
Interrelations between Serum N-Terminal Pro B-Type Natriuretic Peptide (Nt-Probnp) Levels and Early Cardiovascular Risk Factors and Echocardiographic Parameters in Obese Adolescents
*Corresponding Author(s):
Ozgur PirgonDepartment Of Pediatric Endocrinology And Diabetes, Faculty Of Medicine, Süleyman Demirel University, Isparta, Turkey
Tel:+90 2462119302,
Email:ozgurpirgon@gmail.com
Abstract
Keywords
INTRODUCTION
Natriuretic peptide signaling may actively influence differential body fat distribution. N-Terminal-pro-Brain Natriuretic Peptide (NT-proBNP) and brain natriuretic peptide are useful for the diagnosis of heart failure, and their high levels in serum and plasma, respectively, are related to wall stress, which is often increased in severe obesity. High brain natriuretic peptide as well as high NT-proBNP are new promising cardiovascular risk markers and have been associated with high blood pressure, and LV hypertrophy [9,10]. These are sensitive markers of cardiac dysfunction and may be useful in the early diagnosis of cardiac loading.
NT-proBNP is extremely reliable due to the high negative predictive value so it is used more frequently than from brain natriuretic peptide [11,12]. Recent findings on the relationship between NT-proBNP and metabolic parameters, morphologic and dynamic cardiac abnormalities in adolescent obesity are still inconsistent and controversial. Therefore, the aim of the present study was to evaluate the associations of serum NT-proBNP levels to cardiovascular risk factors, echocardiographic and metabolic parameters in obese adolescents.
MATERIALS AND METHODS
Patients
The adolescents receiving treatment for any reason, syndromic ones and patients having either an endocrinological disease or familial dyslipidemia were dismissed from the study. Patients were excluded if they had any systemic disease, including type 1 or type 2 diabetes mellitus, taking medications, or had a condition known to effect insulin action, or insulin secretion (e.g., glucocorticoid therapy, hypothyroidism, Cushing’s disease). This study was conducted in accordance with the guidelines proposed in the Helsinki Declaration and was received the approval of Istanbul Sisli Etfal Research Hospitals Ethics Committee on 19 July, 2011. The informed consent form was obtained from the patients or the legal guardians.
Anthropometric variables
Blood pressure
Laboratory analyses
N-Terminal Pro B-Type natriuretic peptide measurements
Insulin sensitivity measurement
Echocardiographic evaluation
Conventional echocardiography measurements were performed by using recommendation of the American Society of Echocardiography [18]. The LV mass and the LV mass index were calculated by using the method of Woythaler and his colleagues, which is also a modification of the method of Devereux and Reichek [1].
Pulsed-wave tissue Doppler imaging
The thickness of the epicardial adipose tissue was measured from the right ventricular free wall in the parasternal long axis view. The epicardial adipose tissue was identified as an echo-free space in the pericardial layers on the two-dimensional echocardiography, and its thickness was measured perpendicularly on the free wall of the right ventricle at end diastole [19,20].
To standardize the set point of measurement between different observers, the aortic annulus was used as the anatomic reference. The measurement was performed at a point on the free wall of the right ventricle along the midline of the ultrasound beam perpendicular to the aortic annulus. The average value from three cardiac cycles was used for the statistical analyses.
