
Low Ejection Fraction Aortic Stenosis Comparing Transcatheter to Surgical Aortic Valve Replacement
*Corresponding Author(s):
Gregory Duncan RushingDivision Of Cardiac Surgery, Wexner Medical Center, The Ohio State University, Columbus, United States
Tel:+1 6142939327,
Email:Gregory.Rushing@osumc.edu
Abstract
Objective
The purpose of this study was to assess the improvement in Left Ventricular Ejection Fraction (LVEF) in patients With Low-Flow, Low-Gradient (LFLG) aortic stenosis treated with Transcatheter Aortic Valve Replacement Technique (TAVR) compared to Surgical Aortic Valve Replacement (SAVR).
Methods
Patients undergoing aortic valve replacement by TAVR or SAVR between January 2012 and December of 2015 were included. Severe low-flow, low-gradient aortic valve stenosis was defined as: Aortic valve area <1.0 cm2, mean aortic valve gradient <40 mmHg, peak aortic valve velocity of <4 m/s and LVEF <50%. LV dysfunction was considered moderate if the LVEF was 50% to 25%, and severe if it was <25%. Clinical and echocardiographic outcomes were measured at 1 and 12 months.
Results
Ninety-One patients (27.4% female) were included in the study. Forty-four patients (48.3%) underwent SAVR and forty-seven patients had TAVR. In the TAVR group, LVEF improved from baseline 28.4%±7.4 to 34.9%± 12.7, (p<0.0001) as well as in the SAVR group, 28.4%±9.8 to 37.0%±11.2, (p<0.0001). Thirty day mortality was 6.8% (3 patients) in the SAVR group, and 4.3% (3 patients) in the TAVR group. Two patients (4.5%) in the SAVR group and 6 patients (12.8%) in the TAVR group required a permanent pacemaker. In the TAVR group, LVEF was improved at 1 month (32.4%, p<0.05) and 1 year (37.76%, p<0.05).
Conclusion
Aortic valve replacement can be performed safely in patients with low-flow, low-gradient aortic stenosis, using both techniques. TAVR results in early and one-year improvement of LVEF.
INTRODUCTION
Low flow, low gradient aortic stenosis is described in patients with left ventricular systolic dysfunction (Left Ventricular Ejection Fraction (LVEF) <50%) and low mean aortic valve gradient (less than 40 mm Hg) [1]. It is present in 5-10% of patients with severe aortic stenosis and is known to carry poor prognosis [2-4]. Low ejection fraction is often due to end-stage ventricular dysfunction from long-standing after load mismatch. Surgical Aortic Valve Replacement (SAVR) carries a higher mortality rate in this population. In spite of a higher risk of mortality, surgical valve replacement has shown to improve clinical symptoms, ventricular function, and provide a survival benefit in this subset of patients [2,3].
Transcatheter Aortic Valve Replacement (TAVR) has brought an alternative treatment option to these patients. The results of large randomized trials have demonstrated an equivalence or superiority to surgical aortic valve replacement [5-7]. As a consequence, an increasing number of patients with low flow, low gradient aortic stenoses are being treated with TAVR, including patients who would never have come to surgical treatment of their aortic valve stenosis. However, it is still unknown as to whether removing the after load mismatch with TAVR patients results in a similar improvement in Left Ventricular Ejection Fraction (LVEF) as with SAVR. The purpose of this study was to characterize the changes in LVEF in patients with moderate to severe left ventricular dysfunction after TAVR, when compared to SAVR.
PATIENTS AND METHODS
Study design and patient population
SAVR patient data was obtained from retrospective review of patient records and the institution Society of Thoracic Surgeons database. In both groups, baseline clinical and echocardiographic characteristics were obtained from review of clinical records.
Preoperative LVEF, Left Ventricular End Systolic Dimension (LVESD), Left Ventricular End Diastolic Dimension (LVEDD), Peak Aortic valve Velocity (PAV), Mean aortic valve Gradient (MG) and Aortic Valve Area (AVA) as measured by transthoracic echocardiogram were reviewed. Patients were considered to have severe low-flow low-gradient aortic valve stenosis if their aortic valve area was ≤1 cm2, mean aortic valve gradient <40 mmHg, peak aortic velocity of <4 m/s, and LVEF <50%. Left ventricular dysfunction was considered moderate if the LVEF was 50% to 25%, and severe, if it was <25%.
