
Light Emitting Diode (LED) Illumination for Enhanced Growth and Cellular Composition in Three Microalgae
*Corresponding Author(s):
Sibi GDepartment Of Biotechnology, Indian Academy Degree College-Autonomous, Bengaluru, India
Tel:+91-99864-52875,
Email:gsibii@gmail.com
Abstract
Light wavelength is an essential parameter of microalgal growth and light spectrum quality affects the algal metabolism. Light-emitting diodes (LEDs) emit a very narrow wavelength which addresses fundamental as well as applied aspects of light color on biomass and value-added compound formation in microalgae. In this study, Arthospira platensis, Chlorella vulgaris and Scenedesmus obliquus were cultivated in Bold’s basal medium under five different LED illuminations (white, blue, green, yellow and red) to augment growth, pigment, protein, carbohydrates and lipid production. Dry weight of microalgae at the end of 10 days cultivation period revealed that higher content under the LED illumination was in the order of red>white>blue. Highest Chlorophyll a content of 8.4 mg g-1 was recorded in C. vulgaris under red LED. Whereas green LED illumination has resulted higher content of Chlorophyll b with 2.0, 1.98 and 1.68 mg g-1 in A. platensis, C. vulgaris and S. obliquus respectively. Similarly highest carotenoid content of 6.1 mg g-1 in A. platensis was recorded in green LED. Total carbohydrates of microalgae were increased under blue light and the maximum content of 52.1 mg g-1 was found in C. vulgaris. Protein content in the range of 102.7-97.5 mg g-1, 77.9-76.5 mg g-1 and 51.9-49.4 mg g-1 was observed in A. platensis, C. vulgaris and S. obliquus under blue and green LED. Lipid content of 5.68 % and 4.96 % was obtained in C. vulgaris and S. obliquus under blue LED. This was followed by 4.37% and 3.86% under red LED illumination for the same species. Based on the results, selection of blue and red LED illumination to enhance microalgal growth and cellular composition is possible in the selected microalgae.
Keywords
Arthospira; Chlorella; Illumination; LEDs; Microalgae; Scenedesmus
INTRODUCTION
Microalgae are phototrophic organisms and promising resources for the mass production of lipids, biomass and nutraceuticals. Microalgae use sunlight to derive energy and expanded their applications were expanded and diversified in recent years. Light quality and quantity are the most important parameters for microalgae and other phototrophic organisms, as it is required for photosynthesis and the regulation of several cellular processes [1]. As a photoautotroph, microalgae cannot utilize all spectra of light but only some fractions, in particular between 400 and 700 nm, which are called as photosynthetic active radiation [2]. In most laboratories and industries, artificial light is used to control the microalgal growth and physiology. However, the competitiveness of any artificial light-driven microalgal production hinges on energy consumption. Better light energy usage by phototrophs can be achieved by tailoring species-specific emission spectra of artificial light sources [3]. Although artificial light costs more than sunlight, it allows control of microalgal biochemistry and growth, increasing the reliability of industrial processes for the production of high-value biomolecules from microalgae. Artificial light also provides better regulation of the photosynthetic photon flux density, photoperiod, and light spectra in microalgal production [4]. Among the artificial light sources, Light-Emitting Diodes (LEDs) are being used an alternative light source in microalgae cultivation [5].
Light wavelength is an essential parameter of microalgal growth [6]. Algal metabolism and growth can be also affected by spectrum and spectrum quality is known to influence biochemical composition, pigment content and photosynthesis rate of various species [7-9]. It is important to study the effects of different light qualities on microalgae and screen out the most efficient one as there is a possibility that high production efficiency can be achieved by optimizing the artificial light quality [10].
The main objective of the present work is to investigate the effect of LED as a light source to augment growth, pigment, protein, carbohydrates and lipid production of Arthospira platensis, Chlorella vulgaris and Scenedesmus obliquus.
