
Intravascular CO2 Injector: An Initial in vivo Feasibility Study of the Prototype
*Corresponding Author(s):
Luiz LanziottiMedical Research Laboratory Anatomy And Vascular Surgery, School Of Medicine, Universidade De São Paulo (USP), São Paulo, Brazil
Tel:+55 2122454320,
Email:luizlanziotti@gmail.com
Abstract
Purpose: Iodinated contrast is the gold standard in endovascular procedures. However, adverse effects after injection of iodinated contrast, such as contrast-induced nephropathy, limit its use. Intravascular injection of medical-grade Carbondioxide (CO2) is widely accepted as a safe alternative to iodinated contrast, but its handling and manual delivery system may pose risks to patients, such as room air contamination. To address these issues, we developed an automated, microprocessor-based system for delivery of CO2 as a contrast agent. This study aimed to test in an animal model in vivo the clinical feasibility of this prototype system, comparing it with iodinated contrast injection as the reference standard.
Methods: Ten Large White pigs were randomized into two groups: iodinated contrast (n=5) and CO2(n=5). All animals were subjected to angioplasty of the left renal artery with either iodinated contrast or injection of CO2 with the prototype system, according to group assignment. Laboratory blood gas analyses were performed pre, peri and postoperatively. Renal function was monitored by measuring serum creatinine preoperatively and at 48 hours postoperatively. Animals were kept under clinical observation for 48 hours after the procedure.
Results: Angioplasty was technically successful in all animals in both groups, with no need for additional imaging with iodinated contrast in the CO2 group. There were no technical failures associated with the prototype CO2 injector. There was no clinical or radiological evidence of contamination with room air. All laboratory data were within the normal range. Angiographic images obtained in the CO2 group were subjectively considered of inferior quality.
Conclusion: The prototype intravascular CO2 injector was suitable for the proposed procedures and effective in obtaining images, allowing successful procedures without unexpected complications in agreement with previous positive prototype in vitro results. The present results demonstrate the initial technical feasibility of the device.
Keywords
INTRODUCTION
Iodinated contrast with a high viscosity solution is currently the most commonly used contrast agent for DSA. However, it has several side effects with accompanying risks to patients. About 1% of people are allergic to iodine, which may cause anaphylactic reactions on injection resulting in serious toxic effects and even rarely in death [2-6]. In addition, the use of iodinated contrast material may aggravate pre-existing heart failure and lead to acute renal dysfunction, known as contrast-induced nephropathy requiring dialysis in some cases [7-9].
CO2 proved to be an efficient contrast agent over the last decades, and its intravascular use is well supported by the literature [10-24]. Studies in animal models and in humans have shown that intravascular injections of CO2 with a volume of 100 to 200 cm3 cause no significant changes in vital signs [25-28]. With the advent of DSA in 1980, CO2-based angiography became a useful diagnostic tool, particularly for patients hypersensitive to iodine-based contrast media or with renal impairment [23,29]. When injected, CO2 displaces blood creating a small bubble that moves with blood flow before being dissolved and eliminated by the blood passing through the lungs. This short time is sufficient to acquire a series of images similar to those obtained with the use of iodinated contrast [30].
There is at present, a striking disparity between the uses of these two contrast media although it has been long accepted that both agents are safe and effective in obtaining images. This difference is particularly relevant and even intriguing, considering that a significant proportion of patients with cardiovascular disease have or will have kidney disease due to a combination of several clinical factors, such as age, hypertension, diabetes, and even repeated kidney injury due to frequent use of high-osmolar iodine-based contrast media during imaging tests.
This mismatch occurs mainly because the manual preparation of CO2 injections is time consuming and attention demanding, and the delivery systems are often adapted for the capture of CO2 from high-pressure, high-capacity cylinders that are commonly present in the operating room, attached to the laparoscopic device. Even recent injection systems, using a plastic reservoir bag, pose a risk of room air contamination that cannot be detected by the human eye, with potentially catastrophic consequences [31-33]. These risks and technical difficulties, coupled with the benefits of using CO2 instead of iodinated contrast, have prompted the development of a portable, automated, microprocessor-based system for delivery of CO2 as a contrast agent.