DEVICES AND PROCEDURES
TAVR was performed either via femoral or axillary artery access. While we do perform TAVR procedures via a trans-apical approach, none of the low-flow, low gradient underwent this access. Both self-expanding and balloon expanded valves were implanted.
Follow up
STATISTICAL ANALYSIS
Continuous variables are expressed as mean ± SD. Categorical variables are expressed as percentage and were compared by chi-square or Fisher's exact tests as appropriate. Statistical analysis was performed using SAS Statistical Software (SAS Institute, Inc.).
RESULTS
Ninety one patients were included in the study. Forty-four patients underwent SAVR while forty-seven patients had TAVR. Baseline clinical characteristics of the TAVR and SAVR groups are shown in table 1. TAVR patients were older (78.3 years vs 66.4 years, p<0.001) with a higher rate of previous cardiac surgery procedure (36.2% in TAVR vs 18.2% in SAVR, p = 0.045) and higher STS predicted risk of mortality (11.3% in TAVR vs 6.7% in SAVR, p = 0.009). However, they have less coronary artery disease than SAVR (21.2% in TAVR vs 56.8% in SAVR, p <0.03).The prevalence of diabetes (40.4% in TAVR vs 36.4% in SAVR, p =0.69), NYHA class III-IV symptoms (93.6% in TAVR vs 79.5% in SAVR, p=0.07) and COPD (61.7% in TAVR vs 43.2% in SAVR, p =0.74) was similar among the groups.
|
Patient Characteristics |
SAVR (n=44) |
TAVR (n=47) |
p-value |
|
Age (in years, mean, SD) |
66.4 (13.1) |
78.3 (8.9) |
<0.0001 |
| Sex (n %) Male |
34 (77.3) | 32 (68.1) | 0.22 |
|
H/o smoking (n, %) |
17 (38.6) |
22 (46.8) |
0.01 |
|
A trial fibrillation (n %) |
22 (50) |
31 (66) |
0.74 |
|
COPD (n, %) |
22 (43.2) |
29 (61.7) |
0.74 |
|
CAD/MI (n %) |
25 (56.8) |
10 (21.2) |
0.03 |
|
Diabetes (n %) |
16 (36.4%) |
19 (40.4%) |
0.69 |
|
NYHA class I and II |
9 (11.6) |
3 (6.3) |
0.98 |
|
NYHA Class III and IV (n %) |
35 (79.5) |
44 (93.6) |
0.07 |
|
STS PROM |
0.113 |
0.067 |
0.009 |
|
Previous cardiac surgery |
8 (18.2) |
17 (36.2) |
0.045 |
Table 1: Baseline clinical characteristics of patients undergoing SAVR vs TAVR.
Baseline echocardiographic characteristics for both groups are described in table 2. LVEF in TAVR patients was 35.4%±4 in the moderate dysfunction group and 21.2%±5 in the severe dysfunction group. In SAVR patients LVEF was 35.7%±4.5 in the moderated dysfunction group and 20.2%±5.5 in the severe dysfunction group. Aortic valve peak velocity in TAVR patients was 3.67 m/s±0.5 in the moderate dysfunction group and 3.2 m/s±0.6 in the severe dysfunction group. In SAVR patients, aortic valve peak velocity was 3.68m/s±1.15 in the moderate dysfunction group and 3.47 m/s±0.4 in the severe dysfunction group. Mean aortic valve gradient in TAVR patients were 31.4 mmHg±8 in the moderate dysfunction group and 29.9 mmHg±10 in the severe dysfunction group. In SAVR patients, mean aortic valve gradients were 37.1 mmHg±16.9 in the moderate dysfunction group and 28.9 mmHg±7.5 in the severe dysfunction group. Aortic valve area in TAVR patients was 0.75 cm2±0.2 in the moderate dysfunction group and 0.67 cm2±0.2 in the severe dysfunction group. In SAVR patients, aortic valve area was 0.79 cm2±0.25 in the moderate dysfunction group and 0.67 cm2±0.14 in the severe dysfunction group. Left ventricular end systolic diameter in TAVR patients was 4.1 cm±0.9 in the moderate dysfunction group and 4.9 cm±0.8 in the severe dysfunction group. In SAVR patients, left ventricular end systolic diameter was 4.2 cm±0.6 in the moderate dysfunction group and 5.3 cm±0.8 in the severe dysfunction group.
