
Long-Term Auditory Follow-Up of Preterm Infants after Neonatal Hearing Screening
*Corresponding Author(s):
KS De Graaff-KorfDepartment Of Neonatology, Isala Women And Children's Hospital, Netherlands
Tel:+31 641556932 ,
Email:k.s.korf@isala.nl
Abstract
Background
Since introduction of neonatal Automated Auditory Brainstem Response (AABR) hearing screening in Neonatal Intensive Care Unit (NICU) graduates, Hearing Loss (HL) is established during the first few months of age. The diagnostic Auditory Brainstem Response (ABR) is used as the gold standard in establishing HL after birth. Aim of this study was to investigate the predictive value of better ear ABR findings at three months Post Term Age (PTA) in preterm infants with bilateral SensoriNeural (SNHL) or Conductive Hearing Loss (CHL). In Preterm’s with bilateral Auditory Neuropathy Spectrum Disorder (ANSD) the predictive value of Visual Reinforcement Audiometry (VRA) was investigated.
Methods
Outcome data of graduates of a level III NICU, who didn’t pass AABR neonatal hearing screening between 2004-2016 were analysed retrospectively. At follow-up type and hearing level of graduates with bilateral HL was established. Hearing level was investigated at the age of two years using VRA and at four and eight years of age using play-audiometry. The Two One-Sided Tests equivalence procedure for paired means was applied with the magnitude of the region of similarity equal to 10dB.
Results
In all 32 cases ABR at three months PTA correctly predicted the final type of HL. In 8 SNHL children initial ABR was equivalent with the four and eight year’s play-audiometry (p<0.05). In eight SNHL and 15 ANSD children, VRA levels didn’t reflect significantly play-audiometry levels. Almost all cases (89%, N=8/9) with non-syndromic CHL recovered properly.
Conclusion
ABR of the better ear at three months PTA in preterm reliably seems to predict all types of HL in later childhood as well as the hearing level in children with SNHL at follow-up. In case of ANSD the VRA was not predictive for the severity of HL at follow-up. Further research is necessary to substantiate these findings in preterm infants.
Keywords
ABBREVIATIONS
SNHL - SensoriNeural Hearing Loss
CHL - Conductive Hearing Loss
ANSD - Auditory Neuropathy Spectrum Disorder
PTA - Post Term Age
TOST - Two One-Sided Tests
AABR - Automated Auditory Brainstem Response
ABR - Auditory Brainstem Response
NICU - Neonatal Intensive Care Unit
HL - Hearing Loss
VRA - Visual Reinforcement Audiometry
IVH - Intra Ventricular Hemorrhage
NEC - Necrotising Entero Colitis
INTRODUCTION
A two-step Automated Auditory Brainstem Response (AABR) neonatal hearing screening program was gradually introduced in all Neonatal Intensive Care Units (NICU) in the Netherlands between 1998 and 2001 as a first step towards nation-wide neonatal hearing screening [3]. After repetitive referral, audiological diagnostic tests were performed at a Speech and Hearing center to establish neonatal HL as soon as possible, within three months Post Term Age (PTA). This correction for gestational age is extremely relevant, especially in NICU graduates with an extremely low gestational age. Besides the fact that the stage of the ontogenetic development is related to the duration of pregnancy it is reported in literature that the ongoing process of myelination of the auditory pathway may be delayed or deviant in infants who are born prematurely [4-6]. Therefore, we want to investigate the predictive value of the initial diagnostic ABR findings in the better ear at three months PTA versus the Visual Reinforcement Audiometry (VRA) results at two years and the play-audiometry results at four and eight years of age, with regard to the type and severity of bilateral HL.
METHODS
Types of hearing loss
Diagnostic audiological tests
VRA: Visual Reinforcement Audiometry (VRA) is based on the orientation reflex towards a new sound source. It is a subjective auditory test that requires cooperation of the child and parents. Interacoustics Affinity/AC440 was used (Interacoustics A/S, Middelfart, Denmark) to present the acoustical stimuli.
Play-audiometry: Play-audiometry is an auditory examination of children based on conditioned responses. The child is taught to perform an action only when he or she hears a sound. The test determines the minimal intensity of a warble tone at least at 0.5, 1,2 and 4 kHz at which the child is able to detect the stimulus [9]. The tones were presented through a headphone/insert earphone and a bone-conductor. The bone-conductor was placed on the mastoid bone thus obtaining a pure tone audiogram. Interacoustics Affinity/AC440 (Interacoustics A/S, Middelfart, Denmark) was used to present the acoustical stimuli.
