
Latent Genital Tuberculosis in Male - A Possible Cause of Reproductive Failure
*Corresponding Author(s):
Siddhartha ChatterjeeCalcutta Fertility Mission, 21, Bondel Road, Kolkata - 700019, India
Tel:+91 9830387875,
Email:sidchat54@gmail.com
Abstract
Objective: Latent Genital Tuberculosis (LGTB) or tubercular infestation is prevalent in Southeast Asia and even the presence of tubercular bacilli in both male and female genital tract is becoming an important factor for reproductive failure. This study has been undertaken to explore the various undetected causes like LGTB of adverse reproductive outcome which includes infertility and recurrent pregnancy loss in both the male and female partners and the concerned route of sexual transmission of the same.
Methods: 250 couples aged between 25-45 yrs old who visited Department of Reproductive Medicine at Calcutta Fertility Mission with complaints of infertility or recurrent pregnancy loss, over a period of 36 months (January 2017 to November 2019) were enrolled in this retrospective observational study and grouped into Group A, B and C. Along with the baseline tests depending on the clinical history both male and female partners were screened for LGTB by PCR test of the endometrial aspirate and semen. Sperm function tests like Vital staining, Hypo-osmotic swelling test, sperm DNA Fragmentation Index (DFI) were also performed on the semen samples.
Results: 53 infertile couples (55.79%) in Group A, 23 couples (45.1%) in Group B and 64 couples (61.54%) in Group C, tested positive, when screened by PCR, for tubercular bacilli in endometrial aspirate as well as semen. Male partners who were screened to have LGTB by PCR had essentially abnormal sperm function test, especially high sperm DFI which has been analysed and found as statistically significant (p value<0.001) factor for poor reproductive outcome.
Conclusion: LGTB in males has been demonstrated as an important factor associated with poor sperm function test, especially, high sperm DFI which result in serious negative consequences to natural conception. The sexual transmission of the tubercular bacilli from asymptomatic males to their female counterparts is quite evident by the clinical scenario of our patients.
Keywords
Endometrial aspirate; Latent genital tuberculosis; PCR; Semen; Sperm DNA fragmentation; Sperm function test
INTRODUCTION
Female Genital Tuberculosis (FGTB) is a major health problem in many developing countries in Asia and Africa and has been proved to be responsible for a significant proportion of female infertility. The detection of FGTB often goes unnoticed as it is asymptomatic in majority of the affected women and is diagnosed only during infertility work-up [1]. Previous literature show genital tuberculosis as a cause of infertility in 3-16% of women, varying to about 20% in India [2,3]. Now-a-days a strong association of Latent Genital Tuberculosis (LGTB) has been noticed with early endometriosis, minor tubal defects or even both in a substantial number of patients with apparently “unexplained” infertility” [4]. It has been observed that quite a large number of cases of Recurrent Pregnancy Loss (RPL) are affected with latent FGTB or tubercular infestation of the endometrium as a sole or strongly associated factor for the development of such an unfortunate situation [5]. There is paucity of evidence regarding the sexual route of transmission of the tubercular bacilli either in latent or overt stage of the disease except in a study by Brian J. Angus et al., in which sexual transmission of Mycobacterium tuberculosis from patients with asymptomatic endometrial tuberculosis and tuberculous penile ulcer has been demonstrated by means of molecular methods [6].
Conventional semen analysis has been considered as the cornerstone laboratory examination during the initial evaluation of male factor infertility [7]. WHO 2010 thresholds for progressive motility and vitality are superior to the 1999 thresholds in predicting sperm DNA fragmentation, whereas the cut-off value of sperm concentration, total sperm number and normal morphology may need further revisions to increase their accuracy [8]. However, these parameters do not reliably predict either male fertility or the likelihood of pregnancy after infertility treatment. Ideally, the sequential analysis of sperm functions can help the clinicians in planning therapeutic approach, but the assessment of sperm function tests lack standardized protocols [9-11]. The one-step eosin-nigrosin technique can be run with ordinary bright-field microscopy and it appears preferable in terms of standardization and quality control management to assess sperm vitality [12]. Sperm plasma membrane integrity is a necessary condition for motility and fertilization; semen samples with abnormal motility usually show a HOS-E result indicative of a defective plasma membrane [13]. The results of this test correlate with other semen assessments such as morphology and motility [14]. Abnormal sperm DNA Fragmentation Index (DFI) affects the embryo quality and development that decrease the implantation rates and increase the rates of early miscarriage in Assisted Reproductive Techniques (ART) as well [15]. Thus more comprehensive sperm function tests like vital staining, HOS test, DFI, have been performed in our study, to identify the sperm dysfunction at the cellular and molecular level and its relation with LGTB, for better treatment of male infertility.