|
TAVR |
SAVR |
|||||
|
LV Dysfunction |
LV Dysfunction |
|||||
|
Moderate (n=28) |
Severe (n=19) |
P value |
Moderate (n=23) |
Severe (n=21) |
P value |
|
|
LVEF (%) |
33.3±4 |
21.2±5 |
<0.01 |
35.7±4.5 |
20.2±5.5 |
<0.001 |
|
Aortic peak velocity (m/s) |
3.67±0.5 |
3.2±0.6 |
0.42 |
3.67±1.15 |
3.47±0.46 |
0.48 |
|
Aortic mean gradient (mmHg) |
31.4±8 |
29.9±10 |
0.29 |
37.1±16.9 |
28.9±7.5 |
0.03 |
|
AVA (cm2) |
0.75±0.2 |
0.67±0.20 |
0.35 |
0.79±0.25 |
0.67±0.14 |
0.03 |
|
LVEDD, cm |
5.2±0.8 |
5.7±0.8 |
0.32 |
5.3±0.5 |
6.06±0.75 |
0.002 |
|
LVESD, cm |
4.1±0.9 |
4.9±0.8 |
0.51 |
4.2±0.6 |
5.35±0.8 |
0.0001 |
Table 2: Baseline echocardiograph characteristics for SAVR and TAVR group stratified by the severity of LV dysfunction.
OPERATIVE CHARACTERISTICS
TAVR was performed with a self-expanding bioprosthesis (Medtronic, Minneapolis, MN) in 37 patients and with a balloon expanded bioprosthesis (Edwards Life Sciences, Irvine, CA) in 10 patients. The valve was delivered through femoral access in 44 patients and an axillary approach in 3 patients.
Operative mortality was similar among the groups (8% in SAVR vs 4.2% in TAVR, p=0.02). Permanent pacemaker rates were also similar. In the SAVR group, 2 patients (4.5%) required a permanent pacemaker; while 6 patients (12.7%) undergoing TAVR required one (p=0.095).
One month follow up
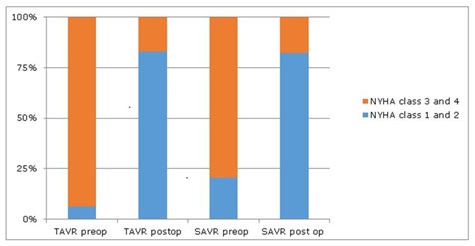 Figure 1: NYHA class preoperative vs. postoperative, in TAVR and SAVR group.
Figure 1: NYHA class preoperative vs. postoperative, in TAVR and SAVR group.TAVR group: One month after TAVR, LVEF improved from 27%±3.9 to 35%±10.5%, p=0.019. As in SAVR, this improvement occurred in both the moderate (33%±3 to 42%±12, p=0.002) and the severe LV dysfunction groups (21.3%±4.89 to 26.7%±9.0, p=0.01) (Table 3 and 4). TAVR was not associated with reduction in LVEDD and LVESD (Table 3 and 4). NYHA class improved after TAVR in both moderate and severe LV dysfunction groups. After TAVR the proportion of patients in NYHA class I and II increased from 17.9% to 89.2% (p<0.001) in the moderate LV dysfunction group and from 15.1% to 72.2% (p<0.001) in the severe group (Tables 3,4 and Figure 1). BNP decreased from baseline in both moderate (1015 pg/dl±821 to 659 pg/dl±145, p=0.02) and severe LV dysfunction (1596 pg/dl±635 to 838 pg/dl±174, p=0.019, Table 3 and 4). At one year follow up, echocardiogram continued to show improvement in LVEF. Patients with moderate LV dysfunction improved from 33%±3 to 40.2%±9 (p=0.002) and those with severe LV dysfunction improved from 21.3%±4.89 to 33.4%±7, (p<0.001), (Figure 2). There were 2 deaths within 30-day period, and 7 subsequent deaths within 1 year.