MATERIALS AND METHODS
Microalgae strains and culture conditions
Three microalgae, Arthospira platensis, Chlorella vulgaris, Scenedesmus obliquus were used in this study. Initial concentration of microalgal cells was adjusted to 0.05 g/L and the microalgae were cultured in sterilized Bold’s basal medium in 2 L flasks with a 1.5 L working volume at 20 ± 1°C and a 12/12-h light/dark cycle. Light emitting diodes (LEDs) strips were used as light source for the cultivation of microalgae for a period of 10 days. The LED lights emitting blue (465 nm), green (520 nm), yellow (640 nm), red (660 nm) and white light were used.
Dry weight determination
Microalgae grown under different LED wavelengths were harvested by centrifugation at 5000 rpm for 10 mins. The algal pellets were dried at 95°C in a hot air oven until a constant weight was obtained to determine the dry weight.
Cellular pigments Assay
Determination of chlorophyll a, chlorophyll b, and total carotenoid contents was done according to Lichtenthaler [11]. The determination of levels in whole pigment extract of microalgae using a UV–Vis spectrophotometer was performed using the following equations:
Chl a (μg ml−1) = 11.24 × OD662 – 2.04 × OD645
Chl b (μg ml−1) = 20.13 × OD645 – 4.19 × OD662
Ct (μg ml−1) = (1000 × OD470 – 1.90 × Chl a – 6.31 × Chl b) / 214
whereChl a is the chlorophyll a, Chl b is the chlorophyll b, and Ct is the total carotenoids (μg mL−1).
Protein Assay
The extraction of proteins from microalgae was performed using alkali method. Aliquots of algal sample were centrifuged and 0.5 N NaOH was added to the pellet followed by extraction at 80?C for 10 mins. The mixture was centrifuged and protein content of the supernatant was estimated using Bovine Serum Albumin (BSA) as standard [12].
Carbohydrate Assay
Cellular carbohydrates were estimated using the anthrone method after hot alkaline extraction [13, 14]. Briefly, microalgal pellets were resuspended in distilled water and then heated in 40% (w/v) KOH at 90?C for 1 h. After cooling down, ice cold ethanol was added and stored at −20?C overnight followed by centrifugation. The pellet was resuspended in distilled water and then reacted with anthrone reagent. D-glucose was used as standard and the colour development was read at 578 nm in a spectrophotometer.
Total Lipid estimation
Lipid extraction from dried algal cells were carried out by chloroform:methanol extraction method [15]. Dried algal cells added with distilled water were ultrasonicated and mixed with chloroform: methanol (2:1). The mixture was left for 30 mins in a water bath (30°C) and filtered through a Whatman No.1 filter paper. The filtrate was transferred to another screw cap tube containing NaCl solution (0.9%) and the purified chloroform layer was evaporated to a constant weight in a fuming hood under vacuum at 60°C. The total lipid content of dry weight was calculated using the following equation.
Lipid content % = (m2-m0) / m1 × 100 ………….. Eq. (3)
where m1 is the weight of the dried algal cells, m0 is the weight of the empty new screw cap tube and m2 is the weight of the new screw cap tube with the dried lipids.
RESULTS AND DISCUSSION
The quality and regulation of the illumination is crucial in microalgae cultivation processes. Light-emitting diodes (LEDs) emit a very narrow wavelength address fundamental as well as applied aspects of light color on algae biomass and value-added compound formation. In this respect, effect of various LEDs on microalgal growth and biochemical characteristics were examined in this study. Three microalgae namely Arthospira platensis, Chlorella vulgaris and Scenedesmus obliquus were cultivated in Bold’s basal medium under five different LED illuminations (white, blue, green, yellow and red) for a period of 10 days.
