Introduction of Polymer Nanoparticles for Drug Delivery Applications
*Corresponding Author(s):
Garry L RempelDepartment Of Chemical Engineering, Waterloo Institute For Nanotechnology, University Of Waterloo, Waterloo, Ontario N2L 3G1, Canada
Tel:+1 5198884567, 32702
Fax:+1 5197464979
Email:grempel@uwaterloo.ca
Abstract
Keywords
INTRODUCTION
In the fields of molecular biology and medicine, cancer has been the leading cause of death and a serious threat to the body health of human beings. Until now, the main techniques to fight cancer are non-targeted chemotherapy and radiation. However, it is unavoidable to prevent systematic side effects to the human body due to non-specific uptake by normal, healthy, noncancerous tissues because of the instinctive properties of the chemotherapy chemical agent featured with high toxicity and a lack of tumor specificity.
In order to overcome the limitations of free chemotherapeutic agents, targeting of tumors with nanoparticulate drug carriers has received much attention and expectance [1,2]. Nanocarriers can offer many avenues over free drugs for the following aspects [3]: (1) protect the drug from premature degradation; (2) prevent drugs from prematurely interacting with the biological environment; (3) enhance absorption of the drugs into a selected tissue (for example, solid tumour); (4) control the pharmacokinetic and drug distribution profile; and (5) improve intracellular penetration.
Let us first recall the important moments in the short but rapid development history of drug delivery systems (Figure 1). Lipid is the first nanotechnology based drug delivery system, which was discovered in the 1960s and later known as liposomes [4]. After that, biomaterials made of a variety of organic and inorganic substances were developed for drug delivery. In 1976, the first controlled release polymer drug delivery system was reported [5]. In 1980, pH stimuli drug delivery systems to trigger drug release [6] and cell specific targeting of liposomes were reported [7,8]. In 1987, the first long circulating liposome named “stealth liposomes” was described [9]. Subsequently, the use of Polyethylene Glycol (PEG) was known to increase circulation times for liposomes [10] and polymer nanoparticles [11] in 1990 and 1994, respectively, which established a solid foundation for the development and subsequent approval of DOXIL (Doxorubicin Liposome) in 1995 for the treatment of epidemic (AIDS-related) Kaposi sarcoma [12].
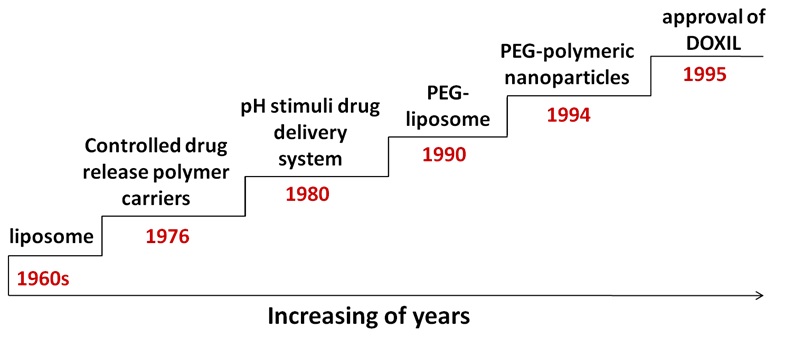
Figure 1: Highlights in the development history of nanoparticle based drug delivery system.
After fifty-years of development of drug delivery systems, biocompatible polymer nanoparticles have been regarded as a substantial promising effective drug delivery system characterized by the self-assembly of amphiphilic block copolymer surfactants (frozen micelles), dendrimers, vesicles, liposome, emulsions, microemulsion, and latex particles. Drug delivery systems based on polymer nanoparticles generally have the following properties [13-17]: (1) decreased immunogenicity; (2) protection from alteration and inactivation of the active drug; (3) altered biodistribution to reduce systemic toxicity; (4) elimination of multidrug resistance; (5) increased tumor cytotoxicity; (6) passive targeting by Enhanced Permeability and Retention (EPR); and (7) site-specific delivery of drugs by active targeting. On the other hand, in order to reach the rapid and effective clinical translation, these nanocarriers must (1) be made from a material that is biocompatible, well characterized, and easily functionalized; (2) exhibit high differential uptake efficiency in the target cells over normal cells or tissue; (3) be either soluble or colloidal under aqueous conditions for increased effectiveness; and (4) have an extended circulating half-life, a low rate of aggregation, and a long shelf life.