Carotid intima-media thickness measurements
Statistical analysis
RESULTS
| Leans | Obese | ||
| Mild-moderate | Severe | ||
| n | 63 | 95 | 43 |
| Age (mo) | 170.3 ± 27.0 | 169.6 ± 20.1 | 162.1 ± 23.2 |
| BMI(kg/m2) | 20.1 ± 1.3 | 33.9 ± 9.3‡ | 39.4 ± 4.0†.* |
| BMI-SDS | 1.2 ± 0.2 | 2.2 ± 0.2‡ | 2.7 ± 0.2* |
| Waist circumference (cm) | 67.0 ± 5.8 | 102.7 ± 8.7‡ | 112.3 ± 11.1†.* |
| Hip circumference (cm) | 91.0 ± 7.6 | 114.4 ± 8.5‡ | 124.3 ± 10.0†.* |
| Fasting glucose (mg/dl) | 84.5 ± 10.3 | 90.1 ± 9.3‡ | 91.6 ± 9.2† |
| Fasting insulin (mIU/ml) | 10.9 ± 2.5 | 26.6 ± 15.2‡ | 27.0 ± 11.5† |
| HOMA-IR | 2.2 ± 0.6 | 5.9 ± 3.9‡ | 5.7 ± 2.7† |
| HDL-C (mg/dl) | 48.2 ± 16.8 | 46.1 ± 10.2 | 45.3 ± 11.3 |
| LDL-C (mg/dl) | 80.4 ± 29.2 | 110.1 ± 142.5‡ | 100.8 ± 25.3† |
| Triglycerides (mg/dl) | 144.5 ± 37.3 | 122.8 ± 62.0‡ | 131.7 ± 59.9† |
| Total cholesterol (mg/dl) | 164.1 ± 36.2 | 168.9 ± 35.7 | 174.4 ± 35.0† |
| NT- proBNP (pg/ml) | 44.3 ± 23.3 | 67.2 ± 64.4‡ | 76.0 ± 49.7† |
| High sensitivity CRP (mg/L) | 1.7 ± 0.8 | 1.9 ± 1.1‡ | 2.7 ± 1.0†.* |
| Systolic Blood Pressure (mm Hg) | 105.2 ± 11.7 | 128.0 ± 14.3‡ | 128.0 ± 14.3‡ |
| Diastolic Blood Pressure(mm Hg) | 71.8 ± 7.0 | 83.0 ± 10.3‡ | 86.7 ± 10.8† |
| Left ventricular mass index (gr/m2) | 62.4 ± 18.2 | 88.5 ± 23.0‡ | 87.5 ± 34.8† |
| Myocardial performance index | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1†.* |
| Carotid intima-media thickness (mm) | 0.52 ± 0.08 | 0.88 ± 0.18‡ | 0.91 ± 0.23† |
| Epicardial adipose tissue thicknesses (mm) | 4.28 ± 0.79 | 7.38 ± 1.76‡ | 7.42 ± 1.55† |
‡ p < 0.05 Leans vs. mild-moderate obese
† p < 0.05 Leans vs. severe obese
BMI: Body Mass Index; HDL-C: High Density Lipoprotein Cholesterol; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; LDL-C: Low Density Lipoprotein Cholesterol; NT- proBNP : N-Terminal Pro B-Type Natriuretic Peptide; Values are mean ±SD
The average LV mass index was greater in the mildly-moderately and severely obese group than in the control group (88.5 ± 23, 87.5 ± 34.8and 62.4 ± 18.2 g/m2 respectively, p:0.012), but there was no difference between the groups in obesity (p>0.05).
The average carotid IMT was 0.88 ± 0.18 and 0.91 ± 0.23 mm respectively in the mildly-moderately and severely obese group and 0.52 ± 0.08 mm in the control group (p:0.002). However, significant differences were not observed between obesity groups (p>0.05). The average EATT was 7.38 ± 1.76 and 7.42 ± 1.55 mm respectively in the mildly-moderately and severely obese and 4.28 ± 0.79 mm in the control group (p:0.0032). The mitral valve pulsed-wave Doppler analyses show a statistically significant difference between leans and obese groups in terms of diastolic early wave peak velocity (E'), diastolic late wave peak velocity (A'), (E'/A'), isovolumic relaxation time, average MPI, accelaration time and average decelaration time. The average acceleration time, average deceleration time and average MPI were greater in severe obese group than mild-moderate obese group (p<0.05). The results of the tissue Doppler imaging studies from the mitral annulus for three groups are summarized in Table 2. In the all obese group, there were statistically significant correlations between BMI and LV mass index (r:0.38, p:0.03), MPI (r:0. 72, p:0.003 and) carotid IMT (r:0.34, p:0.033) and EATT (r:0.45, p:0.023). Moreover; a statistically significant positive correlation was found between EATT and the carotid IMT (r:0.60, p:0.002).