The current study was therefore designed to test in a porcine model in vivo the initial feasibility of a microprocessor-based prototype system for CO2 delivery during DSA, named “intravascular CO2 injector”, by comparing it with injection of iodinated contrast as the reference standard.
MATERIALS AND METHODS
Eleven adult male large white pigs weighing 42 to 53 kg were used in the study. One animal was selected for a pilot study aimed at determining the optimal parameters of the intensity of radiation produced by the fluoroscopy unit and the optimal frequency of image acquisition while injecting CO2. In this in vivo feasibility evaluation, we chose to simulate an endovascular procedure of balloon angioplasty of the left renal artery in 10 animals, which were randomized to the prototype intravascular CO2 injector or to the intravascular iodinated contrast injector. The latter was chosen as the parameter for comparison because iodinated contrast angiography is the gold standard method for vascular imaging. All experimental procedures were performed using a mobile C-Arm digital subtraction imaging system (Philips BV 25 Gold, Philips Medical Systems, Netherlands).
The animals were randomly divided into two groups
Group 2 (n=5): Angiography of the aorta just below the diaphragm and left renal artery and renal balloon angioplasty with injection of CO2 as the contrast agent using the prototype CO2 injector developed for this study which was operated in continuous mode for delivery of a continuous micro flow of CO2 aiming to prevent contamination with room air (Figure 1).
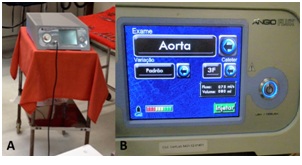 Figure 1: A) Prototype intravascular CO2 contrast injector. B) LCD touch screen monitor of the prototype CO2 injector.
Figure 1: A) Prototype intravascular CO2 contrast injector. B) LCD touch screen monitor of the prototype CO2 injector.Serial laboratory blood gas analyses were performed at three predetermined time points to monitor blood parameters for possible changes caused by CO2 injection: 1 - At the beginning of the procedure; 2 - Immediately after the completion of angiography (with CO2 or iodinated contrast); 3 - At the end of the procedure, immediately before removal of the arterial sheath. Arterial blood was withdrawn through the side port of the vascular introducer inserted in the femoral artery. Renal function was monitored by measuring serum creatinine. The reference values for normal serum creatinine levels in pigs are 1.0 to 2.7 mg/dL [34]. Blood samples were collected the night before and 48 hours after the procedure. During the 48-hour observation period, the animals remained under care at the laboratory, including monitoring of animal behavior and basic motor skills, with free access to water and regular pig chow.
Animals underwent daily clinical assessments throughout the study period to monitor their general health status. Only animals considered clinically healthy were used in the study. At the end of the study period, all animals were killed under general anesthesia.
Surgical technique: All anesthetic procedures were performed under the supervision of a veterinarian. The animals were preanesthetized with intramuscular ketamine (5.0 mg/kg) and midazolam (0.25 mg/kg) mixed in the same syringe. Fifteen minutes after injection, the marginal ear vein was catheterized with a 20G catheter for induction of anesthesia with 20 mg/kg of sodium thiopental. Animals were intubated with size 6 endotracheal tubes, and anesthesia was maintained with halothane. Analgesia was maintained with fentanyl, at an initial dose of 5 µg/kg, followed by continuous intravenous infusion of 0.4 µg/kg/min. During mechanical ventilation, pulse oximetry and capnometry were used for continuous evaluation of pulmonary function.
