
Laryngomalacia: The Importance of Disease Severity
*Corresponding Author(s):
Randa BaraziDepartment Of Otolaryngology Head And Neck Surgery American University Of Beirut Medical Center, Beirut, Lebanon
Tel:+961 3419627,
Fax:+961 1370793
Email:ra110@aub.edu.lb
Abstract
Introduction
Assessment of Laryngomalacia (LM) disease severity is a complex task. To date, there are severity scoring systems that use subjective findings to stratify disease. We propose to create an objective scoring system based on an objective scale, to improve disease stratification, treatment and follow up.
Objectives
1. Illustrate the importance of LM disease severity. 2. Propose an objective scoring system to standardize LM management.
Method
We performed a literature review of the most common symptoms of Laryngomalacia. We assigned adjusted points to those symptoms based on their severity as follows: Inspiratory stridor-1, retractions-1, choking/gagging-1, difficulty feeding-1, failure to thrive-2, apnea-3, and pectus excavatum-3. Disease severity was stratified as follows: mild 1-3, moderate 4-6, severe>6. A retrospective analysis of 182 LM charts at a single tertiary care center and we applied our scoring system to first encounter. We then investigated for a correlation between markers of disease severity such as reflux therapy, incidence of surgery and higher scores.
Results
In our overall cohort, 148 patients had mild disease, 24 moderate, and 10 severe. Reflux therapy (p<0.008) and incidence of surgery (p<0.001) both significantly correlated with higher scores. The Chi-square analysis of a score of 4 was the highest (25.3) and significance was attained with p
Conclusion
We successfully showed that our scoring system correlated with disease severity. Also, we suggest a cut-off score of 4 to decide for surgery. Our scoring simplifies disease severity categorization and should be validated in a larger multi-center prospective study.
Keywords
Laryngomalacia, Score, Severity Level of Evidence: 4
INTRODUCTION
The characteristic inspiratory stridor is secondary to the supraglottic collapse of the airway. The common findings seen on exam that illustrate the disease are the “prolapse of the posteriorly positioned arytenoid cartilages and mucosa into the airway during inspiration, shortening of the distance between the arytenoid and epiglottis, and an “omega-shaped” or “retroflexed epiglottis” [2,3]. All or just one of these characteristics in the supraglottis may be observed on direct visualization. Olney et al. created a scoring system of the type of laryngomalacia based on the site of supraglottic collapse. Type 1 consists of arytenoid cartilage prolapse, type 2 consists of shortened Aryepiglottic (AE) folds, and type 3 consists of epiglottis collapse [4].
Diagnosis is highly suspected by clinical history and confirmed by direct visualization. Direct visualization is gold standard for diagnosis. This is achieved by flexible laryngoscopy in office, or Direct Laryngoscopy and Bronchoscopy (DLB) in the operating room.
The most common comorbidity associated with laryngomalacia is acid reflux occurring in 65% to 100% of patients [2]. The nature of the relationship between acid reflux and laryngomalacia has been investigated without any conclusive evidence suggesting that it contributes to the etiology of laryngomalacia [5]. Several papers showed that the supraglottic collapse of the airway creates a negative intrathoracic pressure which promotes acid reflux onto the laryngeal tissues causing laryngeal edema [4-6]. Literature is clear on the fact that disease severity is correlated with incidence of reflux symptoms [7]. The second most common finding associated with laryngomalacia is a Secondary Airway Lesion (SAL), whose incidence is recorded as 7.5%-64% [1]. The presence of a SAL may worsen the disease process at times [1].
Management of laryngomalacia is determined by clinical judgment of progression of disease and its severity. The severity of disease is characterized by the gravity of the symptoms. It is categorized into mild, moderate or severe. Mild laryngomalacia usually presents with inspiratory stridor and hardly any feeding difficulties. Moderate to severe laryngomalacia presents with failure to thrive, apnea/ALTE (Apparent Life-Threatening Event), choking, gagging, and/or retractions [2,4]. Although several attempts have been made to produce an objective classification system, most centers continue to classify disease based on clinical symptoms and judgment [2,4].