BIODEGRADABLE POLYMERS
Biodegradable polymers used in drug delivery studies can be broadly classified into two categories: natural and synthetic polymers. The majority of investigations into the use of natural polymers as drug delivery systems have concentrated on the use of proteins such as collagen, gelatin, insulin, and albumin and polysaccharides such as starch, dextran, cellulose, and hyaluronic acid.
Various synthetic degradable polymers have been reported for the formulation of controlled drug delivery systems, since they can be synthesized with specific properties to meet particular applications. Probably the most widely investigated biodegradable synthetic polymers are linear aliphatic polyesters Poly (Lactic Acid) (PLA), Poly (Glycolic Acid) (PGA), Poly (Lactic Acid-co-Glycolide) (PLGA), and Poly (ε-Caprolactone) (PCL). They exhibit important advantages of biocompatibility, predictability of biodegradation kinetics, ease of fabrication, and regulatory approval.
Polyanhydride polymers and copolymers have also received considerable interest for the preparation of drug delivery systems due to their labile anhydride linkages in the polymer structure. In addition, poly (ortho ester)’s and polyphosphazenes have generated a lot of research interest for the formulation of nanoparticulate drug delivery systems [18,19]. Poly (ortho ester)’s have the advantage of undergoing controllable degradation. The degradation of polyphosphazenes and the linkage of reactive drug molecules to the polymer backbone are controlled by the side-group modification. Langer and coworkers reported a family of synthetic biodegradable Poly (Polyol Sebacate) (PPS) via the bulk polycondensation reactions of the polyol and sebacic acid monomers [20]. Material properties including the physicochemical and mechanical properties as well as the degradation rates of the obtained PPS can be tuned by altering the reacting stoichiometric ratio of monomers polyol and sebacic acid. Through the assessment of biocompatibility in vitro and in vivo, PPS polymers were found have comparable biocompatibility to Poly (L-Lactic-co-Glycolic Acid) (PLGA) which is a type of material approved for human use.
DISCUSSION
FMH can occur as an acute or chronic event. In the chronic presentation, the hemorrhage has been prolonged or repeated during pregnancy, anemia developed slowly, giving the fetus the opportunity to develop hemodynamic compensation. In this case, the diagnosis is often postnatal and these infants may manifest only pallor at birth with no other complications [5].
In opposition, in acute FMH, perinatal hypoxia and intrauterine death or severe anemia and hypoxia at birth can be present [5].
Neonatal anemia was the first manifestation of FMH in 35% of the reported cases. In severe ones, shock and circulatory failure may be present [6].
In our clinical report, it’s more likely to be a chronic transfusion given the rapid ability of the newborn to adapt to extra uterine life despite the hemoglobin value at birth. The absence of fetal movements noted by the mother was not due to the FMH but it was caused by the circular of the umbilical cord around the arms that made it impossible to the fetus to move.
Abdominal trauma and invasive techniques of prenatal diagnosis are related to FMH [1]. Physicians should consider alternative diagnosis to neonatal anemia such as isoimmune hemolytic anemia, congenital infections that result in bone marrow suppression (TORCH), sepsis, congenital erythrocyte defects and congenital hemoglobinopathies [6]. Clinical and laboratory evaluation of infection, Coombs test and viral serology should be performed.
In this case, the diagnosis of FMH was confirmed by the KB test. Pink fetal red blood cells are observed and counted in the mother’s peripheral blood smear because fetal hemoglobin is resistant to acid elution, leaving discolored maternal cells (patients with sickle cell anemia or hereditary persistence of fetal hemoglobin may lead to a false positive result and ABO incompatibility may produce a false negative result) [2].