(Figure 1) shows the dry cell weight of microalgae under the different LED wavelengths ranging from white to red. Dry weight of microalgae at the end of 10 days cultivation period revealed that higher content under the LED illumination was in the order of red>white>blue. Highest dry weight of 1.43 g l-1 was recorded in S. obliquus under red light illumination followed by 1.41 g l-1 by A. platensis. Whereas, white LED illumination resulted in highest dry weight content in C. vulgaris. Both green and yellow LEDs produced lower biomass in the form of dry weight. In a study by Ajayan, highest biomass (5.2 g L−1) was observed under red illumination with reflector in Chlorella species [16]. At the same time, combination of Blue-Red LED combination gave maximum biomass in terms of cell density in Chlorella sp found that the combination of red and blue light is more conducive to the growth of Arthrospira platensis[17, 18]. In another study, red LED has produced higher biomass in Picochlorumatomus[19]. It was also reported that binary combination of blue and red LEDs could produce the high biomass and photosynthetic pigments in the four microalgae [20].
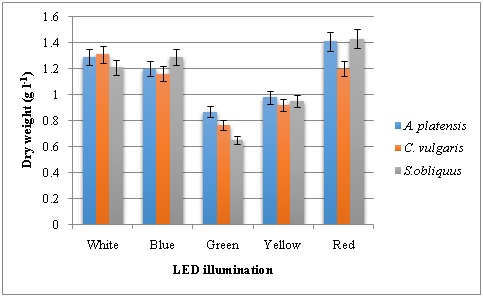 Figure 1: Dry cell weight of microalgae grown under various LED illumination.
Figure 1: Dry cell weight of microalgae grown under various LED illumination.
To understand the relations between pigment concentrations under various light sources, chlorophyll and carotenoid contents were measured and recorded (Figure 2, 3 and 4). The Chlorophyll content of the green, yellow and blue LEDs was significantly lower than that produced under the red and white light. Among the LED lights tested on pigment production of three microalgae studied, red LED has resulted in higher chlorophyll a followed by white LED. Highest Chl a content of 8.4 mg g-1 was recorded in C. vulgaris. Both red and white LED illumination produced 7.8 mg g-1 Chla in A. platensis. In the case of Chl b, green LED illumination has resulted higher content in all the three microalgae with 2.0, 1.98 and 1.68 mg g-1 in A. platensis, C. vulgaris and S. obliquus respectively. Similarly, carotenoids were high in microalgae grown in the presence of green LED illumination with highest carotenoid content of 6.1 mg g-1 in A. platensis. The second highest was recorded with yellow LED illumination.
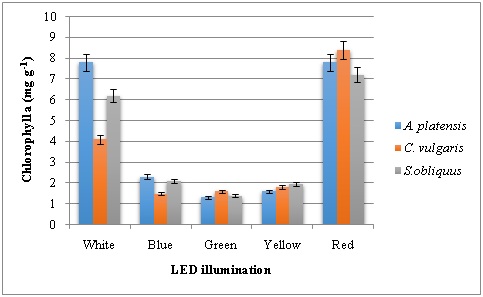 Figure 2: Chlorophyll a content of microalgae grown under various LED illumination.
Figure 2: Chlorophyll a content of microalgae grown under various LED illumination.
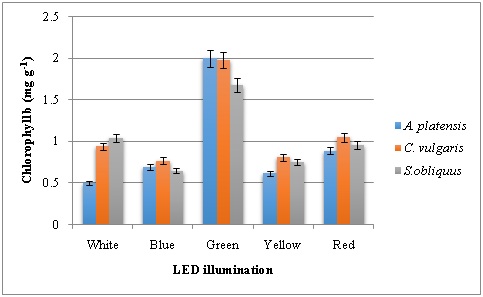 Figure 3: Chlorophyll b content of microalgae grown under various LED illumination.
Figure 3: Chlorophyll b content of microalgae grown under various LED illumination.
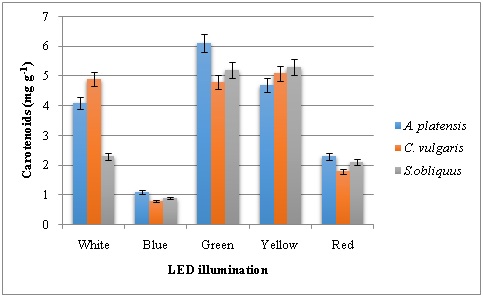 Figure 4: Carotenoid content of microalgae grown under various LED illumination.
