Magnetic Nanoparticle for Biomedicine Applications
*Corresponding Author(s):
Gang LiuKey Laboratory Of Magnetic Materials And Devices, Ningbo Institute Of Materials Technology And Engineering, Chinese Academy Of Sciences, Ningbo, Zhejiang 315201, China
Tel:+86 57486685030,
Fax:+86 57486685163
Email:liug@nimte.ac.cn
Run-Wei Li
Key Laboratory Of Magnetic Materials And Devices, Ningbo Institute Of Materials Technology And Engineering, Chinese Academy Of Sciences, Ningbo, Zhejiang 315201, China
Tel:+86 57486685134,
Fax:+86 57486685163
Email:runweili@nimte.ac.cn
Yu Chen
Key Lab For Advanced Materials, Institute Of Applied Chemistry, East China University Of Science And Technology, 130 Meilong Road, Shanghai 200237, China
Tel:+86 2164253765,
Fax:+86 2164252485
Email:chentangyu@yahoo.com
Abstract
Keywords
INTRODUCTION
The aim of this review article is to summarize the recent progress in the synthesis and functionalization of magnetic nanoparticles and their applications in biomedicine, such as bioseparation, molecular detection, drug delivery, Magnetic Resonance Imaging (MRI) and hyperthermia. With the development in the synthesis and functionalization of the MNPS, the above applications are demonstrating great vitality and have been or may be used in clinical practice.
SYNTHESIS OF MAGNETIC NANOPARTICLES
The most common wet chemical method used for the preparation of MNPs is co-precipitation. This approach offers a wide range of advantages: 1) It is low-cost because of the use of inexpensive chemicals and mild reaction conditions, 2) It is environmentally friendly because the MNPs can be directly synthesized in water, 3) The method is extremely flexible when it comes to the modulation of the core and surface properties by controlling the experimental parameters such as the reaction temperature, pH value and ionic strength of the media; 4) It is extremely repeatable if the experimental parameters are fixed [14]. For example, by using this method, size-controllable iron oxides (either Fe3O4 or γ-Fe2O3) nanoparticles can be efficiently synthesized from aqueous Fe2+/Fe3+ salt solutions by the addition of a base at room temperature [15].
Other approaches including microemulsions [16], sol-gel synthesis [17], sonochemical reactions [18], hydrothermal reactions [19], hydrolysis and thermolysis of precursors [20], flow injection synthesis [21], electrospray synthesis [22], and graft copolymer method [23] are reported in the literature as well. For instance, in order to increase the loading amount of the reactive groups on the magnetic particles and to improve the stability and dispersibility of magnetic particles, several magnetic particles have been synthesized by the graft copolymer method, with the flexibility and diversity to control the chemical composition and functional groups on the surface of nanoparticles. XF Sun et al., reported the synthesis of a novel hemicelluloses based magnetic hydrogel by using such a graft copolymer method, showing that the Fe3O4 particles can be well dispersed in the hydrogel matrix and the as-prepared hydrogels had promising paramagnetic properties for potential biomedical application. A brief summary of various MNPs synthesis methods is shown in table 1.
| Synthetic method | Synthesis condition | Size distribution | Shape control |
| Co-Precipitation |
Very simple, ambient conditions |
relatively narrow | not good |
| Thermal decomposition |
complicated, inert atmosphere |
very narrow | very good |
| Microemulsion |
complicated, ambient conditions |
relatively narrow | good |
| Hydrothermal synthesis |
simple, high pressure |
very narrow | very good |
Table 1: Summary comparison of various magnetic nanoparticle synthesis methods [14].
FUNCTIONALIZATION OF MAGNETIC NANOPARTICLES
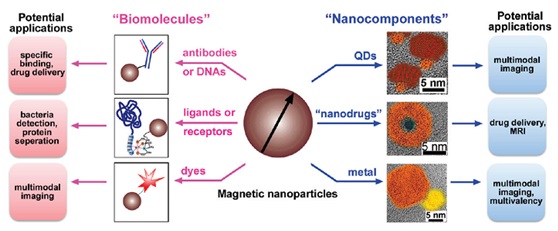
Other ways of functionalization of MNPs, such as combining them with other functional nanostructures by sequential growth or coating, have also been reported. For example, MNPs combined with quantum dots or metallic species lead to a promising candidate for molecular imaging [27-29]. The encapsulation of a potential anticancer drug by iron oxide nanoshells affords the yolk-shell nanostructures, which is potentially promising for controlled drug delivery (Figure 1). In addition, owing to the large surface area to volume ratios and strong dipole-dipole interactions, MNPs tend to agglomerate and form larger clusters with limited application [5,30]. By functionalizing MNPs, it is possible to avoid this phenomenon and achieve desirable dispersibility in the liquid state as well (Figure 1,Table 2) [31,32].
| Biomolecule coating agent
|
Applications |
|
Adenoviral vectors Antibodies |
|
| Aptamers | Recognition processes. |
| Dextran | Stabilizer agent. Enhances blood circulation. |
| DNA or RNA | Recognition processes. Drug delivery. |
| Doxorubicin | Drug for cancer treatment. |
| Enzymes or proteins |
|
| Folic acid | Effective tumor targeting agent. |
| Nitrilotriacetic acid | Protein separation. |
| Oligonucleotides | Probes for detecting or separating DNA or RNA. Nanoswitches. |
| Paclitaxel | Drug for cancer treatment. |
| Polyethylene glycol | Widely used for protein conjugation. |
| Silica | Stabilizer agent that can be loaded (within the pores). Useful in the fabrication of multifunctional MNPs. |
Table 2: Examples of different coating agents and biomolecules used in the fabrication of magnetic biocomposites [32].