Many patients with mild laryngomalacia can be treated symptomatically whereas patients with mild-moderate disease may need symptomatic treatment and surgical intervention at times [1,2,5]. Patients with severe laryngomalacia may require a surgical intervention such, as a supraglottoplasty or epiglottopexy [1,4,5]. To date there is no objective quantitative measure assessing the severity of Laryngomalacia on which to base treatment. In our literature search, there have been attempts to create an algorithm to the approach of treatment of a child with laryngomalacia based on general characterizations of severity of disease [1]. By introducing a scoring system to the severity of disease, we hope to provide a more quantitative algorithm to aid physicians in the treatment approach for laryngomalacia patients.
The scope of disease and management of laryngomalacia has varied in the literature. Primary objective of this study is geared towards developing an algorithm for the treatment of laryngomalacia after reviewing the disease process of 274 patients at our institution. Our algorithm will be based on a disease severity scoring system based on presenting symptoms. Based on this new scoring system, we will aim to provide guidelines on the surgical management of these patients.
MATERIALS AND METHODS
Inclusion criteria included: Age 0-3 years, complete follow up throughout the duration of the disease, diagnosis of disease by visualization only. Exclusion criteria included: Age > 3 years, incomplete follow up, and co-morbidities that contribute to airway compromise (craniofacial anomalies or underlying neurologic anomalies).
A scoring system was created to classify the disease severity of laryngomalacia based on the presenting symptoms. Symptoms that are common and in a milder form were assigned a score of 1. These symptoms include inspiratory stridor, retractions, gagging/choking, and difficulty feeding (defined as insufficient duration of feeding or inability to breathe appropriately while feeding). More severe symptoms were assigned higher scores and those include apnea/ALTE (Apparent Life-Threatening Event - Assigned a score of 3), Failure to Thrive (FTT - Assigned a score of 2), and pectus excavatum (Assigned a score of 3). Failure to thrive was defined as BMI or weight less than the 3rd percentile. The individual symptom scores were added up to a total. The maximum total score attainable in the presence of all symptoms is 12. The total scores were then categorized as mild (score 1-3), moderate (4-5), or severe (6 or greater).
The means of visually confirming diagnosis consisted of flexible fiberoptic in the office or direct visualization in the OR. If no direct visualization was performed, patient was excluded.
There were a total of five surgeons whose patients were reviewed. Patient confidentiality was protected by assigning a letter to each patients’ initials included in the study which was entered in a master list on a locked computer.
Since this is a retrospective chart review, the symptom severity score of those who have received a surgical intervention will be compared to those who did not receive a surgical intervention. The long-term plan of this research is to perform a prospective confirmatory analysis of the disease severity score identified in this research.
Statistics: Initial analyses in this project include performing an independent t-test on the two independent groups (surgical intervention vs. no surgical intervention) disease severity score. It is hypothesized that children who received a surgical intervention will have a significantly higher disease severity score. We have a priori hypothesized that this score will be above 4 for those who received surgical intervention and below 4 for those who did not. Thus, a Pearson’s chi-square test was performed for the different cutoff scores. We report the analysis of a 2 x 2 contingency table: surgical intervention Yes/No vs. a disease severity score ≤ or > 4.
RESULTS
|
Number of Patients (Valid Percent) |
|
|
Race |
|
|
Black |
61 (35.7%) |
|
White |
72 (42.1%) |
|
Hispanic |
4 (2.3%) |
|
Asian |
2 (1.2%) |
|
Middle Eastern |
1 (0.6%) |
|
Indian |
1 (0.6%) |
|
Other |
13 (7.6%) |
|
Unknown |
17 (9.9%) |
|
Missing data |
11 |
|
Gender |
|
|
Female |
113 (62.8%) |
|
Male |
67 (36.8%) |
|
Missing data |
2 |
|
Term at Birth |
|
|
Term |
134 (77.9%) |
|
Pre-term |
38 (22.1%) |
|
Missing data |
10 |
|
Total |
182 |
On average, reported symptoms lasted for 17.8 weeks (SD = 20.2, range = 0.4 – 156.0 weeks). Laryngomalacia diagnosis was made using a flexible fiberoptic scope in the office or DLB in the operating room. Flexible fiberoptic diagnosis was made in 43% of the patients and 41% diagnoses were made in the operating room. The remaining patients had a magnified airway as their initial diagnostic procedure, but still subsequently undewerwent flexible laryngoscopy. The anatomic abnormalities identified during exam included Omega-shaped epiglottis (49%; N = 88), shortened aryepiglottic folds (56%; N = 101), arytenoid prolapse into airway (58%; N = 105), and supraglottic edema (40%; N = 73). Just over one fourth of the sample (27%; N = 48) had a secondary airway lesion (tracheomalacia, bronchomalacia, subglottic stenosis, or vocal fold paralysis).
