
Intravenous Administration of Nicotinamide Adenine Dinucleotide Alleviates Tremors Associated with Parkinson’s Disease A Case Report
*Corresponding Author(s):
Broom SLSchool Of Natural And Behavioral Sciences, Department Of Psychology, William Carey University, Hattiesburg, Mississippi, United States
Tel:+1 2283040522,
Email:sgibson@wmcarey.edu
Abstract
Introduction: Parkinson’s Disease (PD) is a neurodegenerative disorder characterized by Lewy body formation and dopaminergic neuronal death in the substantia nigra. Current pharmacological dopamine agonists and dopamine replacement therapy for PD treatment has shown adverse effects including hallucinations, cardiovascular complications, psychosis and further dyskinesia. The co-factor Nicotinamide Adenine Dinucleotide (NAD+) serves a vital role in cell functionality and dopaminergic neuronal replenishment, where NAD+ depletion has been associated with the onset of neurodegenerative disease such as PD. Additionally, in a previous case study in a PD patient, an Intravenous (IV) NAD+ administration protocol showed a rapid and sustained alleviation of PD-related symptoms, providing rationale for further investigating the positive effects of IV NAD+ through quantifiable measurements on tremors and cognitive function associated with PD.
Method: A 59 year old male was diagnosed with PD four years prior to entering an outpatient clinic for treatment, reporting to have used PD medications with no alleviation of symptoms. Patient received a specific protocol of NAD+ (called BR+NAD) for a total of six days, with two days of 1,500mg IV NAD+, followed by four days of 500-750mg IV NAD+. Tremors were recorded in the client’s dominant right hand using an accelerometer and gyroscope. Researchers recorded periodic measurements (Hz) beginning on Day 2 through the end of Day 5 with 13 total measurements, on three axes; vertical, horizontal and anterior-posterior. Following Day 6, client received sublingual NAD+ tablets (300mg, twice per day X 14 days) after finishing IV treatment.
Results: 1) Vertical axis tremors decreased by 75.9%, horizontal axis tremors decreased by 83.0% and anterior-posterior axis tremors decreased by 9.1%. 2) Mean tremor on Day 2 was 44.5Hz, while on Day 6 was 20.6Hz, resulting in a decline of 54.7% over the 4 day span. 3) Patient self-report of tremors continued to decline in the clients left and right hands two weeks post BR+NAD (average for both hands 12Hz) with maintenance sublingual supplementation and application of a relaxation technique.
Conclusion: These data show the effectiveness and endurance of the initial IV BR+NAD followed by sublingual tablets (300mg, twice per day) in maintaining decline and alleviation of PD symptoms. Additionally, these results substantiate previous research and case study findings, while establishing a protocol for empirically measuring PD symptom changes of IV NAD+.
Keywords
Accelerometer and gyroscope; Nicotinamide adenine dinucleotide; Parkinson’s disease; Parkinson’s symptoms; Tremors
INTRODUCTION
Parkinson’s Disease (PD) is a progressively disabling neurodegenerative disease marked by the formation of Lewy body aggregates and the death of dopaminergic neurons within the brain’s substantia nigra. The symptoms of PD-related neurodegeneration often involve noticeable disturbances in motor functionality, including tremors and bradykinesia, as well as cognitive impairments in executive functioning, including compromised working memory, attention and problem-solving [1,2]. Current pharmacological treatment options involve the use of dopamine agonists and dopamine replacement agents, yet such treatments have produced significant adverse effects for patients, including visual hallucinations, dyskinesia and psychosis [3]. In consideration of alternate treatments for PD, prior research has shown that supplementation with the co-factor Nicotinamide Adenine Dinucleotide (NAD+), a critical molecule involved in maintaining healthy cellular redox metabolism and mitochondrial functionality, can replenish the neuronal loss implicated in PD and other neurodegenerative diseases [4]. With respect to prion and prion-like neurodegenerative diseases including Alzheimer’s, Parkinson’s, and Huntington’s diseases, researchers found that NAD+ starvation in a murine model induced neurodegeneration, whereas NAD+ supplementation displayed neuroprotective qualities in delaying neurodegeneration in prion-infected mice [5]. Additionally, a recent study found a direct link between the administration of an NAD+ precursor in the prevention of dopaminergic neuronal loss amongst patient-derived induced pluripotent stem cells of those with Parkinson’s disease [6]. This increasing evidence supports the rationale for further investigating the therapeutic potential of NAD+ as an alternative form of treatment in PD.