| Leans | Obese | ||
| Mild-moderate | Severe | ||
| n | 63 | 95 | 43 |
| Left ventricular systolic function | |||
| Ejection fraction (%) | 65.3 ± 3.8 | 64.6 ± 4.1 | 64.5 ± 3.7 |
| Fractional shortening (%) | 35.4±2.9 | 35.3 ± 3.2 | 34.9 ± 2.7 |
| Left ventricular diastolic function | |||
| Diastolic early wave peak velocity (cm/sn) | 17.8 ± 2.9 | 16.8 ± 3.1‡ | 16.9 ± 3.1† |
| Diastolic late wave peak velocity (cm/sn) | 6.5 ± 1.3 | 7.3 ± 1.5‡ | 7.7 ± 1.7† |
| E’/A’ | 2.8 ± 0.7 | 2.4 ± 0.6‡ | 2.3 ± 0.5† |
| Isovolumic relaxation time (ms) | 49.1 ± 8.3 | 50.7 ± 8.3‡ | 53.7 ± 10.3† |
| Acseleration time (ms) | 53.9 ± 8.5 | 52.6 ± 9.0‡ | 48.6 ± 8.4†,* |
| Deceleration time (ms) | 74.3 ± 9.6 | 73.6 ± 9.8‡ | 66.0 ± 6.0†,* |
| Left ventricular systolic and diastolic function | |||
| Myocardial performance index | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1†,* |
* p < 0.05 Mild-moderate obese vs. severe obese
‡ p < 0.05 Leans vs. mild-moderate obese
† p < 0.05 Leans vs. severe obese
NT-proBNP measurement in relation to obesity, metabolic and hemodynamic risk factors
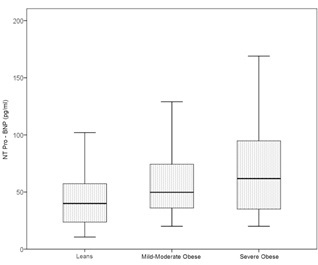 Figure 1: Comparisons of the plasma N-terminal pro B-type natriuretic peptide levels in leans, mild-moderate and severe obese groups.
Figure 1: Comparisons of the plasma N-terminal pro B-type natriuretic peptide levels in leans, mild-moderate and severe obese groups.| Obese | ||||
| Mild-moderate | Severe | |||
| r | p | r | p | |
| Body composition | ||||
| BMI (kg/m2) | 0.484 | 0.001 | 0.667 | <0.001 |
| BMI-SDS | 0.384 | 0.004 | 0.667 | <0.001 |
| Waist circumference (cm) | 0.226 | 0.04 | 0.545 | <0.001 |
| Hip circumference (cm) | 0.315 | 0.01 | 0.516 | <0.001 |
| Metabolic factors | ||||
| Fasting glucose (mg/dl) | 0.122 | NS | 0.135 | NS |
| Fasting insulin (mIU/L) | 0.133 | NS | 0.123 | NS |
| HOMA-IR | 0.116 | NS | 0.084 | NS |
| HDL-Cholesterol (mg/dl) | 0.020 | NS | 0.023 | NS |
| LDL-Cholesterol (mg/dl) | 0.114 | NS | 0.119 | NS |
| Triglyceride (mg/dl) | -0.183 | NS | -0.112 | NS |
| Total Cholesterol (mg/dl) | 0.056 | NS | 0.077 | NS |
| High sensitive CRP (mg/dl) | 0.063 | NS | 0.067 | NS |
| Hemodynamic factors | ||||
| Systolic Blood Pressure (mm Hg) | 0.128 | NS | 0.289 | 0.04 |
| Diastolic Blood Pressure (mm Hg) | 0.319 | 0.004 | 0.598 | <0.001 |
| Heart rate | 0.088 | NS | 0.022 | NS |
| Left ventricular thickness | ||||
| LVPWd(cm) | 0.155 | NS | 0.424 | <0.001 |
| IVSs (cm) | 0.134 | NS | 0.114 | NS |
| IVSd (cm) | 0.394 | <0.001 | 0.495 | <0.001 |
| LVPWs (cm) | 0.451 | <0.001 | 0.456 | <0.001 |
| LVPWt (cm) | 0.435 | <0.001 | 0.544 | <0.001 |
| LV mass index (gr/m2) | 0.383 | 0.0019 | 0.649 | <0.001 |
| Left ventricular systolic function | ||||
| Ejection fraction (%) | 0.043 | NS | 0.297 | 0.021 |
| Fractional shortening (%) | 0.015 | NS | 0.025 | NS |
| Left ventricular diastolic function | ||||
| Diastolic early wave peak velocity (cm/sn) | 0.021 | NS | 0.040 | NS |