Although the KB test is inexpensive and requires no special equipment, it lacks standardization and is imprecise [3]. Flow cytometry, based on the use of anti-fetal hemoglobin for detection of fetal cells with fetal hemoglobin, represents an improvement of KB test since is more specific and precise [7].
Although the prognosis of massive FMH is poor, it can be improved if physicians early recognize this condition. When the infant is near-term gestation, immediate cesarean delivery is indicated. If, on the other hand, the fetus is still preterm, in uterus transfusion can be considered and has been shown to be effective and improves the prognosis [6].
Long term outcome for infants affected by massive FMH is unfavorable with death or neurological dysfunction [6]. The prognosis is more directly related to initial hemoglobin value and clinical manifestations post-delivery than with the transfused volume of blood [8].
The case reported emerged from a pregnancy with no risk factors. Mother’s perception of decreased fetal movements, recognition of fetal distress on the CTG, immediate caesarean section and prompt hemodynamic and respiratory support to the newborn with early red blood cells transfusion contributed for this good outcome.
LIPOSOME
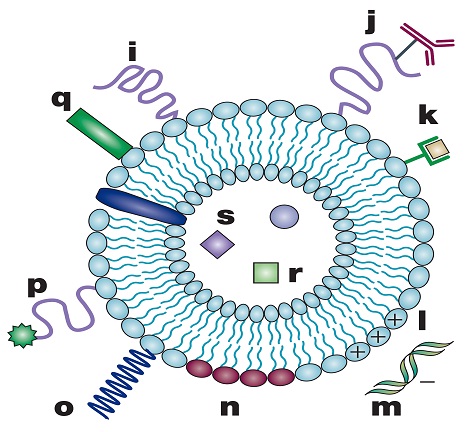
For the liposome to act as a useful drug carrier, it should as a minimum be able to retain an encapsulated drug for a sufficiently long time after its administration in order to appropriately alter the pharmacokinetics of the drug. However, there have been major drawbacks existing in the use of liposomes for targeted drug delivery, most notably due to poor control over drug release from the liposome (i.e., the potential for leakage of the drug into the blood), low encapsulation efficiency and manufacturability at the industrial scale, and poor storage stability [23,24]. Numerous studies have been carried out to meet these challenges and considerable progress has been achieved, as seen from Torchilin’s review articles [25,26]. Figure 2 represents the illustrative structure of a new generation of liposomes, which can be tailor-made to carry different types of functional groups with an aim to reach the optimal interplay of various biophysicochemical parameters as denoted by the letters in figure 2.