In a prior case study conducted by Gadol and colleagues [7], one Parkinson’s patient showed significant improvement in PD symptomology, in addition to the discontinuation of PD-related medication, following an 8-day treatment course of intravenously delivered NAD+ with monthly IV maintenance. Based on these findings, the present case study sought to quantitatively measure and further elaborate on similar symptom alleviation effects found in another PD patient [8] through directly measuring PD symptoms and corresponding symptom changes over a 6-day treatment course of intravenously delivered NAD+ [9].
METHOD
We present a case of a 58-year-old male who had been diagnosed with PD five years prior to entering an outpatient clinic specializing in IV (Intravenous) NAD+ therapy. Before beginning treatment, the patient reported the following PD symptoms: gait rigidity (an impairment in ability to walk fluidly), bilateral hand tremors with a more pronounced tremor in the right hand, impaired movement of fingers and hands, difficulty typing, and difficulty opening and closing a fist. Additionally, the patient indicated that in the past four years, his increased levels of anxiety and stress, as well as lack of consistent, healthy sleep, had greatly affected his quality of life and led to an increase of PD symptomology. According to the patient, his Parkinsonian symptoms began manifesting with severity over these last four years. The patient reported taking PD medications in the class of dopamine agonists during these four previous years, yet decided to discontinue his PD-related medication before entering NAD+ treatment, reporting that he had minimal alleviation of symptoms.
The treatment (known as BR+NAD, or Brain Restoration Plus NAD) was comprised of IV infusions of NAD+, as well as added oral supplementation of Vitamin C, B6, Folate, Selenium, N-acetyl-L-cysteine, N-acetyl-L-tyrosine and electrolytes. The patient received BR+NAD for a total of six treatment days. He received 1,500mg of BR+NAD for Days 1 and 2, followed by 500-750mg of BR+NAD for Days 3-6 (Table 1). Tremor measurements were recorded in his right, more compromised hand beginning on Day 2 using a computerized program that included a gyroscope and accelerometer, called Toozon Tremor. Empirical measurements of tremor severity (Hz) were analyzed on the vertical, horizontal and anterior-posterior axes. Additionally, researchers used a standardized battery of computerized tests using Lumosity to assess cognitive performance in the areas of memory, attention and problem solving on Day 1 (baseline score) and Day 5. Lastly, in addition to the BR+NAD infusions, the client learned and practiced a combination of relaxation techniques, including deep diaphragmatic breathing, progressive muscle relaxation, and a form of guided self-hypnosis (e.g., “With each breath I become more relaxed,” etc.,). The results of the patient’s cognitive performance on Day 5 of his IV BR+NAD treatment were compared to his baseline scores on Day 1 for observable, quantifiable evidence of improvements in cognitive abilities following his IV BR+NAD infusion. Following Day 6, for additional maintenance of his Parkinson’s symptoms, the patient received sublingual NAD+ tablets (300mg, twice per day) for 14 days. The patient recorded mean tremors for 14 days and provided additional self-report of changes through email follow-up. Data were prepared in table and figure formats and analyzed by descriptive analysis using Microsoft Excel.
RESULTS
Baseline symptomology, treatment dose/timeline and qualitative changes in PD symptoms are summarized in table 1.
|
Treatment Day |
BR+NAD Dose (mg) |
PD Symptoms and Symptom Changes |
|
Baseline |
0 |
Tremors in both hands; more pronounced in right hand, subtle but present in left hand; shuffling gate/gait rigidity; difficulty writing/making a fist; mood and cognitive ability rated appropriate |
|
Day 1 |
1, 500 |
Baseline Lumosity tests conducted; bilateral tremors present; patient reporting stress in am; By noon, patient reports decreasing tremors “Something is happening!” |
|
Day 2 |
1,500 |
Average tremors (left hand) declining from 47-24 HZ; Patient actively participating in treatment plan discussion |
|
Day 3 |
750 |
Mood and Sleep quality good; Average tremors right hand continue to decline, lowest point at 16.9Hz |
|
Day 4 |
750 |
Average tremors (right hand) stabilizing at 22.17 by mid-day; Patient noted that stressors caused an increase in tremors late in the day |
|
Day 5 |
500 |
Average tremors (right hand) final recording at 20.63HZ |
|
Day 6 |
750 |
Steady gait; Tremors reduced in combination with breathing exercises/relaxation "I feel good." Second round of Lumosity tests conducted. |
|
Day 20 |
300mg 2X day |
Steady gait; Patient reported tremors present initially at wake up time, then drop off with relaxation exercises |
Table 1: Summary of treatment days and corresponding PD symptoms.