|
TAVR (n=28) |
p-Value |
SAVR (n=23) |
p-Value |
|||
|
Baseline |
1 Month |
Baseline |
1 Month |
|||
|
LVEF, % |
33±3 |
42±2 |
0.0029 |
35.7±4.5 |
42.7±12.1 |
0.0018 |
|
LVEDD, cm |
5.3±0.82 |
5.2±0.92 |
0.75 |
5.39±0.5 |
5.27±0.73 |
0.28 |
|
LVESD, cm |
4.2±0.98 |
4.3±0.94 |
0.62 |
4.22±0.6 |
4.04±0.75 |
0.07 |
|
AVA |
0.75±0.16 |
0.78±0.25 |
||||
|
Aortic mean gradient, mmHg |
31.4±7.8 |
8.4±4.2 |
<0.001 |
37.1±17 |
12.4±5.8 |
<0.001 |
|
Peak aortic velocity |
3.7±0.52 |
1.9±0.53 |
<0.001 |
3.68±1.15 |
2.34±0.58 |
0.0007 |
|
NYHA Class I and II, n (%) |
5 (17.9) |
25 (89.2) |
<0.001 |
5 (21.7%) |
18 (78.2%) |
<0.001 |
|
NYHA Class III and IV, n (%) |
23 (82.1) |
2 (7.1) |
<0.001 |
18 (85.7) |
3 (14.2%) |
<0.001 |
|
BNP levels (pg/dl) |
1015.3±821 |
659±145 |
0.02 |
1407.5±362 |
189.5±208 |
0.02 |
|
Mortality, n (%) |
0 |
1 (3.5%) |
2 (8.7%) |
|||
Table 3: Echocardiographic and clinical characteristic of patients with moderate LV dysfunction (LVEF 25- 40%) at 1 month post procedure.
|
TAVR (n=19) |
p-Value |
SAVR (n=21) |
p-Value |
|||
|
Baseline |
1 Month |
Baseline |
1 Month |
|||
|
LVEF, % |
21.3±4.89 |
26.7±9.0* |
0.01 |
20.3±5.5 |
30.8±11.6 |
0.0004 |
|
LVEDD, cm |
5.72±0.8 |
5.61±0.88 |
0.4 |
6.04±0.75 |
5.7±0.8 |
0.02 |
|
LVESD, cm |
4.7±1.8 |
4.8±1.1 |
0.4 |
5.38±0.84 |
4.7±1.03 |
0.012 |
|
Aortic valve area |
0.67±0.2 |
0.67±0.14 |
||||
|
Aortic mean gradient, mmHg |
30.3±9.9 |
7.1±1.73 |
<0.001 |
28.7±7.5 |
9.7±4 |
0.001 |
|
Peak aortic velocity, (m/s) |
3.5±0.55 |
1.8±0.29 |
<0.001 |
3.4±0.45 |
2.0±0.5 |
<0.001 |
|
BNP levels |
1596±635 |
838±174 |
0.019 |
973.6±212 |
542±124 |
0.12 |
|
NYHA Class I and II, n (%) |
3 (15.7) |
14 (72.2) |
<0.001 |
3 (14.3) |
15 (78.9) |
<0.001 |
|
NYHA Class III and IV, n (%) |
16 (84.3) |
6 (33.8) |
<0.001 |
18 (85.7) |
4 (21) |
<0.001 |
|
Mortality, n (%) |
1 (5.2) |
1 (4.7) |
||||
Table 4: Echocardiographic and clinical characteristic of patients with severe LV dysfunction (LVEF <25%) at 1 month post procedure.
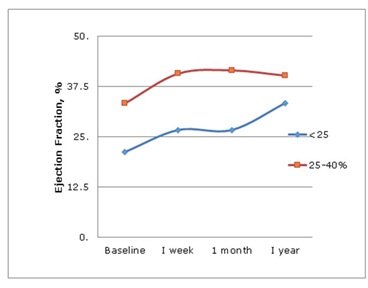 Figure 2: LVEF over 1 year in TAVR group.
Figure 2: LVEF over 1 year in TAVR group.SAVR vs TAVR: Both groups showed similar improvements in LVEF. Measurements of LVEDD and LVESD by echocardiogram showed improvements in patients with severe LV dysfunction undergoing SAVR but not TAVR. TAVR patients had a higher need for permanent pacemakers postoperatively. Operative mortality was similar in both groups.
DISCUSSION
This study evaluates the clinical and echocardiograph outcomes of patients with low flow low gradient aortic stenosis treated either with TAVR or SAVR. The main findings of this study are that 1) Patients with low flow, low gradient aortic stenosis can be safely treated with both SAVR and TAVR, 2) Both TAVR and SAVR improved LVEF immediately after the procedure, 3) LVEF continue to improve in TAVR patients up to one year after the procedure, 4) This improvement is seen, even in patients with severe LV dysfunction.
Preoperative left ventricular dysfunction has been identified as a strong prognostic indicator for operative mortality and long term outcomes in patients with aortic stenosis. In spite of the increased operative mortality, previous reports have demonstrated that SAVR can be performed with an acceptable operative mortality in this group of patients. There are a few reports on the safety of TAVR in low flow low gradient aortic stenosis patients. These studies have reported 1-year mortality ranging from 25.9% to 36% [5,8].