STIMULI-RESPONSIVE AMPHIPHILIC BLOCK COPOLYMERS
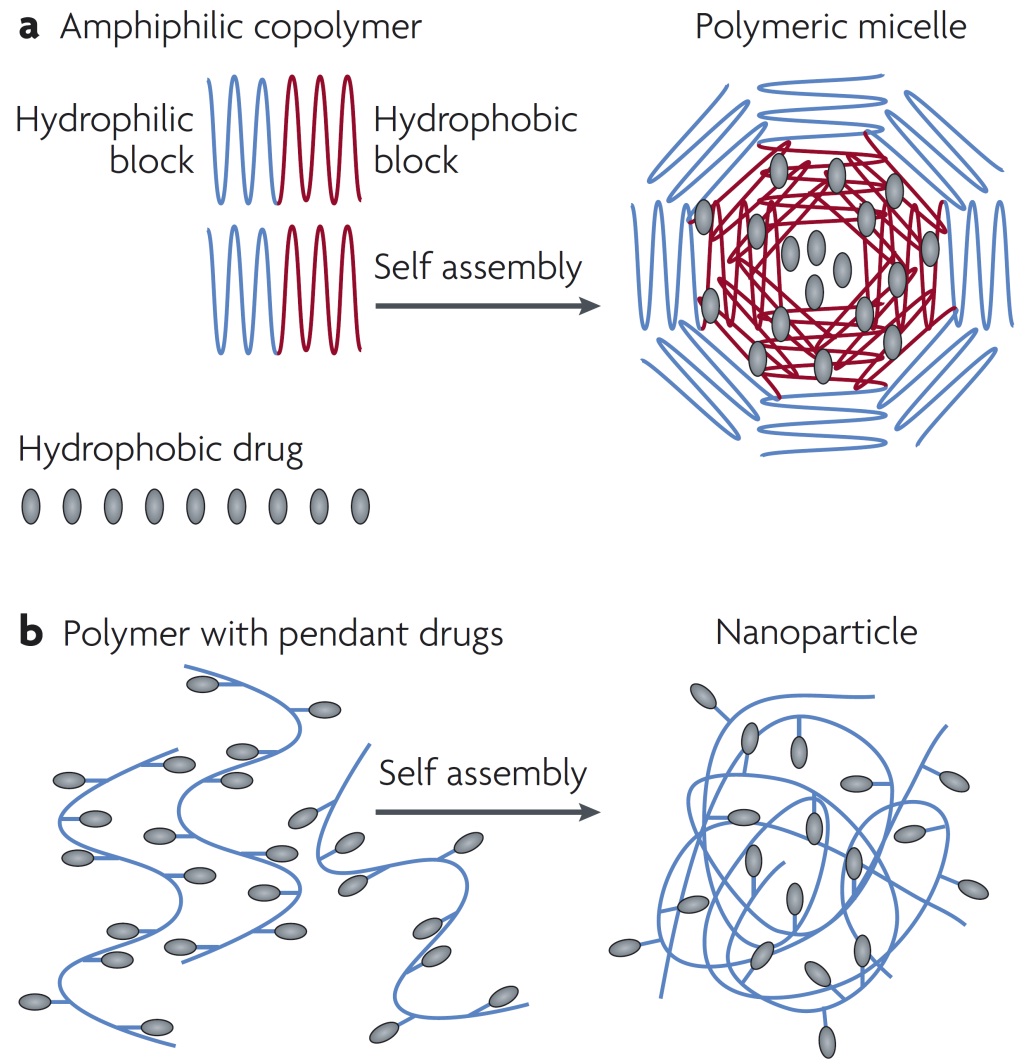
The drug delivery carriers prepared from the block copolymers are obtained by taking advantage of the feature of an amphiphilic self-assembled property, by which a core-shell structured micelle can form and provide a loading space to accommodate mainly hydrophobic drugs in aqueous solution. The drug can be incorporated into the micelle core either by the covalent attachment to the hydrophobic fragment of the amphiphilic unimers or via a non-covalent interaction into the hydrophobic core of the micelle (Figure 3). The nanoscale polymer micelles should exhibit prolonged systematic circulation times and reduced uptake by the mononuclear phagocyte system. Anticancer drugs that are incorporated into core-shell micelles have to show improved stability and efficiency [15].
pH-responsive amphiphilic block copolymers
Tam and coworkers [41] synthesized a pH-responsive Poly (Methacrylic Acid) -b-Poly ((2-Diethylamino) Ethyl Methacrylate) (PMAA-b-PDEAEMA) diblock copolymer via Atom Transfer Radical Polymerization (ATRP) technique using protected group chemistry of tert-butyl methacrylate, followed by hydrolysis under acidic conditions. This copolymer system is believed to have the potential for drug-delivery applications because of the biocompatibility of PMAA and PDEAEMA. PMAA is a weak acid (pKa = 5.4), which can be ionized and becomes soluble at high pH, whereas PDEAEMA is a weak base (pKa = 7.3), which can be protonated at low pH and makes the block hydrophilic polymer. Because both blocks have different pKa values and different chain lengths, the Hydrophile-Lipophile Balance (HLB) values of the block copolymer at high or low pH values are different. Recently, Tam and coworkers also reported α-Cyclodextrin (α-CD) induced assembly of a double hydrophilic block copolymer Poly (Ethylene Oxide)-b-Poly (Acrylic Acid) (PEO-b-PAA). A vesicular nanostructure was produced with a hydrodynamic diameter of 330 nm, which may have potential applications as a drug delivery vehicle [42].