All symptoms at presenting visit were recorded. All 182 patients had inspiratory stridor on presentation. Table 2 lists the presenting symptoms from most frequent to least frequent. Most of the initial visits took place in the office (N = 144). There were 22 inpatient consultations in which the diagnosis was made, 8 ER consultations, and 4 patients which were diagnosed after an intraoperative consultation with a diagnostic DLB.
|
Frequency of symptoms (Percentage) |
|
|
Stridor (1 point) |
182 (100%) |
|
Choking/Coughing (1 point) |
85 (47%) |
|
Retractions (1 point) |
66 (37%) |
|
Difficulty feeding (1 point) |
20 (20%) |
|
Apnea (3 point) |
22 (12%) |
|
Failure to thrive (2 point) |
9 (5%) |
|
Pectus excavatum (3 point) |
0 (0%) |
Reflux therapy (p < 0.008) and surgery (p < 0.001) were highly correlated with higher disease severity scores. Chi-square analysis and odds ratio were calculated for each score of 3, 4, 5, and 6. The Chi-square analysis of a score of 4 was the highest at 25.3, and significance was attained with p < 0.001. The odds ratio of a score of 4 was 9.6 with a 95% confidence interval ranging from 3.5 to 26. Tables 3a and 3b show the 2 x 2 contingency tables for observed and expected results. Figure 1 shows the chi square analysis and odds ratio of the subsequent scores. To further evaluate score cut-point, an ROC analysis was performed (Figure 2). Using a cut-point of 4 had the highest level of specificity without losing too much sensitivity.
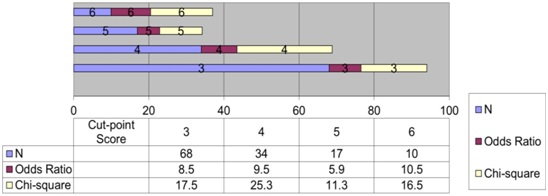
Figure 1: Cut-point Score Analysis.
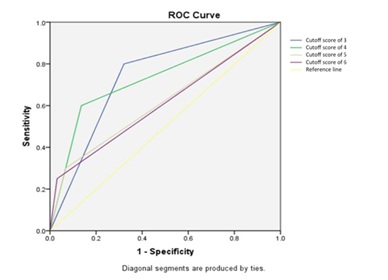
Figure 2: ROC Curve for different cut-off scores.
|
Surgery |
No Surgery |
Total |
|
|
Score > 4 |
12 |
22 |
34 |
|
Score < 4 |
8 |
140 |
148 |
|
Total |
20 |
162 |
182 |
|
Surgery |
No Surgery |
Total |
|
|
Score > 4 |
4 |
30 |
34 |
|
Score < 4 |
16 |
132 |
148 |
|
Total |
20 |
162 |
182 |
DISCUSSION
Laryngomalacia: Demographics and symptoms
Symptoms of laryngomalacia are numerous the most common of which is inspiratory stridor [2,8,12]. All our patients had inspiratory stridor on presentation. Feeding problems, which include choking/coughing, are the second most common set of symptoms according to the literature [2,8,10,12]. Indeed the second most common symptom in our sample was choking/coughing happening at a rate of 47% (Table 3b).