The same surgical team following standard endovascular techniques performed all procedures. Before surgery, the skin in the femoral region was shaved and cleaned with chlorhexidine gluconate 2%. Access to the right femoral aorta was established with an 18G needle y ultrasound-guided puncture, and a short 5F sheath was then implanted. After systemic heparinization with 5000 IU of sodium heparin, a 5F pigtail angiographic catheter was introduced and advanced over a hydrophilic guide wire for catheterization of the aorta, followed by aortography to locate the renal arteries. The contrast agent was then injected, according to group assignment, as follows: iodinated contrast - 10 mL, at a flow rate of 10 mL/s (Figure 2); CO2 - 20 mL, at a flow rate of 10 mL/s (Figure 3).
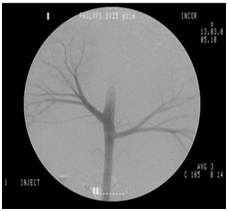 Figure 2: Initial iodinated contrast angiography of the aorta.
Figure 2: Initial iodinated contrast angiography of the aorta.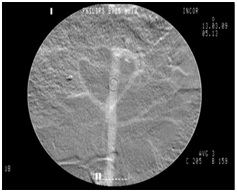 Figure 3: Initial CO2 contrast angiography of the aorta.
Figure 3: Initial CO2 contrast angiography of the aorta.Evaluation of the quality of angiographic images: Arteriograms obtained with CO2 and iodinated contrast agents were subjectively evaluated for the quality of images produced. No quantitative score was used. Two vascular surgeons involved in the study analyzed the acquired images to determine whether they could identify the animals’ renal arteries and perform renal angioplasty with technical success. A positive outcome was defined as the successful performance of angioplasty with CO2 without the need to use additional images obtained with iodinated contrast as a complement.
RESULTS
There were no technical failures associated with the use of the prototype intravascular CO2 injector. During the procedures using the prototype system, the device was operated stably with no clinical or laboratory evidence of contamination with room air.
The only type of complication was vasospasm of the distal renal artery, occurring in four cases (two related to the use of iodinated contrast and two related to the use of CO2). The occurrence of these spasms was attributed to catheter manipulation of the renal artery, with spontaneous resolution in all cases as observed on the control arteriogram.
Blood pH and arterial partial pressure of carbondioxide (PaCO2) values obtained pre, peri and postoperatively are shown in table 1 and 2 respectively. In both groups, all parameters of blood gas analysis were relatively stable over time and laboratory data were within the normal range, with no difference between groups.
|
n |
Contrast agent |
Initial |
Post-contrast |
Final |
|
1 |
CO2 |
7.40 |
7.17 |
7.39 |
|
2 |
Iodinated |
7.27 |
7.25 |
7.28 |
|
3 |
CO2 |
7.38 |
7.30 |
7.31 |
|
4 |
CO2 |
7.26 |
7.28 |
7.36 |
|
5 |
Iodinated |
7.38 |
7.30 |
7.36 |
|
6 |
CO2 |
7.40 |
7.38 |
7.39 |
|
7 |
Iodinated |
7.26 |
7.28 |
7.35 |
|
8 |
Iodinated |
7.37 |
7.30 |
7.37 |
|
9 |
CO2 |
7.46 |
7.40 |
7.42 |
|
10 |
Iodinated |
7.40 |
7.36 |
7.38 |
|
n |
Contrast agent |
Initial |
Post-contrast |
Final |
|
1 |
CO2 |
35.1 |
72.4 |
38.3 |
|
2 |
Iodinated |
51.4 |
53.2 |
50.4 |
|
3 |
CO2 |
43.9 |
54.6 |
52.6 |
|
4 |
CO2 |
57.9 |
58.3 |
45.4 |
|
5 |
Iodinated |
46.5 |
52.2 |
49.0 |
|
6 |
CO2 |
40.6 |
43.2 |
41.2 |
|
7 |
Iodinated |
61.9 |
58.1 |
52.5 |
|
8 |
Iodinated |
45.2 |
47.0 |
49.2 |
|
9 |
CO2 |
37.0 |
38.9 |
41.6 |
|
10 |
Iodinated |
46.7 |
47.5 |
48.8 |
The greatest variation in pH was observed in animal no. 1 from the CO2 group, which showed a decrease in pH from 7.4 (initial) to 7.17 (immediately after CO2 injection), stabilizing at 7.39 at the end of the procedure (Table 1, Figure 4). This variation was attributed to the presence of a CO2 concentration of 72.4 mmHg in the blood sample collected immediately after injection of CO2 (before metabolization in the lung), which decreased to 38.3 mmHg postoperatively, a level close to the initial value of 35.1 mmHg (Table 2, Figure 5). Nevertheless, both the initial and final values were within the normal range.