Figure 4: Carotenoid content of microalgae grown under various LED illumination.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments found in plants and algae. Chlorophyll a is the primary molecule responsible for photosynthesis, while chlorophyll b is an accessory pigment, the level of which increases upon exposure to a broad spectrum of light that transfers the energy to Chl a. Carotenoids as photosynthetic pigments play a role of excess energy disposal. The absorbance maxima of chlorophylls and carotenoids are in the red and blue wavelength regions of the light spectrum (Richmond, 2003). Microalgal photosystem II can be enhanced by red light wavelength, whereas the microalgal photosystem I can be induced by blue light wavelength [21, 22]. Therefore, blue and red light wavelengths are more suitable for microalgal growth than other wavelengths and should be adequately and selectively provided for microalgal photosynthesis [23]. The ability of microalgae to use different spectra is possibly related to the photosynthetic pigment compositions [24]. Red light can be efficiently absorbed by chlorophyll and phytochrome, and then be transmitted for photosynthesis Hohm[25]. Blue light can be efficiently absorbed by chlorophyll and carotenoids, and green light can be used by phycocyanin[26].
Total protein content of microalgae grown under LED illumination revealed that blue and green LED produced higher amounts. Protein content in the range of 102.7-97.5 mg g-1, 77.9-76.5 mg g-1 and 51.9-49.4 mg g-1 was observed in A. platensis, C. vulgaris and S. obliquus respectively (Figure 5). In general, blue LED has increased the protein content followed by green light. The results were in contradictory with who found the highest content of protein in microalgae under green light and the lowest under blue light. Total carbohydrates of microalgae were increased under blue light and the maximum content of 52.1 mg g-1 was found in C. vulgaris[21, 27]. The second highest carbohydrate content was produced under red LED illumination in A. platensis and S. obliquus(Figure 6). The results were in accordance to findings in which blue light increased the carbohydrate content, and adding green light can improve protein content in Arthrospira platensis[18]. Blue light influences gene expression and several metabolic pathways in algae via photoreceptors and several other growth factors. But low carbohydrate content was observed in Isochrysis sp. when exposed to the blue light [28, 29].
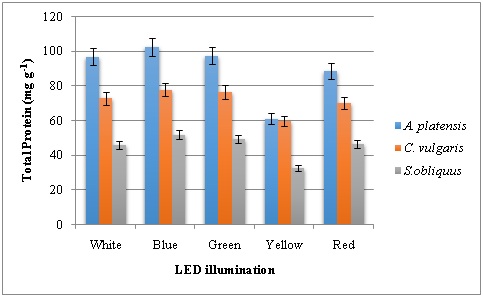 Figure 5: Total protein content of microalgae grown under various LED illumination.
Figure 5: Total protein content of microalgae grown under various LED illumination.
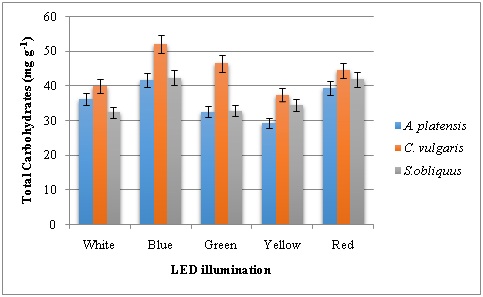 Figure 6: Total carbohydrate content of microalgae grown under various LED illumination.
Figure 6: Total carbohydrate content of microalgae grown under various LED illumination.