Rempel and coworkers prepared a type of pH-responsive ellipticine-loaded poly (DEAEMA)-poly (PEGMA) core-shell structured nanosphere with an average particle diameter of 96.2 nm via a two-step semibatch emulsion polymerization technique for the purpose of carrying out, controlled release, and delivery of hydrophobic antitumoral agent ellipticine [43]. DEAEMA and PEGMA represent 2-(diethylamino) ethyl methacrylate and poly (ethylene glycol) methacrylate, respectively. Ellipticine (5,11-dimethyl-6H-pyrido [4,3-b] carbazole) is a natural antineoplastic plant alkaloid with a green fluorescence property, which can be extracted from natural sources including Ochrosia elliptica, strychnos dinklagei and Bieekeria vitiensis[44-46]. The release of ellipticine from core-shell nanospheres at 37°C was found to be a highly pH-responsive and controlled release process. An increase in the acid strength will accelerate the rate of release. The ellipticine-loaded nanospheres can be triggered at pH’s of 3, 4, and 5 to achieve a significant ellipticine release of 88% after 98 h, 83% after 98 h, and 79% after 122 h, respectively. The release of ellipticine from the polymer matrix in low pH media was thought to be controlled by the deformation of the polymer matrix and electrostatic repelling force between the protonated ellipticine and poly (DEAEMA) segment. In addition, the PEG corona derived from PEGMA polymers can impart stealth like properties on the nanoparticles, which can enhance the lifetime circulation of nanocarriers.
Temperature responsive block copolymers
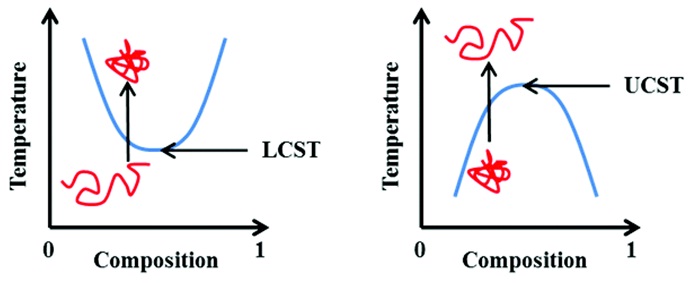
Reproduced from Phillips and Gibson’s work [27] with permission from Royal Society of Chemistry.
Recently, Sugihara and coworkers synthesized a series of random copolymers poly (HOBVE1-x-ran-VEMx) containing poly (4-hydroxybutyl vinyl ether) and 2-(vinyloxy) ethyl methacrylate, and thermo responsive coacervate droplets were obtained [47]. These initially formed coacervate droplets. Slightly above the phase separation temperature with cross-linking by UV irradiation in water to form fine hydrogel microspheres.
pH/temperature/salt multi-responsive block copolymers
Recently, Lee and coworkers synthesized a novel pentablock copolymer Oligomeric Sulfamethazine (OSM)-b-Poly (?-Caprolactone-co-Lactide) (PCLA)-b-PEG-b-PCLA-b -OSM using Br-PCLA-b-PEG-b-PCLA-Br as a macroinitiator for ATRP, in which Sulfamethazine Methacrylate monomer (SM) was used as a pH responsive moiety. PCLA-b-PEG-b-PCLA was used since it is biodegradable and temperature sensitive, as well as a type of amphiphilic triblock copolymer. The block copolymer solution shows a sol-gel transition in response to a slight pH change over the range of 7.2-8.0 [48].
Li and coworkers reported a type of self-assembled thermo- and pH-responsive polymer micelle from Poly (Acrylic Acid)-b-Poly (n-Isopropylacrylamide) (PAA-b-PNIPAM) using ATRP techniques and Doxorubicin (DOX) was used for entrapment and release experiments [49].