| Diastolic late wave peak velocity (cm/sn) | 0.028 | NS | 0.024 | NS |
| E’/A’ | 0.012 | NS | 0.021 | NS |
| Acceleration time (ms) | 0.305 | 0.034 | 0.279 | 0.045 |
| Deceleration time (ms) | 0.059 | NS | 0.044 | NS |
| Left ventricular systolic and diastolic function | ||||
| Myocardial performance index | 0.033 | NS | 0.288 | 0.042 |
| Indicators of early atherosclerosis | ||||
| Carotid IMT (mm) | 0.299 | 0.029 | 0.325 | 0.003 |
| EATT (mm) | 0.233 | NS | 0.339 | 0.022 |
Nt-proBNP measurement in relation to subclinical cardiovascular damage
BMI: Body Mass Index; EATT: Epicardial Adipose Tissue Thickness; HDL-C: High Density Lipoprotein - Cholesterol; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance; LDL-C: Low Density Lipoprotein Cholesterol; LV: Left Ventricular; LVPWd: Left Ventricular Posterior Wall diastolic thickness; LVPWs: Left Ventricular Posterior Wall systolic thickness; LVPWt: Left Ventricular Posterior Wall thickness; IV: Interventricular; IVSs: Interventricular Septum systolic thickness; IVSd: Interventricular Septum diastolic thickness
NT-proBNP measurement in relation to hypertension
| Obese | |||
| Hypertensive | Normotensive | p | |
| n | 34 | 104 | |
| NT-proBNP (pg/ml) | 86.5 ± 39.7 | 63.2 ± 44.4 | 0.023 |
| Left ventricular mass index (gr/m2) | 78.5 ± 27.0 | 68.4 ± 16.8 | 0.042 |
| Myocardial performance index | 0.42 ± 0.1 | 0.40 ± 0.1 | 0.860 |
| Carotid intima-media thicknesses (mm) | 0.88 ± 0.2 | 0.71 ± 0.13 | 0.012 |
| Epicardial adipose tissue thicknesses(mm) | 7.12 ± 1.45 | 7.28 ± 1.56 | 0.980 |
Table 4: Comparison of the plasma N-terminal pro B-type natriuretic peptide levels, left ventricular mass index values, myocardial performance index, carotid intima-media, and epicardial adipose tissue thicknesses in obese subjects with normal and elevated blood pressures.
DISCUSSION
We found significant differences in NT-proBNP levels among the groups. In children there are a few studies examining the relations between NT-proBNP levels and obesity. In a study, NT-proBNP concentrations were found to be higher in obese children than the control group similar results of our study [23]. Childhood obesity causes to changes in the heart’s structure and function and it is associated with cardiovascular risk factors [24]. Starting from childhood, myocardial mass parallels the increase in BMI. It is shown that LV hypertrophy occurs in obesity and that this hypertrophy is associated with an increased risk of cardiovascular diseases [25,26]. In the current study, the LV mass index was higher in the obese group than in the control group. This difference was statistically significant because it is known that LV hypertrophy affects diastolic function negatively. On the other hand, in obese patients with no ventricle hypertrophy, cardiac functions may deteriorate. Moreover, this deterioration may be seen in each ventricle [27,28].