Reflux symptoms are commonly associated with laryngomalacia, with 90.1% of our patients suffering from them, in concordance with the rate of 65-100% mentioned in the literature [2]. Secondary airway lesion is also prevalent co-morbidity associated with laryngomalacia occurring at a rate of 28.7% in our sample. Sources in the literature approximate the rate of secondary airway lesions to be ranging from 7.5-64% [1,12].
Laryngomalacia: Attempt at classification
The retrospective nature of this paper leads to some discrepancies in our results. Indeed, of the 20 patients who proceeded to surgery, 8 were classified as mild. This discrepancy can be explained by the fact that those patients were only scored upon presentation and not immediately before surgery. All in all, we think that this score should be validated by a prospective multicenter study.
CONCLUSION
FINANCIAL DISCLOSURE
POTENTIAL CONFLICTS OF INTEREST
FUNDING
THIS PAPER WAS PRESENTED
REFERENCES
- Thompson DM (2010) Laryngomalacia: Factors that influence disease severity and outcomes of management. Curr Opin Otolaryngol Head Neck Surg 18: 564- 570.
- Landry AM, Thompson DM (2012) Laryngomalacia: Disease presentation, spectrum, and management. Int J Pediatr 2012: 753526.
- Belmont JR, Grundfast K (1984) Congenital laryngeal stridor (laryngomalacia): Etiologic factors and associated disorders. Ann Otol Rhinol Laryngol 93: 430-437.
- Olney DR, Greinwald JH, Smith RJ, Bauman NM (1999) Laryngomalacia and its treatment. Laryngoscope 109: 1770-1775.
- Ayari S, Aubertin G, Girschig H, Van Den Abbeele T, Mondain M (2012) Pathophysiology and diagnostic approach to laryngomalacia in infants. Eur Ann Otorhinolaryngol Head Neck Dis 129: 257-263.
- Wright CT, Goudy SL (2012) Congenital laryngomalacia: Symptom duration and need for surgical intervention. Ann Otol Rhinol Laryngol 121: 57-60.
- Hartl TT, Chadha NK (2012) A systematic review of laryngomalacia and acid reflux. Otolaryngol Head Neck Surg 147: 619-626
- Thompson DM (2007) Abnormal sensorimotor integrative function of the larynx in congenital laryngomalacia: A new theory of etiology. Laryngoscope 117: 1-33.
- Simons JP, Greenberg LL, Mehta DK, Fabio A, Maguire RC, et al. (2016) Laryngomalacia and swallowing function in children. Laryngoscope 126: 478-484.
- Giannoni C, Sulek M, Friedman EM, Duncan NO 3rd (1998) Gastroesophageal reflux association with laryngomalacia: A prospective study. Int J Pediatr Otorhinolaryngol 43: 11-20.
- McSwiney PF, Cavanagh NP, Languth P (1977) Outcome in congenital stridor (laryngomalacia). Arch Dis Child 52: 215-218.
- Thorne MC, Garetz SL (2016) Laryngomalacia: Review and Summary of Current Clinical Practice in 2015. Paediatr Respir Rev 17: 3-8.
- Kilpatrick LA, Boyette JR, Hartzell LD, Norton JA, Boswell JB, et al. (2014) Prospective quality of life assessment in congenital laryngomalacia. Int J Pediatr Otorhinolaryngol 78: 583-587.
- Thottam PJ, Simons JP, Choi S, Maguire R, Mehta DK (2016) Clinical relevance of quality of life in laryngomalacia. Laryngoscope 126: 1232-1235.
- van der Heijden M, Dikkers FG, Halmos GB (2015) The groningen laryngomalacia classification system--based on systematic review and dynamic airway changes. Pediatr Pulmonol 50: 1368-1373.
- Shah UK, Wetmore RF (1998) Laryngomalacia: A proposed classification form. Int J Pediatr Otorhinolaryngol 46: 21-26
Citation: Shah VS, Haupert M, Haddad G, Barazi R (2019) Laryngomalacia: The Importance of Disease Severity. J Otolaryng Head Neck Surg 5: 028.
Copyright: © 2019 Sweeti V Shah, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