Recent trends aim at developing materials that respond to more than one stimulus or try to broaden the applicability and expand such phenomena to other solvent systems and have given rise to several other types of novel stimuli responsive systems, for example, the redox-mediated reversible micellization of block copolymers. Manners and coworkers reported a type of Polystyrene-b-Polyferrocenylsilane (PS-PFS) block copolymer wherein PFS can be selectively oxidized in a controlled manner [50]. The micellization was induced through monitoring the polarity of the charged PFS segment in the oxidization process. Another example is the incorporation of the light responsive segment into the polymer chains. Zhao and coworkers synthesized a block copolymer composed of a side-chain liquid crystalline azobenzene-containing Polymethacrylate and Poly (tert-Butyl Acrylate) (PAzoMA-b-PtBA). The reversible changes in the micellar aggregates for both the core-shell micelles and vesicles took place upon irradiation as a result of the reversible trans-cis photoisomerization of azobenzene mesogens in PAzoMA [51].
It is worthwhile to note that the polymeric drug delivery types mentioned above sometimes are not used individually, but will be combined to fabricate a better-performing drug delivery system. For instance, Farokhzad and coworkers synthesized PLGA-lecithin-PEG core-shell nanoparticles by a modified nanoprecipitation method coupled with self-assembly for controlled drug delivery through combining the beneficial properties of liposomal and polymeric nanoparticles. These hybrid nanoparticles consist of (a) a PLGA hydrophobic core, (b) a soybean lecithin monolayer, and (c) a hydrophilic PEG shell. These types of hybrid nanoparticles assemble the respective merits of the polymer and liposomes, which may represent a useful controlled release drug delivery system.
EXPECTED CRITERIA FOR THE PREPARATION OF POLYMER NANOPARTICLES FOR DRUG DELIVERY
(1) The size of the particulate drug carriers has been proven to be the primary key in determining their biodistribution. The smaller particle size can dramatically decrease the chances of uptake by macrophages of Reticuloendothelial Systems (RES) and then reduce the accumulation in the liver and spleen. The optimal size of nanoparticles is typically considered to be between 25 and 100 nm [52].
(2) The Critical Micelle Concentration (CMC) value is expected to be as low as 10-3 molar or even lower;
(3) Better biodistribution of nanoparticles;
(4) Longer blood circulation period;
(5) Controlled release profile for the incorporated drug ;
(6) Good compatibility between the core-forming block and the incorporated drug;
(7) Coordination of moieties of nanoparticles. In order to prepare “smart” multifunctional pharmaceutical nanocarriers, various chemical moieties were required to provide certain required individual properties via simultaneous attachment on the nanocarriers. Therefore, it is a central challenge on how to optimize the biophysicochemical parameters to work coordinately to reach a desired combination of useful properties.
(8) A higher loading and encapsulation efficiency toward the aimed drug should be satisfied.
SUMMARY
ACKNOWLEDGEMENT
REFERENCES
- Tong R, Langer R (2015) Nanomedicines Targeting the Tumor Microenvironment. Cancer J 21: 314-321.
- Fattal E, Tsapis N, Phan G (2015) Novel drug delivery systems for actinides (uranium and plutonium) decontamination agents. Adv Drug Deliv Rev 90: 40-54.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2: 751-760.
- Bangham AD, Standish MM, Watkins JC (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13: 238-252.
- Langer R, Folkman J (1976) Polymers for the sustained release of proteins and other macromolecules. Nature 263: 797-800.
- Yatvin MB, Kreutz W, Horwitz BA, Shinitzky M (1980) pH-sensitive liposomes: possible clinical implications. Science 210: 1253-1255.
- Leserman LD, Barbet J, Kourilsky F, Weinstein JN (1980) Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature 288: 602-604.
- Heath TD, Fraley RT, Papahdjopoulos D (1980) Antibody targeting of liposomes: cell specificity obtained by conjugation of F(ab')2 to vesicle surface. Science 210: 539-541.