MATERIALS AND METHODS
A total of 250 couples of male and female partners aged between 25-45 yrs old who visited Department of Reproductive Medicine at Calcutta Fertility Mission, over a period of 36 months (January 2017 to November 2019) were enrolled in this retrospective observational study and grouped into Group A, B and C. In our present study, the women satisfying the inclusion criteria, and their partners were screened by multiplex PCR for presence of tubercular bacilli in the endometrial aspirate or semen. Then, following miscarriage, DNA analysis was repeated from samples obtained from the Product of Conception (POC), women’s endometrium and male partners’ semen. Thereafter, statistical analyses were performed on these DNA amplification data.
Experimental design
Inclusion criteria
- • Couples who were infertile and the female partner tested positive repeatedly for LGTB even after anti-tubercular treatment (Group A)
- • Couples in whom female partner presented with missed abortion with previous history of one pregnancy loss (Group B)
- • Couples who had two or more pregnancy losses (>=2) or Recurrent Pregnancy Loss (RPL) (Group C)
- • All male partners had essentially normal basic semen analysis (WHO 2010) and near normal endocrinological profile (Serum FSH/LH/Testosterone/ TSH/ Prolactin)
Exclusion criteria
- • Couples with history of pulmonary or extra-pulmonary tuberculosis or either partner being on anti-tubercular drugs
- • Couples in whom the male partner has history of testicular pathology
- • Couples in whom female partners had diagnosed uterine malformations, chromosomal defects, thrombophilia, diagnosed endometriosis or Polycystic Ovarian Syndrome (PCOS) on treatment
Couples were divided into three groups: Groups A, B, and C in which Group A (n = 95 couples) included patients with apparently unexplained infertility, who had only latent GTB as the etiological factor in both partners, among the baseline male and female factors screened in this study. Group B (n = 51 couples) consisted of women, who presented with missed abortion to us and were found to have tubercular bacilli in the Product of Conception (POC) as well as in the endometrial aspirate of the mother and semen of the father. Group C (n = 104 couples) included patients with recurrent miscarriage (>=2), who were diagnosed to have LGTB in both male and female partners as one of the etiology for RPL. These couples had undergone routine tests for infertility and RPL along with the TB-PCR test with an endometrial aspirate as well as semen of the male partner.
Sample collection for DNA PCR test: Female participants were advised to attend clinic on the 2nd day of her menstrual cycle and also during mid-luteal phase (21st or 23rd day) of the same cycle for collection of menstrual blood and endometrial aspirates. POC samples were collected aseptically after suction and evacuation procedure (Group B only). After collection, both specimens were carefully transferred to available lysis buffer for DNA extraction using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and eluted in 200µl elution buffer and was stored at -20°C. Male participants were advised to attend clinic after a couple of days of last intercourse for semen PCR. Semen sample were collected according to the WHO 2010 guideline. After collection, semen was transferred to the incubator to liquify for 15mins at 370 C. This sample was then centrifuged at 3000rpm for 10mins on the same day. Thereafter, the supernatant fluid was discarded and the pellet was used for DNA extraction.
Semen sample for Sperm function test: All the semen samples were transferred to Embryology section of our institute to pursue the sperm function tests including Vital Staining, Hyposmotic Swelling Test (HOS) and DNA Fragmentation Index (DFI). The data thus obtained were statistically analysed.
Ethical consideration
The Ethical Committee of Calcutta Fertility Mission has given clearance for the retrospective study of a prospective database on 12/01/2020 (code: CFM/2020/036). Written informed consent has been obtained from all couples who participated in the study.
Statistical analysis
Categorical variables are expressed as number of patients and percentage of patients and compared across the groups using Pearson’s Chi Square test for Independence of Attributes/ Fisher's Exact Test as appropriate. The statistical software SPSS version 20 has been used for the analysis. An alpha level of 5% has been taken, i.e., if any p value is less than 0.05 it has been considered as significant.
RESULTS
Our data-interpretations clearly show that the loss of pregnancies was due to transmission of the tubercular bacilli via the sexual route as demonstrated in figure 1. It can be suggested from our study that sexual transmission of LGTB can take place even from asymptomatic males harbouring tubercular bacilli in their semen leading to male factor infertility and even recurrent pregnancy loss in the infertile couples.