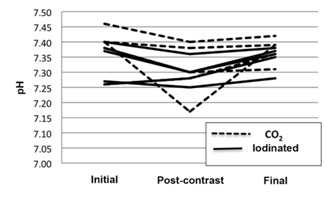 Figure 4: Graphical presentation of the 3 blood pH measures performed in each animal of the two experimental groups (arterial blood gas analysis).
Figure 4: Graphical presentation of the 3 blood pH measures performed in each animal of the two experimental groups (arterial blood gas analysis).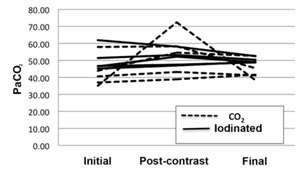 Figure 5: Graphical presentation of blood PaCO2 (in mmHg) in the two experimental groups (arterial blood gas analysis).
Figure 5: Graphical presentation of blood PaCO2 (in mmHg) in the two experimental groups (arterial blood gas analysis).|
n |
Contrast agent |
Preop |
48 h Postop |
|
1 |
CO2 |
1.66 |
1.45 |
|
2 |
Iodinated |
1.45 |
1.3 |
|
3 |
CO2 |
1.61 |
1.7 |
|
4 |
CO2 |
1.7 |
1.8 |
|
5 |
Iodinated |
1.55 |
1.6 |
|
6 |
CO2 |
2.6 |
2.3 |
|
7 |
Iodinated |
1.5 |
1.8 |
|
8 |
Iodinated |
1.7 |
1.7 |
|
9 |
CO2 |
1.9 |
1.7 |
|
10 |
Iodinated |
1.8 |
1.6 |
None of the animals had contrast-induced nephropathy, regardless of the type of contrast used. As measured by digital capnography, there was no sustainable change in pulmonary function in either group during the procedures, except for a slight increase in the 2-3 breaths that followed CO2 injection, as expected, returning to the initial parameters in all animals.
During the 48-hour observation period, all animals resumed their normal behavior, with regular chow intake and water drinking and no clinical changes as established by the veterinarian. The renal arteries could be correctly identified on all angiographic images. In the subjective analysis of the quality of angiographic images, CO2 was considered to provide inferior image quality compared to iodinated contrast, without, however, preventing the procedure from being performed. No quantitative method was used for image comparison.
DISCUSSION
The in vivo evaluation of a prototype device, even in a small number of animals, allows the detection of possible design errors and issues related to the practical applicability of the prototype that have not been identified in vitro. As previously demonstrated in vitro, the intravascular CO2 injector proved to be consistent in delivering scheduled CO2 injections, with no need to interrupt the procedure due to equipment failure. in vitro tests have also shown that the device has achieved adequate accuracy for use in arteriographic studies, since there is a wide margin of safety regarding the volume of CO2 injected. In the in vitro assay, with the gas flow adjusted to 20 mL/s, the difference between the total volume adjusted and total volume injected was less than 10%. This margin of error increased proportionally to higher adjusted volumes, without, however, affecting the intended use of the device, since it is recommended that the device be adjusted at a flow rate of 10 mL/s or 20 mL/s for angiography.