As stated previously, the patient initially presented symptoms including bilateral hand tremors (significantly more pronounced in right hand), shuffling gate/gait rigidity and impairments in movements of the hands and fingers. The patient indicated that he did not experience cognitive or mood impairments typical of many PD patients. The first set of tremor measurements (in the left hand) occurred on Day 2 of the BR+NAD treatment. The patient took the first measurement at 12:00pm, approximately four hours after beginning his second BR+NAD infusion, or approximately 35 hours into the treatment process. The scores for Day 2 were 54.307Hz for vertical, 47.985Hz for horizontal and 31.216Hz for anterior-posterior. The patient took the second measurement at 1:00pm. These scores were 31.988Hz for vertical, 20.255Hz for horizontal and 30.133Hz for anterior-posterior. To note, within this one-hour timeframe, there was a 58% decrease in tremors on the vertical axis, a 42% decrease in tremors on the horizontal axis, and finally a 1% decrease on the anterior-posterior axis (Figure 1). On Day 3 of the patient’s BR+NAD treatment, the second set of measurements was gathered. The patient took the first measurement at 1:00pm, with measured tremor scores of 23.168Hz for vertical, 14.538Hz for horizontal and 30.143Hz for anterior-posterior. At 2:00pm, the second measurement was taken with scores of 22.343Hz for vertical, 13.084Hz for horizontal and 29.674Hz for anterior-posterior. At 3:00pm, the third measurement was taken with scores were 20.628Hz for vertical, 11.275Hz for horizontal and 18.718Hz for anterior-posterior. At 4:00pm, the fourth measurement was taken, with scores of 12.212Hz for vertical, 16.278Hz for horizontal and 20.802Hz for anterior-posterior (Figure 1). On Day 4 of receiving his BR+NAD infusion, the third set of measurements was recorded starting at 1:00pm. His scores were 24.2Hz for vertical, 13.1Hz for horizontal and 29.7Hz for anterior-posterior. At 3:00pm, the second measurement was taken with scores of 23.1Hz for vertical, 12.1Hz for horizontal and 33.3Hz for anterior-posterior. At 6.30pm, the third measurement was taken with scores of 37.0Hz for vertical, 16.1Hz for horizontal and 27.5Hz for anterior-posterior.
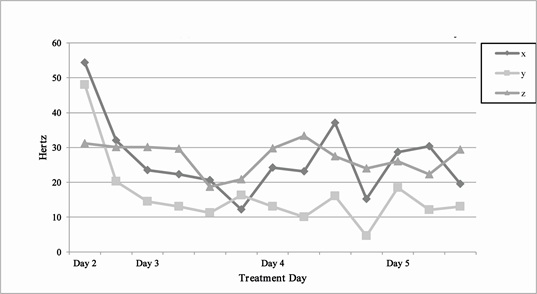 Figure 1: Tremors measured in hertz on vertical (X) horizontal (Y) and anterior-posterior (Z) axes as a function of IV BR+NAD treatment day.
Figure 1: Tremors measured in hertz on vertical (X) horizontal (Y) and anterior-posterior (Z) axes as a function of IV BR+NAD treatment day.
The patient reported that he felt the results for the vertical dimension were influenced by psychological stressors at the time, and therefore not quite as accurate a measurement as the prior measurements. At 6:45pm, the fourth measurement was taken, with scores of 15.2Hz for vertical, 4.6Hz for horizontal and 24.0Hz for anterior-posterior. On Day 5 of the six-day treatment, his fourth set of measurements was recorded starting at 8:15am. His scores were 28.6Hz for vertical, 18.5Hz for horizontal and 26.0Hz for anterior-posterior. At 12:30pm, the second measurement was taken with scores of 30.3Hz for vertical, 17.03Hz for horizontal and 22.3Hz for anterior-posterior. At 1:30pm, the third measurement was taken with scores of 19.5Hz for vertical, 13.0Hz for horizontal and 29.4Hz for anterior-posterior. As shown in figure 1, overall, the vertical axis tremors decreased by 75.9%, the lateral axis decreased by 83.0% and the anterior-posterior axis decreased by 9.1%. Collectively, the average decline from baseline to Day 6 was 54.7%. Patient self-report of tremors continued to decline two weeks post IV BR+NAD (average 12Hz) by utilizing maintenance NAD+ sublingual tablets, along with the application of relaxation techniques (data not shown).