- Allen TM, Chonn A (1987) Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett 223: 42-46.
- Klibanov AL, Maruyama K, Torchilin VP, Huang L (1990) Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268: 235-237.
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, et al. (1994) Biodegradable long-circulating polymeric nanospheres. Science 263: 1600-1603.
- Farokhzad OC, Langer R (2009) Impact of nanotechnology on drug delivery. ACS Nano 3: 16-20.
- Mitragotri S, Burke PA, Langer R (2014) Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 13: 655-672.
- Torchilin VP (2014) Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 13: 813-827.
- Yang D, Ma P, Hou Z, Cheng Z, Li C, et al. (2015) Current advances in lanthanide ion (Ln(3+))-based upconversion nanomaterials for drug delivery. Chem Soc Rev 44: 1416-1448.
- d'Angelo I, Conte C, Miro A, Quaglia F, Ungaro F (2015) Pulmonary drug delivery: a role for polymeric nanoparticles? Curr Top Med Chem 15: 386-400.
- Biju V (2014) Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev 43: 744-764.
- Malda J, Woodfield TB, van der Vloodt F, Kooy FK, Martens DE, et al. (2004) The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 25: 5773-5780.
- Chia SL, Gorna K, Gogolewski S, Alini M (2006) Biodegradable elastomeric polyurethane membranes as chondrocyte carriers for cartilage repair. Tissue Eng 12: 1945-1953.
- Bruggeman JP, de Bruin BJ, Bettinger CJ, Langer R (2008) Biodegradable poly (polyol sebacate) polymers. Biomaterials 29: 4726-4735.
- Willis M, Forssen E (1998) Ligand-targeted liposomes. Adv Drug Deliv Rev 29: 249-271.
- Yih TC, Al-Fandi M (2006) Engineered nanoparticles as precise drug delivery systems. J Cell Biochem 97: 1184-1190.
- Levchenko TS, Hartner WC, Torchilin VP (2012) Liposomes in diagnosis and treatment of cardiovascular disorders. Methodist Debakey Cardiovasc J 8: 36-41.
- Sawant RR, Torchilin VP (2012) Challenges in development of targeted liposomal therapeutics. AAPS J 14: 303-315.
- Torchilin VP (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4: 145-160.
- Torchilin VP (2006) Multifunctional nanocarriers. Adv Drug Deliv Rev 58: 1532-1555.
- Phillipsa DJ, Gibson MI (2015) Towards being genuinely smart: ‘isothermally-responsive’ polymers as versatile, programmable scaffolds for biologically-adaptable materials. Polymer Chemistry 6: 1033-1043.
- Davis ME, Chen ZG, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7: 771-782.
- Rodríguez-Hernández J, Lecommandoux S (2005) Reversible inside-out micellization of pH-responsive and water-soluble vesicles based on polypeptide diblock copolymers. J Am Chem Soc 127: 2026-2027.
- Liu Q, Yu Z, Ni P (2004) Micellization and applications of narrow-distribution poly [2-(dimethylamino) ethyl methacrylate]. Colloid and Polymer Science 282: 387-393.
- Zeng F, Shen Y, Zhu S, Pelton R (2000) Synthesis and Characterization of Comb-Branched Polyelectrolytes. 1. Preparation of Cationic Macromonomer of 2-(Dimethylamino) ethyl Methacrylate by Atom Transfer Radical Polymerization. Macromolecules 33: 1628-1635.
- Lee SB, Russell AJ, Matyjaszewski K (2003) ATRP synthesis of amphiphilic random, gradient, and block copolymers of 2-(dimethylamino) ethyl methacrylate and n-butyl methacrylate in aqueous media. Biomacromolecules 4: 1386-1393.
- Fournier D, Hoogenboom R, Thijs HML, Paulus RM, Schubert US (2007) Tunable pH-and temperature-sensitive copolymer libraries by reversible addition-fragmentation chain transfer copolymerizations of methacrylates. Macromolecules 40: 915-920.