STATISTICAL ANALYSIS
R Version 3.5.1 with library equivalence was used for statistical analysis. The Two One-Sided Tests (TOST) equivalence procedure for paired means was applied with the magnitude of the region of similarity equal to 10dB. The null hypothesis was that the differences in mean dB levels for the different tests were not equal. P-values <0.05 were considered significant.
Within the group of patients with etiologically temporary conductive HL we defined the ABR level to be predictive if at least 75% have normal hearing (<35dB) on the play-audiometry (at four and eight years).
RESULTS
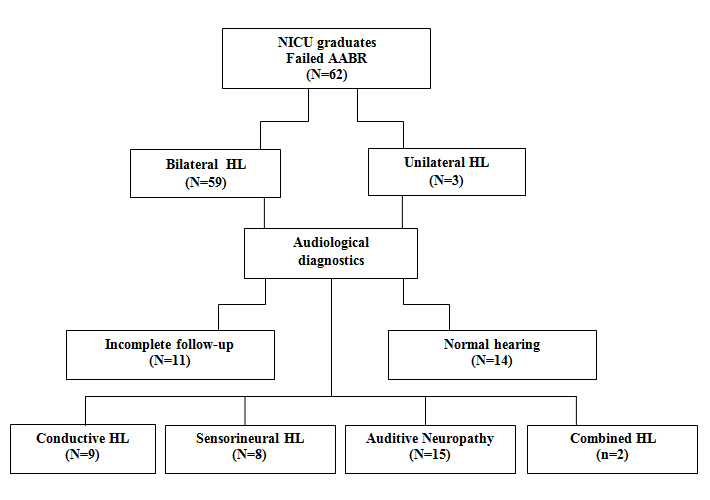
Long term follow-up data were available for 34 (N?34/59) newborns with diagnosed bilateral HL at a PTA of three months. In 14 (N=14/59) no HL was diagnosed and in 11 (N?11/59) the follow-up data set was incomplete due to referral to Speech and Hearing Centers outside the region.
The type and severity of bilateral HL could be diagnosed in 34 NICU graduates: Nine (N?9/34) had CHL, eight (N?8/34) had SNHL, 15 (N?15/34) had ANSD and two (N?2/34) had a combination of ANSD and SNHL.
In this study the last two patients with combined HL were not included in the results, leaving follow-up data of 32 infants for analysis (Table 1).
|
Characteristics |
NICU graduates N = 32 |
|
Gestationalage, mean, weeks |
32 (24 - 41) |
|
Birthweight, mean, grams |
1912 (665 - 4210) |
|
Gender, male, number (%) |
17/32 (53) |
|
Hospitalization NICU, mean, days |
29 (1 - 78) |
|
Mechanicalventilation, number (%) |
24/32 (75) |
|
Mechanicalventilation, mean, days |
9 (1 - 34) |
|
IVH, number (%) |
11/32 (34) |
|
NEC, number (%) |
1/32 (3) |
|
Sepsis (bloodculture proven), number (%) |
5/32 (16) |
|
IVH: Intraventricular Hemorrhage |
|
Overall, at follow-up there was no change in type of HL in 32 (100%, N?32/32) NICU graduates. Figures 2, 3 and 4 show the follow up results for each type of HL in box plots.
SensoriNeural hearing loss
In contrast, the observed differences in means between the VRA level at two years of age in the SNHL group and play-audiometry level at four and eight years of age did not fall within the equivalence bounds of 10dB (resp. p=0.34 and p=0.41) (Figure 2).
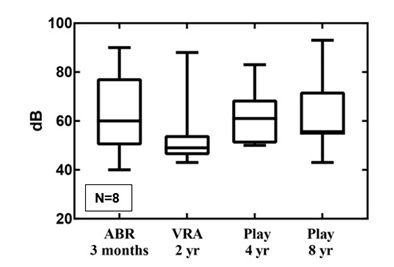 Figure 2: The follow-up results for SNHL in boxplots.
Figure 2: The follow-up results for SNHL in boxplots.Auditory neuropathy spectrum disorder
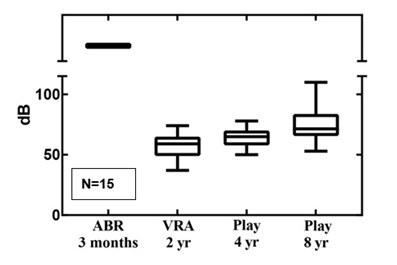 Figure 3: The follow-up results for ANSD in boxplots.