On Day 1 and Day 5 of the patient’s IV BR+NAD, cognitive performance was measured in the areas of problem solving, attention and memory (Figure 2). Figure 2 shows raw scores on these three cognitive performance measures. Values range on a scale of 0-20,000 (zero being the lowest possible score; 20,000 being the highest possible score). On Day 1 of treatment, the patient’s score on the memory test was 3560, where on Day 5, the patient’s score increased by 29.8% to 4620. On Day 1 of treatment, the patient’s score on the attention test was 1500, where on Day 5, the patient’s score increased by 320% to 6300. On Day 1 of treatment, the patient’s score on the problem solving test was 17260, where on Day 5, the patient’s score increased by 7.5% to 18550.
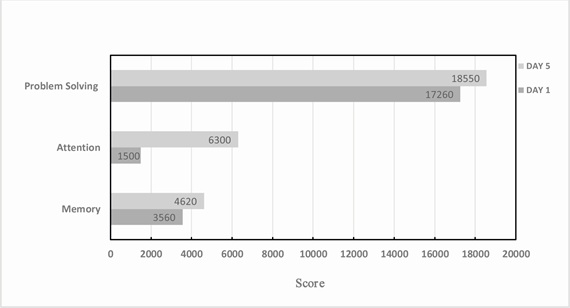 Figure 2: Cognitive performance scores on Day 1 and Day 5 of IV BR+NAD treatment day.
Figure 2: Cognitive performance scores on Day 1 and Day 5 of IV BR+NAD treatment day.
DISCUSSION
As the number of individuals diagnosed with Parkinson’s disease continues rising, with numbers of diagnosed cases nearly doubling globally between the years of 1990 and 2015, the importance of finding effective therapeutic approaches in disease treatment and prevention is paramount [10]. Prior research demonstrates the neuro-regenerative and neuroprotective capacity of NAD+ supplementation in both in vitro and in vivo studies, providing rationale for further investigation of the effects of NAD+ on neurodegenerative diseases such as PD [5,6]. Additionally, as intracellular NAD+ levels of this neuroprotective co-factor are known to progressively lower with age, where markedly low levels are indeed present in adult-onset neurodegenerative diseases of Alzheimer’s and Parkinson’s diseases, utilizing this co-factor for PD lends additional evidence for potentially promising treatment results [11,12].
In this case report, further evidence of the therapeutic potential of IV NAD+ [8] was indeed documented, supporting the decreased PD-related symptom findings of a previous case study of tremor reduction associated through NAD+ use [7]. Yet, this case study builds on these prior findings by providing quantifiable evidence of both tremor reduction and cognitive improvements. Over the course of the six NAD+ treatment days, the patient’s PD symptoms overall improved, as shown by a significant decline in hand tremors, development of a steady gate, increased sociability, increased cognitive functioning and improved sleep (Table 1). Significant tremor improvements on the vertical, horizontal, and anterior-posterior axes hold strong support in NAD+ supplementation in this individual. Additionally, the cognitive improvements in memory, attention, and problem solving from Days 1 to 5 may lend further support for the neuroprotective quality of NAD+ use. Although the increased cognitive scores are consistent with the Practice Effect (i.e., an individual may score better on a test when taken the second time in comparison to the first), the dramatic increase in his attention score by 320% may not be easily explained through this phenomenon. Moreover, in a murine study of Alzheimer’s affected mice, utilizing an NAD+ precursor for the building of intracellular NAD+ showed significant improvements in a number of cognitive performance tests [13]. Therefore, it is possible to attribute the improvement in this patient’s cognitive performance to be due in part to his NAD+ treatment program.
Additionally, the overall effectiveness and lasting effects of NAD+ in this particular patient can be understood by the continued decline in PD-related tremors both during treatment and through NAD+ sublingual tablets post-treatment (dosage was approximately 300mg of NAD+ per tablet, twice per day). If this trend in PD-symptom alleviation continues throughout the course of the patient’s follow ups, not only is strong evidence for the effectiveness of the IV infusion supported in this particular case, it also poses possible effectiveness for oral NAD+ supplementation in maintaining PD symptom stabilization. Likewise, as stress was a predominant trigger for the patient’s tremor severity, the positive patient self-reports of utilizing a combination of deep diaphragmatic breathing, progressive muscle relaxation, and guided imagery/self-hypnosis techniques on lowering his hand tremors supports relaxation techniques as additional treatment for PD sufferers.