Obesity is an important risk factor for atherosclerotic cardiovascular disease. Neeland IJ et al., demonstrated a significant association between higher levels of natriuretic peptides and adiposity, including decreased visceral and liver fat and increased lower body fat, independent of age, sex, race, and obesity-status [29]. We found that serum NT-proBNP levels were higher in patients with the obesity attributable to straight relationships between serum NT-proBNP and LV mass index, LV ejection fraction and MPI independently of age, gender, and metabolic and hemodynamic cardiovascular risk factors in obese adolescents. Bradham WS et al., [30] reported that in adult patients, insulin resistance is associated with higher concentrations of NT-pro-BNP, however; we did not find a significant correlation between insulin levels, insulin sensitivity markers and NT-pro-BNP levels.
In adult studies, the EATT was significantly correlated with the severity of coronary artery stenosis for patients with the known coronary artery disease [31]. Obesity seems to be a predisposing factor for the accumulation of excessive epicardial fat, however; we foundno siginificant correlation between EATT and BMI in our study. Carotid IMT measurement is widely used method in the early diagnosis of atherosclerosis [32]. Di Salvo et al., [33] showed that carotid IMT was not greater in obese children than in nonobese control children. Iannuzzi et al., [34] reported that carotid IMT was increased in children with metabolic syndrome, but that this increase was not statistically significant. Numerous studies have shown that carotid IMT was increased in obese children andit is widely agreed that this increase in childhood is related to atherosclerosis in adulthood [35,36]. In our study, the carotid IMT measurements were significantly increased in the obese group compared to the control group. In addition to the study, a statistically significant correlation was found between EATT and carotid IMT. It is shown that the EATT is associated with atherosclerosis.
Natriuretic peptide hormones are very important for the maintenance of extracellular fluid volume within a narrow range despite wide variations in dietary sodium intake. The primary stimulus for natriuretic peptide release is myocyte stretching. Plasma levels of BNP and NT-proBNP are elevated in adult patients with a wide range of heart diseases including LV systolic and diastolic dysfunction. Thus, they serve as markers for heart disease [37]. Increased NT-proBNP levels were found to be closely related to cardiac structure and function and to be a strong independent indicator for long-term outcome in obese patients. In adult studies, increased serum NT-proBNP levels were found in cases with LV systolic and diastolic dysfunction [38,39]. Kim et al., [40] studied adults with hypertrophic cardiomyopathy and found a positive correlation of the serum NT-proBNP levels with the end-diastolic thickness of the interventricular septum and LV mass index. In this study, NT-proBNP levels were found to be significantly higher in obese adolescent than healthy controls and its levels were higher in obese cases with asymptomatic cardiac dysfunction than in normal control subjects. To the best of our knowledge, serum NT-proBNP levels in obese children have been previously reported in two different studies to date regardless of the presence of systolic and/or diastolic dysfunction. Saritas et al., [23] showed that NT-proBNP was greater in obese children than in nonobese control children but they reported no correlations between the serum NT-proBNP levels and the body weight, carotid IMT, EATT, systolic and diastolic blood pressures, LV mass index and MPI values. Contrary to the this study performed with children,we detected statistically significant correlations between the serum NT-proBNP and BMI, blood pressure, LV mass index, carotid IMT and EATT in severely obese adolescent compared with non-obese and control adolescents. The other pediatric study, NT-proBNP levels in obese children were not different from healthy controls [41]. Similar to the studies in adults, statistically significant correlations were detected between the mitral annular MPI values and serum NT- proBNP levels in obese adolescent compared with nonobese control [42].