Recovery of left ventricular function has previously described after SAVR in patients in low LVEF. Morris et al., showed that 72% of the patients improved their LVEF after SAVR [9]. In patients with low-flow, low-gradient aortic stenosis, Connolly et al., showed that after SAVR, LVEF improved from a mean preoperative EF 24±7%; to EF, 32±14% [10]. In addition SAVR also improved long term survival in patients with low-flow, low-gradient aortic stenosis compared to medical management [3,4]. This improvement is seen even in patients with low flow low gradient aortic stenosis without contractile reserve [11].
Improvement in LVEF after AVR is attributed to removal of the after load mismatch on the ventricle. Studies have shown that as long as there is demonstrable contractile reserve in the left ventricle, function is expected to improve by at least 10% [3,12,13].
The advent of TAVR has brought another treatment option to this high risk cohort relieving the after load mismatch with potential lower operative risk and faster recovery. The PARTNER trial and core valve trials have already established the safety and efficacy of TAVR in patient deemed as high risk for surgery [14,15].
The current study demonstrated that TAVR can be accomplished with similar operative mortality as SAVR in patients with low LVEF. Both TAVR and SAVR resulted in early improvement of LVEF and that improvement is seeing in patients with both moderate and severe LV dysfunction. This improvement continues for up to a year in severe LV dysfunction. However, TAVR was not associated with reverse ventricular remodeling as demonstrated by no changes in LV dimensions after TAVR. SAVR showed an improvement not only in LVEF but also in ventricular dimensions, in those patients with severe LV dysfunction.
Our study has several limitations. First, this is a retrospective single institution study with the limitations inherent to any retrospective study. Second, the baseline characteristics of TAVR and SAVR patients showed that TAVR were a higher risk group and therefore predicted to have worse outcomes. Third, the clinical and echocardiographic follow up in SAVR were limited to one year limiting our ability to compare one year outcomes.
In conclusion we show that TAVR can be done safely in patients with low-flow, low-gradient aortic stenosis and LV dysfunction. TAVR not only improves LVEF in patients with moderate and severe left ventricular dysfunction. The improvement in LV function continues up to one year after TAVR in patients with severe LV dysfunction, but also improves functional status similarly as SAVR. In those patients with severe LV dysfunction, LVEF continues to improve out to one year.
ACKNOWLEDGEMENT
Presented at the 53rd Annual Society of Thoracic Surgeons Meeting, Houston, TX, Jan 2017.
REFERENCES
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, et al. (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American college of cardiology/ American heart association task force on practice guidelines. J Am Coll Cardiol 63: 2438-2488.
- Pereira JJ, Lauer MS, Bashir M, Afridi I, Blackstone EH, et al. (2002) Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 39: 1356-1363.
- Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, et al. (2003) Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation 108: 319-324.
- Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, et al. (2008) Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: A European multicenter study. J Am Coll Cardiol 51: 1466-1472.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, et al. (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364: 2187-2198.
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, et al. (2016) Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374: 1609-1620.
- Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, et al. (2015) 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 385: 2477-2484.
- Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, et al. (2013) Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: A placement of aortic transcatheter valves (PARTNER) trial analysis. Circulation 127: 2316-2326.
- Morris JJ, Schaff HV, Mullany CJ, Rastogi A, McGregor CG, et al. (1993) Determinants of survival and recovery of left ventricular function after aortic valve replacement. Ann Thorac Surg 56: 22-29.
- Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, et al. (2000) Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: Result of aortic valve replacement in 52 patients. Circulation 101: 1940-1946.
- Fougères E, Tribouilloy C, Monchi M, Petit-Eisenmann H, Baleynaud S, et al. (2012) Outcomes of pseudo-severe aortic stenosis under conservative treatment. Eur Heart J 33: 2426-2433.
- Pibarot P, Dumesnil JG (2012) Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol 60: 1845-1853.
- Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E (2010) Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 122: 1928-1936.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, et al. (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363: 1597-1607.
- Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, et al. (2012) Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 366: 1696-1704.
Citation: Rushing GD, Malik M, Shareef M, Crestanello J, Lilly S, et al. (2018) Low Ejection Fraction Aortic Stenosis Comparing Transcatheter to Surgical Aortic Valve Replacement. J Surg Curr Trend Innov 2: 006.
Copyright: © 2018 Mahim Malik, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