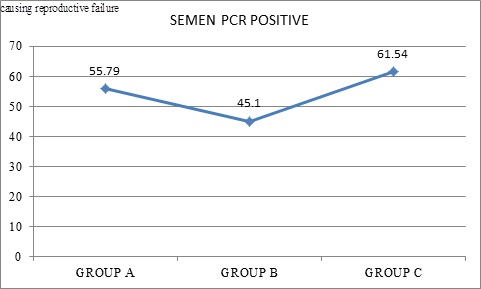 Figure 1: Comparison of Latent genital tuberculosis (%) in seminal fluid of male partners from A, B and C groups.
Figure 1: Comparison of Latent genital tuberculosis (%) in seminal fluid of male partners from A, B and C groups.
Data presented as n (%); Pearson’s Chi Square test for Independence of Attributes. p value - 0.153 (not significant).
DISCUSSION
Latent genital tuberculosis in women is an important cause of subclinical chronic pelvic disease and infertility in developing countries. During the past two decades, the clinicians have faced quite a number of women suffering from LGTB and its consequences such as infertility, so review of these features are considered in differential diagnosis of the various causes of infertility and its consequent interventions and treatment [3]. If the history of previous literature is carefully looked into, there are evidences of sexual transmission in case of extrapulmonary tuberculosis, though not generally regarded as being infectious, but may be due to transmission from one mucosal surface to another during intercourse which has been described in animal models [16,17]. Increased transmission of the disease has also been noted in infected marital couples [18].
Similarly in the present study we have demonstrated that 78% (195) women, satisfying the inclusion criteria, were found to have LGTB with history of either infertility or recurrent pregnancy loss. This clearly correlates with our previous studies which have pointed out time and again that LGTB is one of the important factors causing reproductive failure [5,19] (Figure 2).
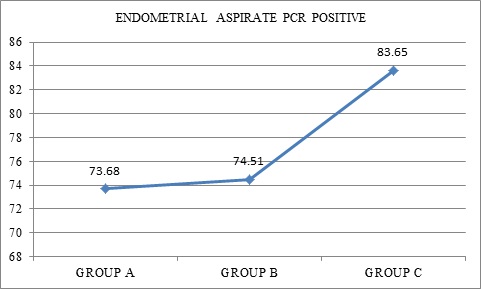 Figure 2: Comparison of Latent genital tuberculosis (%) in female partners of Groups A, B and C.
Figure 2: Comparison of Latent genital tuberculosis (%) in female partners of Groups A, B and C.
Data presented as n (%); Pearson’s Chi Square test for Independence of Attributes; p value>0.05 (not significant) (p value-0.189).
In our study it has been demonstrated that not only women but also their asymptomatic male partners have been affected with LGTB causing adverse reproductive outcome. 53 infertile couples (55.79%) in Group A, 23 couples (45.1%) in Group B and 64 couples (61.54%) in Group C, tested positive, when screened by PCR, for tubercular bacilli in endometrial aspirate as well as semen (Figure 1). These men had a normal basic semen analysis (WHO 2010) and were subjected to sperm function tests. Male partners were also tested for Serum Testosterone, FSH, LH, Prolactin and TSH. About 2-3% male patients had hyperprolactinemia and 8% had hypothyroidism (Figure 3).
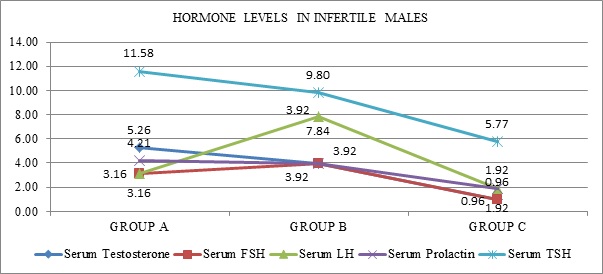 Figure 3: Comparison of Serum FSH/LH/testesterone/TSH/prolactin (%) of male partners from A, B and C groups.
Figure 3: Comparison of Serum FSH/LH/testesterone/TSH/prolactin (%) of male partners from A, B and C groups.
Data presented as n (%); Fisher’s Exact Test; p value>0.05 (not significant).