In adult studies, statistically significant positive correlations have been reported between the serum NT- proBNP and blood pressure and/or LV hypertrophy [9]. In children, NT-proBNP concentrations were found to be lower in the obese than the normal BMI group but higher in the obese hypertensive than the obese normotensive group [43]. In another pediatric study, NT-proBNP levels in hypertensive obese children were not different from non-hypertensive [23]. In this study, the average serum N-terminal pro B-type natriuretic peptide levels, average LVMI and carotid IMT were found to be significantly higher in the hypertensive obese adolescent than the non-hypertensive obese group.
CONCLUSION
SUMMARY POINTS
- Childhood obesity is accompanied with an increased cardiovascular disease risk profile in adulthood
- N-Terminal-pro-Brain Natriuretic Peptide (NT-proBNP) is useful for the diagnosis of heart failure, and their high levels in serum and plasma, respectively, are related to wall stress, which is often increased in severely obesity.
- In this study, serum NT-proBNP levels were found to be significantly higher in obese children than in the control group.
- In this study; we determined statistically significant correlations between the serum NT-proBNP and BMI, blood pressure, LV mass index, carotid IMT and EATT in severely obese adolescents compared with non-obese control adolescents.
REFERENCES
- de Simone G, Devereux RB, Wallerson DC (1994) Echocardiographic assessment of left ventricular hypertrophy in rats using a simplified approach. Am J Hypertens 7: 555-558.
- Eckel RH, Barouch WW, Ershow AG (2002) Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation 105: 2923-2928.
- Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, et al. (2006) Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 47: 2420-2428.
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, et al. (2002) Obesity and the risk of heart failure. N Engl J Med 347: 305-313.
- Alpert MA, Terry BE, Kelly DL (1985) Effect of weight loss on cardiac chamber size, wall thickness and left ventricular function in morbid obesity. Am J Cardiol 55: 783-786.
- Smith HL, Willius FA (1933) Adiposity of the heart. Arch Intern Med 52: 911-931.
- Nakajima T, Fujioka S, Tokunaga K, Hirobe K, Matsuzawa Y, et al. (1985) Noninvasive study of left ventricular performance in obese patients: influence of duration of obesity. Circulation 71: 481-486.
- Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, et al. (1995) Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol 76: 1194-1197.
- Olsen MH, Wachtell K, Tuxen C, Fossum E, Bang LE, et al. (2004) N-terminal pro-brain natriuretic peptide predicts cardiovascular events in patients with hypertension and left ventricular hypertrophy: a LIFE study. J Hypertens 22: 1597-1604.
- Schirmer H, Omland T (1999) Circulating N-terminal pro-atrial natriuretic peptide is an independent predictor of left ventricular hypertrophy in the general population. The Tromsø Study. Eur Heart J 20: 755-763.
- Nasser N, Perles Z, Rein AJ, Nir A (2006) NT-proBNP as a marker for persistent cardiac disease in children with history of dilated cardiomyopathy and myocarditis. Pediatr Cardiol 27: 87-90.
- Pfister R, Scholz M, Wielckens K, Erdmann E, Schneider CA (2004) Use of NT-proBNP in routine testing and comparison to BNP. Eur J Heart Fail 6: 289-293.
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240-1243.
- Sen Y, Kandemir N, Alikasifoglu A, Gonc N, Ozon A (2008) Prevalence and risk factors of metabolic syndrome in obese children and adolescents: the role of the severity of obesity. Eur J Pediatr 167: 1183-1189.
- Hatipoglu N, Ozturk A, Mazicioglu MM, Kurtoglu S, Seyhan S, et al. (2008) Waist circumference percentiles for 7- to 17-year-old Turkish children and adolescents. Eur J Pediatr 167: 383-389.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114: 555-556.
- Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115: 500-503.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A (1978) Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072-1083.
- Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, et al. (2003) Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 11: 304-310.
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, et al. (2003) Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 88: 5163-5168.
- Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, et al. (2001) Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 358: 1400-1404.
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, et al. (2007) Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 23: 75-80.
- Saritas T, Tascilar E, Abaci A, Yozgat Y, Dogan M, et al. (2010) Importance of plasma N-terminal pro B-type natriuretic peptide, epicardial adipose tissue, and carotid intima-media thicknesses in asymptomatic obese children. Pediatr Cardiol 31:792-799.