As the delivery of NAD+ intravenously may not be practical in a growing population of individuals with Parkinson’s disease, this case study shows that the use of alternate means of NAD+ delivery, such as through the use of sublingual tablets, can be another promising method of reducing Parkinson’s disease symptomology. Additionally, the use of NAD+ precursors can be another promising and sustainable option in PD treatment. Previous research indicates that the NAD+ precursor, Nicotinamide Riboside (NR), significantly improves mitochondrial functionality in the cells of those with Parkinson’s disease exhibiting notable dysfunction, as well as prevents further loss of dopaminergic neurons and motor impairment in a fly model of PD [6]. Additionally, a clinical trial is currently underway investigating the role of nicotinamide supplementation in a neurodegenerative disease known to significantly impair motor function [14]. In summary, using such alternative approaches in boosting NAD+ for PD treatment may be promising to employ as well.
Overall, data discussed in this case study show repeatable and quantifiable results, and they also provide a protocol for treating the symptoms of PD in an efficient manner with little to no reported side effects during treatment and follow-up. Results show that IV BR+NAD effectively reduces tremors and improves cognitive performance in a subset of PD patients, further supporting previous findings on tremor alleviation using NAD+. Likewise, the results establish a protocol for empirically measuring the effects of IV NAD+ on PD symptoms, specifically tremors, and show the effectiveness of symptom alleviation in this treatment as compared to traditional PD pharmacotherapies.
ACKNOWLEDGEMENT
Thank you to Springfield Wellness Center for providing the setting and clinical staff in support for this project. Thank you to NAD Research, Inc. for funding this project. Thank you to Rider University for supporting this project. Thank you to William Carey University for providing a Professional Development Grant in sup- port of this project.
REFERENCES
- Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Invest 115: 1449-1457.
- Wojtala J, Heber IA, Neuser P, Heller J, Kalbe E, et al. (2019) Cognitive decline in Parkinson’s disease: The impact of the motor phenotype on cognition. J Neurol Neurosurg Psychiatry 90: 171-179.
- Kvernmo T, Härtter S, Burger E (2006) A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther 28: 1065-1078.
- Pehar M, Harlan BA, Killoy KM, Vargas MR (2017) Nicotinamide Adenine Dinucleotide Metabolism and Neurodegeneration. Antioxid Redox Signal 28: 1652-1668.
- Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, et al. (2015) Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain 138: 992-1008.
- Schöndorf DC, Ivanyuk D, Baden P, Sanchez-Martinez A, De Cicco S, et al. (2013) The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson's Disease. Cell Rep 23: 2976-2988.
- Gadol E, Mestayer RF, Grant R, Grigoryev Y, Gibson SB, et al. (2019) A case of Parkinson’s disease symptom reduction with intravenous NAD+. Case Reports and Literature Review 3: 1-4.
- Rutherford L, Broom SL, Olds T, Mestayer RF, Norris-Mestayer P (2019) Intravenous administration of nicotinamide adenine dinucleotide alleviates tremors associated with Parkinson’s disease: A case report. Poster Presentation at the Society for Neuroscience, Chicago, USA.
- Grant R, Berg J, Mestayer R, Braidy N, Bennett J, et al. (2019) A Pilot Study Investigating Changes in the Human Plasma and Urine NAD+ Metabolome During a 6 Hour Intravenous Infusion of NAD+. Front Aging Neurosci 11: 257.
- GBD 2016 Parkinson’s Disease Collaborators (2018) Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17: 939-953.
- Guest J, Grant R, Mori TA, Croft KD (2014) Changes in Oxidative Damage, Inflammation and [NAD(H)] with Age in Cerebrospinal Fluid. PLoS ONE 9: 85335.
- Hikosaka K, Yaku K, Okabe K, Nakagawa T (2019) Implications of NAD metabolism in pathophysiology and therapeutics for neurodegenerative diseases. Nutr Neurosci 7: 1-13.
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, et al. (2018) NAD+ supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA 115: 1876-1885.
- Reetz K, Hilgers RD, Isfort S, Dohmen M, Didszun C, et al. (2019) Protocol of a randomized, double-blind, placebo-controlled, parallel-group, multicentre study of the efficacy and safety of nicotinamide in patients with Friedreich ataxia (NICOFA). Neurological Research and Practice 1: 1-9.
Citation: Rutherford L, Gadol E, Broom SL, Olds T, Mestayer RF, et al. (2020) Intravenous Administration of Nicotinamide Adenine Dinucleotide Alleviates Tremors Associated with Parkinson’s Disease: A Case Report. J Gerontol Geriatr Med 6: 046.
Copyright: © 2020 Rutherford L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