The design of the intravascular CO2 injector considered the difficulties and risks associated with the use of CO2. The benefits of electronic microprocessor technology, with highly reliable and portable devices, were employed to overcome the risk of contamination of the delivery system with room air, which was demonstrated by the positive CO2 flow while the device was powered on. This constant, very small flow of CO2 assures the device hose is not filled with room air. The apparatus also consists of a one-way valve assembly that allows us to connect the injection hose nozzle to the catheter or introducer, in the same way that has already been used for iodinated contrast injection, without the need for water seal. The sterile extension hose remains connected to the unit throughout the procedure in the sterile surgical field, ready to be used at any time. The microprocessor and valve system ensure the reproducibility of the gas volume at the predetermined flow rate, facilitating image comparison, a goal that cannot be achieved with the same degree of reproducibility by hand injection using a syringe with gas or a plastic reservoir bag.
Medical-grade CO2 costs about USD 50 for a 5-liter gas cylinder refill, which can be used for many procedures, a reduced cost compared to the USD 60 spent on a 50 mL single-use vial of iodinated contrast (Iodixanol, Visipaque 320 mg/mL, 50 mL vial - GE Healthcare). This difference in the price of the primary product allows the device tested here, with initial fixed cost estimated at USD 5,000, to become economically feasible, with recovery of the initial investment in about 3-5 months, considering a conservative scenario of use of three examinations per day. This information is particularly important when considering the potential use of this device in the public health system, which is the case in Brazil.
The easy handling of the intravascular CO2 injector allows the procedure to be performed at the same speed as the procedures commonly performed with iodinated contrast injectors, since the delivery system is ready for use throughout the procedure, with continuous supply of CO2 in the apparatus. The necessary adjustments to the CO2 injector are similar to those required in the iodinated contrast injectors, that is, total volume of CO2 (in mL) and injection flow rate (in mL/s). The device also has pre-stored parameters from the most relevant studies, facilitating change of volume flow configuration for angiography. In the currently available hand-delivering methods for CO2 injection, when using syringes manually loaded under water seal or devices with a plastic reservoir bag, handling is more time consuming, and it is necessary to purge the system when loaded to reduce the risk of room air contamination, compromising agility.
This study has some limitations. The quality of the angiographic images obtained with CO2 was considered, although subjectively analyzed by investigators involved in the procedures, of inferior quality compared to those obtained with iodinated contrast. However, all the proposed procedures with CO2 were successfully performed and no technical difficulties were reported in the identification of the renal arteries when the CO2 injector was used, with a similar number of successfully completed angiographic procedures in both groups (Figures 6,7). The fluoroscopy unit used in the present study dates from the early 1990s and can be considered obsolete, in light of equipment produced nowadays, and this may have been the main cause of inferior image quality reported in the cases using CO2. Hemodynamic and C-arm systems with updated software already have specific programs for image acquisition using CO2, optimizing and providing similar image quality with both contrast agents. Even with the drawbacks of a technologically outdated fluoroscopy unit, the resolution of the images produced in this study was satisfactory in all cases, allowing clear identification of the primary and secondary branches of the renal artery.
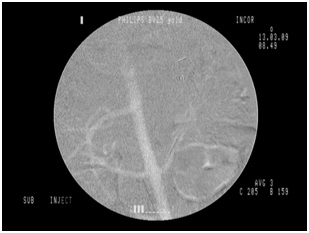 Figure 6: CO2 angiography, animal 4.
Figure 6: CO2 angiography, animal 4.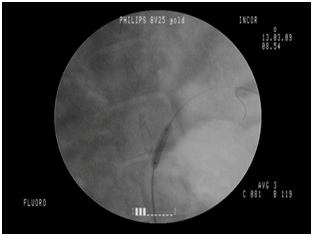 Figure 7: Renal angioplasty, animal 4.
Figure 7: Renal angioplasty, animal 4.The prototype evaluated here should join the automated class of CO2 injectors, once it makes its way into the market.