A correlation between the sperm DNA damage measured by Sperm Chromatin Structural Assay and the severity of semen abnormalities has been reported earlier [20]. Three major factors are known to cause sperm DNA damage: abnormal sperm chromatin assembly, aberrant apoptosis of sperm cells, and excessive oxidative stress [21]. Inflammation in the external genital tracts and varicocele can also increase the risks of Sperm DNA Fragmentation (SDF) by inducing reactive oxygen species in the sperm. Advanced paternal age, poor lifestyle habits including smoking, alcohol consumption, environmental radiation and pollution can all lead to increased SDF [22,23]. The evaluation of oxidative stress and sperm DNA damage would make a solid contribution to standard seminal analysis profile and become diagnostic tools during andrological workup [24]. Sperm DNA fragmentation is being increasingly recognized as an important cause of infertility and is being extensively investigated. The strong association between DNA damage and adverse reproductive outcomes has led to the introduction of sperm DNA integrity testing which in turn is essential for the accurate transmission of genetic information [25]. It appears to be an absolute necessity to obtain a clear diagnosis of the cause and the apply adequate methods of sperm selection pre-ART when high levels of sperm DNA fragmentation are observed, to increase the possibilities to achieve the pregnancy in couples with high sperm DNA fragmentation and repeated assisted reproduction failures [15].
In our present study it been testified with statistically significant data analysis (p value<0.001) that poor DNA fragmentation index (DFI) i.e., high sperm DNA fragmentation in seminal fluid of male partners with LGTB, leads to either infertility or RPL (Figure 4). Any form of sperm chromatin abnormalities or DNA damage may result in male infertility and negative consequences in pregnancies in humans [26,27]. As has been observed in our study also, male patients especially with LGTB, who had infertility or couples with history of recurrent pregnancy loss had statistically significant abnormal Vital Staining and HOS test on analysing sperm function tests (Figures 5 and 6).
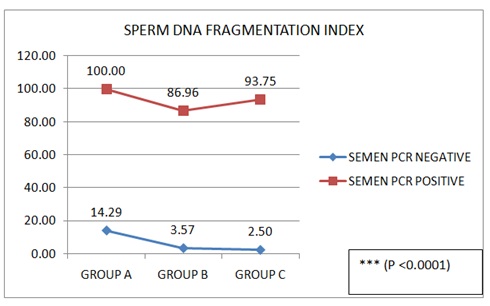 Figure 4: Comparison of Sperm DNA Fragmentation (DFI) (%) of seminal fluid of male partners from A, B and C groups and semen PCR.
Figure 4: Comparison of Sperm DNA Fragmentation (DFI) (%) of seminal fluid of male partners from A, B and C groups and semen PCR.
Data presented as n (%); Fisher's Exact Test
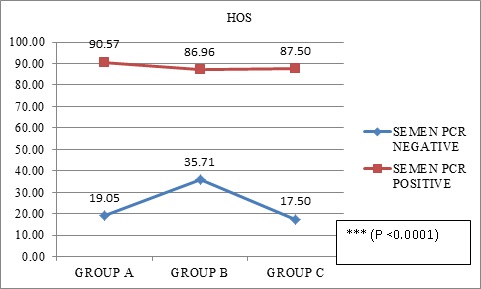 Figure 5: Comparison of Hypo-Osmotic Swelling test (HOS) (%) of seminal fluid of male partners from A, B and C groups and semen PCR.
Figure 5: Comparison of Hypo-Osmotic Swelling test (HOS) (%) of seminal fluid of male partners from A, B and C groups and semen PCR.
Data presented as n (%); Fisher's Exact Test
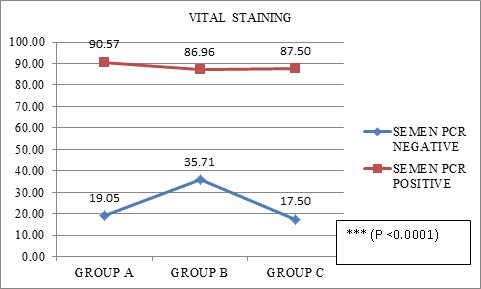 Figure 6: Comparison of vital staining (%) of seminal fluid of male partners from A, B and C groups and semen PCR.
Figure 6: Comparison of vital staining (%) of seminal fluid of male partners from A, B and C groups and semen PCR.
Data presented as n (%); Pearson’s Chi Square test for Independence of Attributes
The sexual route of transmission in case of LGTB still needs to be explored and that it being a sexually transmitted disease should also be a point of research. From the clinical scenario in our group of patients the sexual route of transmission from male to female partners, has been seen to be quite a possibility and hence this area needs to be studied further for increased evidence and accuracy.
CONCLUSION
In our present study, LGTB in males has been demonstrated as an important factor associated with high sperm DFI which result in serious negative consequences to natural conception. Though the exact mechanism has not yet been clarified yet the elaborative statistical analysis of our data shows a significant association of abnormal sperm function test especially sperm DNA fragmentation with LGTB in semen of male partners of couples suffering either from infertility or from recurrent pregnancy losses. Moreover the sexual transmission of the tubercular bacilli is quite evident by the clinical scenario of the patients included in the present study.