- Alpert MA (2001) Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 321: 225-236.
- Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, et al. (2004) Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 43: 1399-1404.
- Wong C, Marwick TH (2007) Alterations in myocardial characteristics associated with obesity: detection, mechanisms, and implications. Trends Cardiovasc Med 17: 1-5.
- Harada K, Orino T, Takada G (2001) Body mass index can predict left ventricular diastolic filling in asymptomatic obese children. Pediatr Cardiol 22: 273-278.
- Sharpe JA, Naylor LH, Jones TW, Davis EA, O'Driscoll G, et al. (2006) Impact of obesity on diastolic function in subjects < or = 16 years of age. Am J Cardiol 98: 691-693.
- Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, et al. (2013) Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol 62: 752-760.
- Bradham WS, Ormseth MJ, Oeser A, Solus JF, Gebretsadik T, et al. (2014) Insulin resistance is associated with increased concentrations of NT-proBNP in rheumatoid arthritis: IL-6 as a potential mediator. Inflammation 37: 801-808.
- Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, et al. (2007) Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 71: 536-539.
- Charakida M, Tousoulis D, Stefanadis C (2006) Early atherosclerosis in childhood: diagnostic approaches and therapeutic strategies. Int J Cardiol 109: 152-159.
- Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, et al. (2006) Abnormal myocardial deformation properties in obese, nonhypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J 27: 2689-2695.
- Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, et al. (2004) Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care 27: 2506-2508.
- Atabek ME, Pirgon O, Kivrak AS (2007) Evidence for association between insulin resistance and premature carotid atherosclerosis in childhood obesity. Pediatr Res 61: 345-349.
- Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, et al. (2007) Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab 92: 3025-3032.
- Mair J, Hammerer-Lercher A, Puschendorf B (2001) The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med 39: 571-588.
- Dong SJ, de las Fuentes L, Brown AL, Waggoner AD, Ewald GA, et al. (2006) N-terminal pro B-type natriuretic peptide levels: correlation with echocardiographically determined left ventricular diastolic function in an ambulatory cohort. J Am Soc Echocardiogr 19: 1017-1025.
- Tschöpe C, Kasner M, Westermann D, Gaub R, Poller WC, et al. (2005) The role of NT-proBNP in the diagnostics of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J 26: 2277-2284.
- Kim SW, Park SW, Lim SH, Kwon SU, Choi YJ, et al. (2006) Amount of left ventricular hypertrophy determines the plasma N-terminal pro-brain natriuretic peptide level in patients with hypertrophic cardiomyopathy and normal left ventricular ejection fraction. Clin Cardiol 29: 155-160.
- Battal F, Ermis B, Aktop Z, Can M, Demirel F (2011) Early cardiac abnormalities and serum N-terminal pro B-type natriuretic peptide levels in obese children. J Pediatr Endocrinol Metab 24: 723-726.
- Micl?u? S, Morno? C, Maximov D, Lupu A, Popa D, et al. (2009) [The myocardial performance index (Tei-Index): correlation with seric NTproBNP levels in patients with dilated cardiomyopathy]. Rev Med Chir Soc Med Nat Iasi 113: 391-396.
- Pervanidou P, Akalestos A, Sakka S, Kanaka-Gantenbein C, Papassotiriou I, et al. (2010) Gender dimorphic associations between N-terminal pro-brain natriuretic peptide, body mass index and blood pressure in children and adolescents. Horm Res Paediatr 73: 341-348.
Citation: Mehmet B, Ozgur P, Bumin D, Bedir A, Nezaket E (2015) Interrelations between Serum N-Terminal Pro B-Type Natriuretic Peptide (Nt-Probnp) Levels and Early Cardiovascular Risk Factors and Echocardiographic Parameters in Obese Adolescents. J Neonatol Clin Pediatr 2: 005.
Copyright: © 2015 Mehmet Boyraz, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