The wavelength and intensity of light play key roles in the process of photosynthesis for photoautotrophic microalgal growth and also affect lipid production by microalgae [30]. Microalgae require optimum irradiation conditions with specific narrow bands of light to achieve maximum photosynthetic rates with minimum energy consumption. LED could be the optimal light source for microalgae systems as they have the characteristics of narrow-band wavelength and cost-effective power consumption. In this study, the percentage of lipid content was determined from the microalgal samples grown under different LED illumination. Blue and red LED was proven to be the best sources for lipid accumulation in A. platensis, C. vulgaris and S. obliquus. Lipid content of 5.68 % and 4.96 % was obtained in C. vulgaris and S. obliquus under blue LED (Figure 7). This was followed by 4.37% and 3.86% under red LED illumination for the same species. Different kinds of algae may require different photon flux density, different light wavelengths for their growth reported that blue LED light was preferable for lipid accumulation as opposed to red LED light in Chlorella species [31-33]. In another study, Skeletonema costatum preferred blue and red light to green light and the saturated light intensity is significantly different under the three monochromatic lights [34]. Severes reported that LED of red light 220 lux wavelength doubled the lipid dry weight in Chlorella sp. Whereas, total lipid (53.3%), lipid productivity (27.7 mg L−1 day−1) and total fatty acid (79.4%) were observed under blue LED with reflector [16,17].
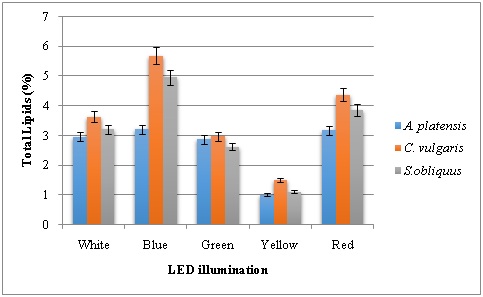 Figure 7: Total lipid content of microalgae grown under various LED illumination.
Figure 7: Total lipid content of microalgae grown under various LED illumination.
CONCLUSION
All the three microalgae produced better growth and had increased biochemical composition under LED light exposure. Blue LED has produced greater total protein, carbohydrate and lipid content. Red light has increased the dry cell weight, chlorophyll a content. Both chlorophyll b and carotenoids were increased with green light illumination. There were not many changes in term of microalgal growth and their cellular composition under yellow LED source. Based on the results, selection of blue and red LED illumination to enhance microalgal growth and cellular composition is possible in the selected microalgae.
REFERENCES
- Oldenhof H, Zachleder V, Van Den Ende H(2006) Blue-and red-light regulation of the cell cycle in Chlamydomonasreinhardtii (Chlorophyta). Eur J Phycol41: 313-320.
- Zhu XG, Long SP, Ort DR (2008)What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? CurrOpin Biotech 19: 153-159.
- Schulze PS, Barreira LA, Pereira HG, Perales JA, Varela JC(2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol32: 422-430.
- Carvalho AP, Silva AO, Baptista JM, Malcata FX(2011) Light requirements in microalgalphotobioreactors: an overview of biophotonic aspects. Appl. Microbiol. Biotechnol 89: 1275-1288.
- Schulze PS, Guerra R, Pereira H, Schüler LM, Varela JCS(2017) Flashing LEDs for microalgal production. Trends Biotechnol 35: 1088-1101.
- Richmond A, Masojidek J, Koblizek M, Torzillo G (2003) Handbook of microalgal culture: biotechnology and applied phycology. Photosynthesis in microalgae. New York: Blackwell Publishers; 20-39.
- Wynne D, Rhee GY,(1986) Effects of light intensity and quality on the relative N and P requirement (the optimum N:P ratio) of marine planktonic algae. J Plankton Res 8: 91-103.
- Rivkin RB(1989) Influence of irradiance and spectral quality on the carbon metabolism of phytoplankton: 1. Photosynthesis, chemical composition and growth. Mar EcolProgSer 55: 291–304.
- Sanchez-Saavedra MP, Voltolina D(2006)The growth rate, biomass production and composition of Chaetoceros sp. grown with different light sources. AquacultEng 35: 161-165.
- Singh J, Gu S(2010) Commercialization potential of microalgae for biofuels production. Renew SustEnerg Rev 14: 2596-2610.
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes Methods. Enzymol 148: 350-382.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. The J of BiolChem193: 265-275.
- Gerhardt P, Murray RG, Wood WA, Krieg NR (1994) Methods for general and molecular bacteriology. ASM, Washington DC.
- Leyva A, Quintana A, Sanchez M, Rodriguez EN, Cremata J, et.al (2008) Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: method development and validation. Biologicals 36: 134-141.