Figure 3: The follow-up results for ANSD in boxplots.Conductive HL
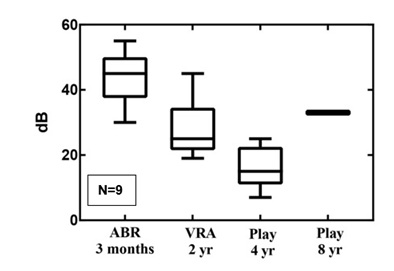 Figure 4: The follow-up results for CHL in boxplots.
Figure 4: The follow-up results for CHL in boxplots.DISCUSSION
In NICU graduates with SNHL the initial ABR and not the VRA was significantly accurate in predicting the severity of HL at four and eight years of age.
ANSD was set as no response at all above a stimulus level of >90dB while OAE’s and/or cochlear microphonics are present. Therefore no level of HL could be established by definition at three months PTA from ABR in children with ANSD. Also in this ANSD group VRA results showed unequal dB levels with play-audiometry at four and eight years of age. In 53% of these children VRA levels were >10dB better than play-audiometry. This means that hearing levels established by VRA were not predictive for hearing levels at four and eight years follow-up.
None of the children with CHL had congenital malformations in the auditory pathway. Nearly all cases recovered from a probably temporary CHL. In one case, CHL was mild and persistent without deterioration due to a chronic middle ear infection.
These findings are of importance in the process of parental guiding. In the SNHL group ABR findings at three months PTA supports future hearing type and level findings while in children with ANSD uncertainty about the level of HL is long lasting.
To our best knowledge this is the first study that takes the PTA of the preterm infant at the time of the initial diagnostic ABR into account as a more physiological milestone for the premature newborn when investigating long-term follow-up results. Due to the recent introduction of neonatal hearing screening, follow-up studies are limited and mostly refer to a (near) term population of newborns. The follow-up period in these studies ranges from six months to six years of age [5,6,10-15]. This study is unique in its insight into data after a lengthy follow-up period, i.e. four and eight years. These follow-up data were related to the PTA of three months which is in terms of pathophysiology more suitable to very preterm newborns. The diagnostic ABR is used as the gold standard in establishing HL after birth [4]. From literature it is known that in premature infants the ongoing process of myelination of the auditory pathway is delayed or alterated [5,6,16-18]. Eventually the maturational delay catches up at the postconceptional age of 40-42 weeks, which is normally the full-term age of healthy newborns [16-18]. Coenraad, et al., showed a prolonged I-V interval at ABR, combined with normal ABR thresholds, which declined with an increased postconceptional age referring to a maturational delay in the auditory pathway [4]. The study of Sleifer, et al., confirms these findings with an inverse correlation between gestational age and ABR inter peak latencies I-III, I-V and III-V with gestational age [5]. Jiang, et al., also found that the maturation in central regions of the brainstem is faster compared to the peripheral regions in preterm infants [6]. Other studies also showed that in preterm infants, <28 weeks of gestation, there is an improvement in HL up until the age of 40 weeks post-term, suggestive of maturational problems of the auditory pathway [17]. The study of Vohr, et al., supports this data [18]. They observed that even in the absence of brain injury there are alterations in maturation and vulnerability of the brain as a consequence of premature birth leading to structural changes within the brain. For these reasons follow-up data in this study were related to the PTA of three months which seems to be proper in the SNHL (very) preterm newborns.
The play-audiometry results at four and eight years of age reflect the most objective stage of HL in the child. The less severe hearing level thresholds at VRA in comparison with play-audiometry levels in the ANSD group may raise questions. The subjective character of the interpretation of the VRA test might possibly bean explanation for the different results between VRA and play-audiometry hearing levels. Another explanation could be a more progressive character or a more heterogeneous course of ANSD in NICU graduates. Robertson, et al., concluded earlier in 2002 that NICU graduates with severe respiratory failure frequently developed late-onset progressive ANSD without a clear explanation for this phenomenon [19]. One can also argue that the more positive results of VRA are partly consequence of the altered or delayed maturation of the brain [5,6]. This study does not support an ongoing deterioration reflected by stable play-audiometry levels between four and eight years of age in children with ANSD. Gardner-Berry, et al., followed twelve infants with ANSD until the age of two years. They found that a reliable VRA was difficult to obtain and resulted in a time delay in further audiological management [15].
Limitation of this study is the limited number of included newborns. We realize there was no sufficient statistical power to detect a lack of difference. Though, the initial results were encouraging for extending this study.
We conclude that the preliminary findings in this study suggests that in NICU graduates ABR of the better ear at three months PTA reliably predicts the type and severity of HL at four and eight years of age in children with SNHL. In the group of children with ANSD VRA levels were lower and not predictive for the severity of HL at four and eight years of age with play-audiometry. These initial results warrant more future research in a larger study group to substantiate reliable predictions with greater precision of the severity of HL in relation to the initial findings at the young age in very preterm NICU graduates to support early intervention and adequately rehabilitation.