The absence of laboratory abnormalities in the intraoperative tests (serial blood gas analysis) and postoperative tests (serum creatinine) is consistent with published data that demonstrate the safety of CO2 injection as an angiographic contrast medium. The animals remained clinically and hemodynamically stable both during surgery and in the first 48 hours postoperatively. Within the scope of this initial technical feasibility study, the results suggest proper functioning of the prototype intravascular CO2injector.
CONCLUSION
FINANCIAL DISCLOSURE
Belczak and Silva have no financial relationships relevant to this article to disclose.
CONFLICT OF INTEREST
REFERENCES
- World Health Organization (2017) The top 10 causes of death. WHO, Geneva, Switzerland.
- Bellin MF, Stacul F, Webb JA, Thomsen HS, Morcos SK, et al. (2011) Late adverse reactions to intravascular iodine based contrast media: an update. Eur Radiol 21: 2305-2310.
- Jensen H, Doughty RW, Grant D, Myhre O (2011) The effects of the iodinated X-ray contrast media iodixanol, iohexol, iopromide, and ioversol on the rat kidney epithelial cell line NRK 52-E. Ren Fail 33: 426-433.
- Scherer K, Harr T, Bach S, Bircher AJ (2010) The role of iodine in hypersensitivity reactions to radio contrast media. Clin Exp Allergy 40: 468-475.
- Khachman D, Gandia P, Sallerin F, Mailly N (2009) [Immediate and delayed hypersensitivity reactions to iodinated radiographic contrast agents: an update]. Therapie 64: 331-339.
- Seitz CS, Pfeuffer P, Raith P, Bröcker EB, Trautmann A (2009) Radiocontrast media-associated exanthema: identification of cross-reactivity and tolerability by allergologic testing. Eur J Radiol 72: 167-171.
- Goossens A, Vander Elst L, Van Haverbeke Y, Muller RN (1995) Mechanical and biochemical cardiac disturbances induced by bolus administration of iodinated contrast media and noniodinated solutions. Invest Radiol 30: 1-9.
- Briguori C, Quintavalle C, De Micco F, Condorelli G (2011) Nephrotoxicity of contrast media and protective effects of acetylcysteine. Arch Toxicol 85: 165-173.
- Wong GTC, Irwin MG (2007) Contrast-induced nephropathy. Br J Anaesth 99: 474-483.
- de Almeida Mendes C, de Arruda Martins A, Teivelis MP, Kuzniec S, Nishinari K, et al. (2014) Carbondioxide is a cost-effective contrast medium to guide revascularization of TASC A and TASC B femoropopliteal occlusive disease. Ann Vasc Surg 28: 1473-1478.
- Chao A, Major K, Kumar SR, Patel K, Trujillo I, et al. (2007) Carbondioxide digital subtraction angiography-assisted endovascular aortic aneurysm repair in the azotemic patient. J Vasc Surg 45: 451-458; discussion 458-460.
- Pérez L, Lecannelier E, Fernández A, Parra J, Aburto G, et al. (2007) [Renal angioplasty with stent using CO2 as contrast medium: report of one case]. Rev Med Chil 135: 365-369.
- Heye S, Maleux G, Marchal GJ (2006) Upper-extremity venography: CO2 versus iodinated contrast material. Radiology 241: 291-297.
- Cronin P, Patel JV, Kessel DO, Robertson I, McPherson SJ (2005) Carbondioxide angiography: a simple and safe system of delivery. Clin Radiol 60: 123-125.
- Teng GJ (2005) [Novel use of CO2 splenoportography: via spleen percutaneous fine needle puncture portography]. Zhonghua Yi Xue Za Zhi 85: 295-296.
- Lorch H, Steinhoff J, Fricke L, Wolny A, Kagel C, et al. (2003) [CO2 angiography of transplanted kidneys]. Rontgenpraxis 55: 26-32.