- FolchJ, Lees M, Stanley GH, (1956) A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry 226: 497-509.
- Ajayan KV, CC Harilal, P Gani (2019) Performance of reflector coated LED Bio-box on the augmentation of growth and lipid production in aerophytictrebouxiophyceaen algae Coccomyxa sp. Algal Research 38: 101401.
- Severes A, Hegde S, D’Souza L, Hegde S (2012) Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae based biofuels. J PhotochemPhotobiol B 170: 235-240.
- Mao R, Guo S(2018) Performance of the mixed LED light quality on the growth and energy efficiency of Arthrospira platensis. ApplMicrobiolBiotechnol 102: 5245-5254.
- Ra CH, Kang CH, Jung JH, Jeong GT, Kim SK (2016) Enhanced biomass production and lipid accumulation of Picochlorumatomus using light-emitting diodes (LEDs). BioresourTechnol 218: 1279-1283.
- Ra CH, Sirisuk P, Jung JH, Jeong GT, Kim SK(2018) Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioprocess BiosystEng 41: 457-465.
- Ravelonandro PH, Ratianarivo DH, Joannis-CassanC, Isambert A, Raherimandimby M(2008) Influence of light quality and intensity in the cultivation of Spirulina platensis from Toliara (Madagascar) in a closed system. J ChemTechnolBiotechnol 83: 842-848.
- You T, Barnett SM(2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19: 251-258.
- Ruyters G(1984) Effects of blue light on enzymes. In: Senger H, editor. Blue light effects in biological systems. Berlin: Springer Veralg 283-301.
- Wang SK, Stiles AR, Guo C, Liu CZ (2014) Microalgae cultivation in photobioreactors: an overview of light characteristics. Eng Life Sci 14: 550-559.
- Hohmann MF, Blankenship RE(2011) Evolution of photosynthesis. Ann Rev Plant Biol 62: 515.
- Iacute M, Dek JI, Iacute K, Zek M, Torzillo G(2004) Photosynthesis in microalgae. Blackwell Publishing Ltd 81: 20-39.
- Markou G(2014) Effect of various colors of light-emitting diodes (LEDs) on the biomass composition of Arthrospira platensis cultivated in semi-continuous mode. ApplBiochem Biotech 172: 2758-2768
- Koc, C, Anderson GA, Kommareddy A(2013) Use of red and blue light-emitting diodes (LED) and fluorescent lamps to grow microalgae in a photobioreactor. Isr JAquacult 65: 2797.
- Marchetti J, Gael B, Thierry J, Sebastien L, Catherine R, et. al (2013) Effects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp. (T-iso). J ApplPhycol 2: 109–119.
- Ugwu CU, Aoyagi H, Uchiyama H (2008)Photobioreactors for mass cultivation of algae. Bioresour. Technol 110: 4021-4028.
- Caner K, Gary AA, Anil K(2013) Use of red and blue light-emitting diodes (LED) and fluorescent lamps to grow microalgae in a photobioreactor. ISR J AQUACULT-BAMID 65: 797-805.
- Glemser M, M Heining, Schmidt J, Becker A, Garbe D, et.al (2015) Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: current state and perspectives. ApplMicrobiolBiotechnol 100: 1077-1088.
- Shu CH, Tsai CH, Liao WH, Chen KY, Huang HC (2012) Effects of light quality on the accumulation of oil in a mixed culture of Chlorella sp. and S. cerevisiae. JChemTechnolBiotechnol 87: 601-607.
- Hongli M, Lina S, Qingzhen T, Shanshan W, Jing W(2012) Study on the effect of monochromatic light on the growth of the red tide diatom Skeletonema costatum. Optics and Photonics Journal 2: 152-156.
Citation: Raqiba H, Sibi G (2019) Light Emitting Diode (LED) Illumination for Enhanced Growth and Cellular Composition in Three Microalgae. Adv Microb Res 3: 007.
Copyright: © 2019 Raqiba Habibi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