REFERENCES
- Dettman SJ, Dowell RC, Choo D, Arnott W, Abrahams Y, et al. (2016) Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otol Neurotol l37: 82-95.
- Marciano E, Laria C, Malesci R, Iadicicco P, Landolfi E, et al.(2013) Newborn hearing screening in the Campania region (Italy): Early language and perceptual outcomes of infants with permanent hearing loss. Acta OtorhinolaryngolItal l33: 414-417.
- van StraatenH,Tibosch C,Dorrepaal C, Dekker F,Kok J (2001)Efficacy of automated auditory brainstem response hearing screening in very preterm newborns. JPediatr 138: 674-678.
- Coenraad S, Hoeve LJ, Goedegebure A (2001) Incidence and clinical value of prolonged I-V interval in NICU infants after failing neonatal hearing screening. EurArch Oto-Rhino-Laryngology 268: 501-505.
- Sleifer P, da Costa S,Cóser P, Goldani M,Dornelles C, et al. (2007) Auditory brainstem response in premature and full-term children. Int J PediatrOtorhinolaryngol 71: 1449-1456.
- Jiang Z, Brosi D,Wu Y, Wilkinson A (2009) Relative maturation of peripheral and central regions of the human brainstem from preterm to term and the influence of preterm birth. Pediatr Res 65: 657-662.
- van Dommelen P, van Straaten H,Verkerk P, Dutch NICU NeonatalHearing Screening Working Group (2011) Ten-year quality assurance of the nationwide hearing screening programme in Dutch neonatal intensive care units. Acta Paediatr100: 1097-10103.
- van Zanten G (2008)Audiologische diagnostiek. In: van Straaten H, Meuwese-Jongejeugd J (eds.). Werkboek Neonatale gehoorscreening. Werkboek Neonatale Gehoorscreening, The Netherlands.
- Hoekstra (2009) Spelaudiometrie. In: LamoreB,Prijs P, Franck V (eds.). Audiologieboek, The Netherlands.
- Bovo R, Trevisi P, Ghiselli S, Benatti A, Martini A (2015) Is very early hearing assessment always reliable in selecting patients for cochlear implants? A case series study. Int J PediatrOtorhinolaryngol79: 725-731.
- Psarommatis I, Florou V, Fragkos M, Douniadakis E, Kontrogiannis A (2011) Reversible auditory brainstem responses screening failures in high risk neonates. Eur Arch Otorhinolaryngol 268: 189-196.
- Yoon PJ, Price M, Gallagher K, Fleisher BE, Messner AH (2003) The need for long-term audiologic follow-up of Neonatal Intensive Care Unit (NICU) graduates. Int J PediatrOtorhinolaryngol67: 353-357.
- Ching TY, Day J, Dillon H, Gardner-Berry K, Hou S, et al. (2013) Impact of the presence of Auditory Neuropathy Spectrum Disorder (ANSD) on outcomes of children at three years of age. Int J Audiol52: 55-64.
- Martínez-Cruz CF, García Alonso-Themann P, Poblano A, Ochoa-López JM (2012) Hearing loss, auditory neuropathy, and neurological co-morbidity in children with birth weight<750 g. Arch Med Res 43: 457-463.
- Gardner-Berry K, Purdy SC,Ching TY,Dillon H (2015) The audiological journey and early outcomes of twelve infants with auditory neuropathy spectrum disorder from birth to two years of age.Int J Audiol54: 524-535.
- American Academy of Pediatrics, Joint Committee on Infant Hearing (2007) Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 120: 898-921.
- Hof JR, Stokroos RJ, Wix E, Chenault M, Gelders E, et al. (2013) Auditory maturation in premature infants: A potential pitfall for early cochlear implantation.Laryngoscope123: 2013-2018.
- Vohr B (2014) Speech and language outcomes of very preterm infants. Semin Fetal Neonatal Med19: 78-83.
- Robertson CM, Tyebkhan JM, Hagler ME, Cheung PY, Peliowski A, et al. (2002) Late-onset, Progressive SensoriNeural Hearing Loss after Severe Neonatal Respiratory Failure. Otol Neurotol 23: 353-356.
Citation: de Graaff-Korf KS, Benard MR, van Dommelen P, van Straaten HLM (2019) Long-Term Auditory Follow-Up of Preterm Infants after Neonatal Hearing Screening. J Neonatol Clin Pediatr 6: 034.
Copyright: © 2019 KS de Graaff-Korf, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