- Kessel DO, Robertson I, Patel J 5th, Peters K, Taylor EJ, et al. (2002) Carbondioxide-guided vascular interventions: technique and pitfalls. Cardiovasc Intervent Radiol 25: 476-483.
- Chanis G (2002) [Carbondioxide angiography: technique and indications]. Rev Med Panama 27: 18-25.
- Beese RC, Bees NR, Belli AM (2000) Renal angiography using carbondioxide. Br J Radiol 73: 3-6.
- Górriz E, Carreira JM, Reyes R, Gallardo L, Pulido JM, et al. (1999) CO2 as a contrast medium in endoluminal treatment of high flow vascular malformations. Eur J Radiol 31: 182-187.
- Barbey MM, Farber A, Marienhoff N, Gmelin E (1999) [Digital subtraction angiography with carbondioxide--basic principles, technique and clinical application]. Vasa 28: 243-249.
- Herrmann K, Waggershauser T, Bonél H, Glaser C, Sittek H, et al. (1998) Kontrastmitteluntersuchungen des Venensystems. Der Radiologe 38: 570-577.
- Hawkins IF, Caridi JG (1998) Carbondioxide (CO2) digital subtraction angiography: 26-year experience at the University of Florida. Eur Radiol 8: 391-402.
- Krasny R, Besgen J, Birkenkamp H, Klose KC, Günther RW (1990) [Arterial DSA using CO2 as a contrast medium: improvement of picture quality using a novel gas injector in an animal experiment]. Rofo 152: 425-429.
- Moore RM, Braselton CW (1940) Injections of Air and of Carbondioxide into A Pulmonary Vein. Ann Surg 112: 212-218.
- Barrera F, Durant TM, Lynch PR, Oppenheimer MJ, Stauffer HM, et al. (1956) In vivo visualization of intracardiac structures with gaseous carbondioxide; cardiovascular-respiratory effects and associated changes in blood chemistry. Am J Physiol 186: 325-334.
- Turner AF, Meyers HI, Jacobson G, Lo W (1966) Carbondioxide cineangiocardiography in the diagnosis of pericardial disease. Am J Roentgenol Radium Ther Nucl Med 97: 342-349.
- Scatliff JH, Kummer AJ, Janzen AH (1959) The diagnosis of pericardial effusion with intracardiac carbondioxide. Radiology 73: 871-883.
- Hawkins IF (1982) Carbondioxide digital subtraction arteriography. AJR Am J Roentgenol 139: 19-24.
- Nicolini A, Lovaria A, Meregaglia D, Palatresi S (2000) [Carbondioxide angiography. A new injection system]. Radiol Med 99: 51-55.
- Kerns SR, Hawkins IF Jr (1995) Carbondioxide digital subtraction angiography: expanding applications and technical evolution. AJR Am J Roentgenol 164: 735-741.
- Kozlov DB, Lang EV, Barnhart W, Gossler A, De Girolami U (2005) Adverse cerebrovascular effects of intraarterial CO2 injections: development of an in vitro/in vivo model for assessment of gas-based toxicity. J Vasc Interv Radiol 16: 713-726.
- Spinosa DJ, Matsumoto AH, Angle JF, Hagspiel KD, Hooper TN (1998) Transient mesenteric ischemia: a complication of carbondioxide angiography. J Vasc Interv Radiol 9: 561-564.
- Jackson PGG, Cockcroft PD (2002) Appendix 3: Laboratory Reference Values: Biochemistry. In: Jackson PGG, Cockcroft PD (eds.). Clinical Examination of Farm Animals. Blackwell Science Ltd, New Jersey, USA.
Citation: Lanziotti L, Belczack SQ, da Silva ES (2018) Intravascular CO2 Injector: An Initial in vivo Feasibility Study of the Prototype. J Angiol Vasc Surg 3: 011.
Copyright: © 2018 Luiz Lanziotti, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