- Xu J, Luo S, Shi W, Liu S (2006) Two-stage collapse of unimolecular micelles with double thermoresponsive coronas. Langmuir 22: 989-997.
- Mao J, Ni P, Mai Y, Yan D (2007) Multicompartment micelles from hyperbranched star-block copolymers containing polycations and fluoropolymer segment. Langmuir 23: 5127-5134.
- Li Z, Kesselman E, Talmon Y, Hillmyer MA, Lodge TP (2004) Multicompartment micelles from ABC miktoarm stars in water. Science 306: 98-101.
- Tadao Nakaya, Yu-Jun Li (1999) Phospholipid polymers. Progress in Polymer Science 24: 143-181.
- Meyer F, Finer M (2001) Gene therapy: progress and challenges. Cell Mol Biol (Noisy-le-grand) 47: 1277-1294.
- Campbell IG, Jones TA, Foulkes WD, Trowsdale J (1991) Folate-binding protein is a marker for ovarian cancer. Cancer Res 51: 5329-5338.
- Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, et al. (1992) Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 52: 3396-3401.
- Dai S, Ravi P, Tam KC, Mao BW, Gan LH (2003) Novel pH-responsive amphiphilic diblock copolymers with reversible micellization properties. Langmuir 19: 5175-5177.
- Liu J, Sondjaja HR, Tam KC (2007) Alpha-cyclodextrin-induced self-assembly of a double-hydrophilic block copolymer in aqueous solution. Langmuir 23: 5106-5109.
- Wang H, Yang L, Rempel GL (2014) Preparation of pH-responsive polymer core-shell nanospheres for delivery of hydrophobic antineoplastic drug ellipticine. Macromol Biosci 14: 166-172.
- Bykadi G, Flora KP, Cradock JC, Poochikian GK (1982) Determination of ellipticine in biological samples by high-performance liquid chromatography. J Chromatogr 231: 137-144.
- Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja SP (2005) Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg Med Chem 13: 5892-5908.
- Nathalie Dias, Hervé Vezin, Amélie Lansiaux, Christian Bailly (2005) Topoisomerase inhibitors of marine origin and their potential use as anticancer agents. In: Waring MJ, Chaires JB (eds.). DNA Binders and Related Subjects. Topics in Current Chemistry, Springer Berlin Heidelberg, Germany 253: 89-108.
- Shinji Sugihara, Masayuki Ohashi, Isao Ikeda (2007) Synthesis of fine hydrogel microspheres and capsules from thermoresponsive coacervate. Macromolecules 40: 3394-3401.
- Dayanandaa K, Hea C, Parka DK, Parkb TG, Lee DS (2008) pH-and temperature-sensitive multiblock copolymer hydrogels composed of poly (ethylene glycol) and poly (amino urethane). Polymer 49: 4620-4625.
- Li G, Song S, Guo L, Ma S (2008) Self-assembly of thermo-and pH-responsive poly (acrylic acid)-b-poly (N-isopropylacrylamide) micelles for drug delivery. Journal of Polymer Science Part A: Polymer Chemistry 46: 5028-5035.
- Rider DA, Winnik MA, Manners I (2007) Redox-controlled micellization of organometallic block copolymers. Chem Commun (Camb) 43: 4483-4485.
- Wang G, Tong X, Zhao Y (2004) Preparation of Azobenzene-Containing Amphiphilic Diblock Copolymers for Light-Responsive Micellar Aggregates. Macromolecules 37: 8911-8917.
- Stuart MA, Huck WT, Genzer J, Müller M, Ober C, et al. (2010) Emerging applications of stimuli-responsive polymer materials. Nat Mater 9: 101-113.
Citation: Wang H, Rempel GL (2015) Introduction of Polymer Nanoparticles for Drug Delivery Applications. J Nanotechnol Nanomed Nanobiotechnol 2: 008.
Copyright: © 2015 Garry L Rempel, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.