].
Conclusion
Injection of HA into the lateral and posterior walls of the vagina is a simple, reproducible and low-cost procedure that proved to be extremely effective in improving the vaginal symptoms associated with the use of tamoxifen in this specific case.
Despite the promising results, however, more studies are needed to standardize the number of sessions, the interval between them and the ideal amount to be injected for the best results.
References
- Dorfman CS, Arthur SS, Kimmick GG, Westbrook KW, Marcom PK, et al. (2019) Partner status moderates the relationships between sexual problems and self-efficacy for managing sexual problems and psychosocial quality-of-life for postmenopausal breast cancer survivors taking adjuvant endocrine therapy. Menopause 26: 823-832.
- Ribi K, Luo W, Walley BA, Burstein HJ, Chirgwin J, et al. (2020) Treatment-induced symptoms, depression and age as predictors of sexual problems in premenopausal women with early breast cancer receiving adjuvant endocrine therapy. Breast Cancer Res Treat 181: 347-359.
- Shelby RA, Dorfman CS, Bosworth HB, Keefe F, Sutton L, et al. (2019) Testing a behavioral intervention to improve adherence to adjuvant endocrine therapy (AET). Contemp Clin Trials 76:120-131
- Berreni N, Salerno J, Chevalier T, Alonso S, Mares P (2021) Evaluation of the effect of multipoint intra-mucosal vaginal injection of a specific cross-linked hyaluronic acid for vulvovaginal atrophy: a prospective bi-centric pilot study. BMC Womens Health 21: 322.
- Garavaglia E, Sala C, Busato M, Bellia G, Tamburlin N, et al. (2020) First Use of Thermal Stabilized Hyaluronic Acid Injection in One-Year Follow-Up Patients with Genitourinary Syndrome. Med Devices (Auckl) 13: 399-410.
- Angelucci M, Frascani F, Franceschelli A, Lusi A, Garo ML (2022) Efficacy of intradermal hyaluronic acid plus polynucleotides in vulvovaginal atrophy: a pilot study. Climacteric 7: 1-7.
- Cohen PR, Riahi RR (2019) Platelet-Rich Plasma and Genital Rejuvenation. Skinmed 17: 272-274.
- Hersant B, SidAhmed-Mezi M, Belkacemi Y, Darmon F, Bastuji-Garin S, et al. (2018) Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with history of breast cancer: a phase 2 pilot study. Menopause 25: 1124-1130.
- Aguilar P, Hersant B, SidAhmed-Mezi M, Bosc R, Vidal L, et al. (2016) Novel technique of vulvo-vaginal rejuvenation by lipofilling and injection of combined platelet-rich-plasma and hyaluronic acid: a case-report. Springer plus 5:1184.
- Yang Y, Sheng H, Gu Q, Su L, Tong H, et al. (2020) Death Caused by Vaginal Injection of Hyaluronic Acid and Collagen: A Case Report. Aesthet Surg J 40: 263-268.
- Han SW, Park MJ, Lee SH (2019) Hyaluronic acid-induced diffuse alveolar hemorrhage: unknown complication induced by a well-known injectable agent. Ann Transl Med 7: 13.
- Park HJ, Jung KH, Kim SY, Lee JH, Jeong JY, et al. (2010) Hyaluronic acid pulmonary embolism: a critical consequence of an illegal cosmetic vaginal procedure. Thorax 65: 360-361.
- Bui KT, Willson ML, Goel S, Beith J, Goodwin A (2020) Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst Rev 3: 13538.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2022) Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol 23: 382-392.
- Fleming L, Agnew S, Peddie N, Crawford M, Dixon D, et al. (2022) The impact of medication side effects on adherence and persistence to hormone therapy in breast cancer survivors: A quantitative systematic review. Breast 64: 63-84.
- Helland T, Hagen KB, Haugstøyl ME, Kvaløy JT, Lunde S, et al. (2019) Drug monitoring of tamoxifen metabolites predicts vaginal dryness and verifies a low discontinuation rate from the Norwegian Prescription Database. Breast Cancer Res Treat 177: 185-195.
- Saghatchian M, Lesur A (2019) Management of side effects related to adjuvant hormone therapy in young women with breast cancer. Bull Cancer 106: 37-42.
- Smith J (2018) FDA warning shines light on vaginal rejuvenation. Ob Gyn News Pg no: 1-5.
- Nusgens BV (2010) Acide hyaluronique et matrice extracellulaire: une molécule primitive? Ann Dermatol Venereol 137: 3-8.
- Fadare O (2011) Vaginal stromal sclerosis: a distinctive stromal change associated with vaginal atrophy. Int J Gynecol Path 30: 295-300.
- Cunningham FG, Leveno KL, Bloom SL (2010) Maternal anatomy. In: Williams obstetrics. New York: McGraw-Hill, USA, Pg no: 17-18.
- Canciani E, Sirello R, Pellegrini G, Henin D, Perrotta M, et al. (2021) Effects of Vitamin and Amino Acid-Enriched Hyaluronic Acid Gel on the Healing of Oral Mucosa: In Vivo and In Vitro Medicina (Kaunas) 57: 285.
Citation: Amaral VC (2023) Injection of Enriched Hyaluronic Acid for Vaginal Dryness in a Tamoxifen User. J Clin Dermatol Ther 9: 0115.
Copyright: © 2023 Vivian de Carvalho Amaral, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