REFERENCES
- Rom W, Garay S (1996) Tuberculosis (1stedn). Little Brown, New York, USA.
- Sharma JB (2015) Current Diagnosis and Management of Female Genital Tuberculosis. J Obstet Gynaecol India 65: 362-371.
- Shahzad S (2012) Investigation of the prevalence of female genital tract tuberculosis and its relation to female infertility: An observational analytical study. Iran J Reprod Med 10: 581-588.
- Chatterjee S, Bagchi B, Chatterjee A, Datta A (2020) Latent Genital Tuberculosis - A Possible Explanation for Unexplained Infertility. Integr Gyn Obstet J 3: 1-4.
- Bagchi B, Chatterjee S, Chowdhury RG (2019) Role of latent female genital tuberculosis in recurrent early pregnancy loss: A retrospective analysis. Int J Reprod BioMed 17: 929-934.
- Angus BJ, Yates M, Conlon C, Byren I (2001) Cutaneous tuberculosis of the penis and sexual transmission of tuberculosis confirmed by molecular typing. Clin Infect Dis 33: 132-134.
- Barratt, Christopher LR (2007) Semen analysis is the cornerstone of investigation for male infertility. Practitioner 251: 8-10.
- Aghazarian A, Huf W, Pflüger H, Klatte T (2020) The 1999 and 2010 WHO reference values for human semen analysis to predict sperm DNA damage: A comparative study. Reproductive Biology 20: 379-383.
- Oehninger S (2011) Clinical management of male infertility in assisted reproduction: ICSI and beyond. Int J Androl 34: 319-329.
- Barratt CLR, Mansell S, Beaton C, Tardif S, Oxenham SK (2011) Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl 13: 53-58.
- González-Marín C, Gosálvez J, Roy R (2012) Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci 13: 14026-14052.
- Björndahl L, Söderlund I, Kvist U (2003) Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum Reprod 18: 813-816.
- Ramirez JP, Carreras A, Mendoza C (1992) Sperm plasma membrane integrity in fertile and infertile men. Andrologia 24: 141-144.
- Goericke-Pesch S, Failing K (2013) Retrospective analysis of canine semen evaluations with special emphasis on the use of the hypoosmotic swelling (HOS) test and acrosomal evaluation using Spermac(®). Reprod Domest Anim 48: 213-217.
- García-Ferreyra J (2015) Sperm DNA Fragmentation and Its Relation With Fertility, New Discoveries in Embryology. Bin Wu, Intech Open.
- Gondzik M, Jasiewicz J (1979) [Experimental study on the possibility of tuberculosis transmission by coitus]. Z Urol Nephrol 72: 911-914.
- Gondzik M, Jasiewicz Z (1979) [Transmission of tuberculosis by sexual route in guinea pigs under experimental conditions]. Ann Acad Med Stetin 25: 505-521.
- Sepkowitz KA (1996) How contagious is tuberculosis? Clin Infect Dis 23: 954-962.
- Chowdhury RG, Paine SK, Bhattacharjee B, Chatterjee S (2010) Infestation Of Endometrium By Mycobacterium Tuberculosis Bacilli-Cause Of Reproductive Failure. Al Ameen J Med Sci 3: 322-331.
- Moskovtsev SI, Willis J, White J, Mullen JB (2009) Sperm DNA damage: correlation to severity of semen abnormalities. Urology 74: 789-793.
- Gomes M, Goncalves A, Rocha E, Sá R, Alves A, et al. (2015) Effect of in vitro exposure to lead chloride on semen quality and sperm DNA fragmentation. Zygote 23: 384-393.
- Wright C, Milne S, Leeson H (2014) Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online 28: 684-703.
- Yang H, Li G, Jin H, Guo Y, Sun Y (2019) The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl Androl Urol 8: 356-365.
- Iommiello VM, Albani E, Di Rosa A, Marras A, Menduni F, et al. (2015) Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int J Endocrinol 2015: 321901.
- Shamsi MB, Imam SN, Dada R (2011) Sperm DNA integrity assays: diagnostic and prognostic challenges and implications in management of infertility. J Assist Reprod Genet 28: 1073-1085.
- Agarwal A, Said TM (2003) Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 9: 331-345.
- Collins JA, Barnhart KT, Schlegel PN (2008) Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril 89: 823-831.
Citation: Chatterjee S, Bhattacharya SM, Bagchi B, Chaudhuri AR, Datta A (2020) Latent Genital Tuberculosis in Male - A Possible Cause of Reproductive Failure. J Reprod Med Gynecol Obstet 5: 057.
Copyright: © 2020 Siddhartha Chatterjee, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