BIOMEDICAL APPLICATIONS OF MAGNETIC NANOPARTICLES
Bioseparation
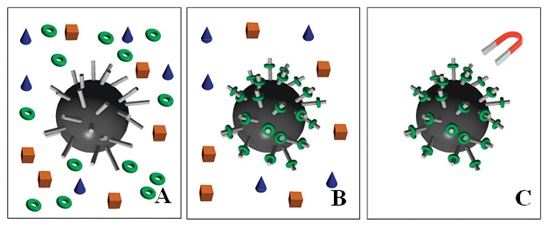
Figure 2: Schematic representation of magnetic separation of DNA or proteins in solution. The grey rods on the surface of the spherical MNP represent the immobilized tag which will tightly bind the DNA strand or protein of interest. The separation process follows the steps: (A) MNPs and the solution with different components are mixed, (B) particular proteins or DNA (green rings) bind to the MNPs and (C) magnetic field is applied to trigger magnetic decantation, followed by further washing steps and collecting the molecule of interest [37].
Depending on the specific functionalization of the MNPs for different targets, this technique has been already applied in the field of the separation and purification of cells [38], proteins [39], bacterial [40] and virus as well. Some work is summarized in table 3. In the study by Yi-Ran Cui et al., Anti-CD 3 monoclonal antibody bioconjugated to core/shell Fe3O4@Au magnetic nanoparticles were synthesized for cell separation (Figure 3). It is showed that the functional Fe3O4@Au MNPs successfully pulled down CD3+ T cells from the whole splenocytes. This method with high efficiency of up to 98.4% demonstrates a more effective cell-capture nanostructure than that obtained by non-oriented strategy [41]. Zhen Liu et al., reported a multifunctional magnetic mesoporous core/shell hetero-nanostructure (designated as Fe3O4@NiSiO3), which combined the capacity of effective protein purification from protein mixture and selective low molecule weight biomolecule enrichment. The hetero-nanoparticles were employed to selectively bind to and magnetically separate of His-tagged proteins from a cell lysate of Escherichia coli[42].
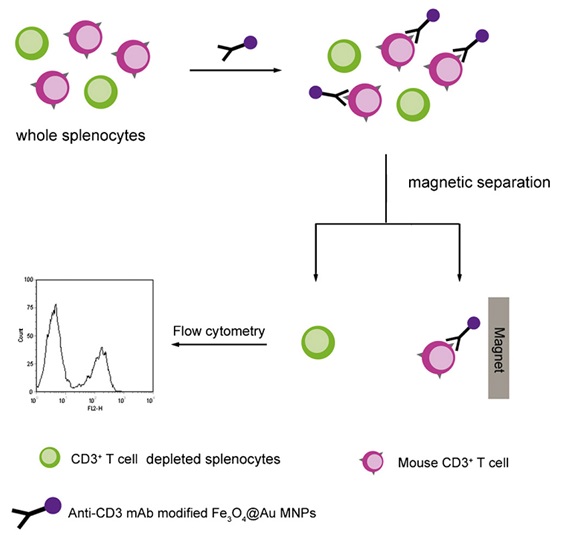
Figure 3: Procedure of magnetic cell separation for whole splenocytes of mouse using anti-CD3 mAb modified Fe3O4@Au MNPs [41].
| Biomolecules | MNPs |
| Cell | core/shell Fe3O4@Au @Au [43] |
| Protein | Fe3O4@NiSiO3/Au/γ-Fe2O3/Fe3O4 [44] |
| Bacterial | Fe3O4@Au [45] |
| Virus | Fe3O4@polymer [46] |
| Gene | Fe3O4-SiO2-Au [47] |
Table 3: Examples of bioseparation by using MNPs.
Biosensing
The modes in which magnetic particles bind to the sensor surface include direct labeling and indirect labeling. The direct labeling method is that MNPs bind to the sensor surface by streptavidin-biotin interaction or complementary DNA sequence recognition. As shown in figure 4, single-stranded DNA receptors are immobilized on the surface of magnetic sensors as a probe. Oligonucleotides of unknown sequence are selectively captured by these complementary probes. Streptavidin-coated MNPs which are capable to bind to the biotin of the hybridized DNA are then introduced into the system. Consequently, magnetic field disturbances because of these MNPs are sensed by the magnetic sensors. The principle of indirect labeling is the sandwich immunoassay such as the Enzyme-Linked Immuno Sorbent Assay (ELISA). The antibodies for the target protein are immobilized on the surface. Then the sample solution containing the target proteins and second biotinylated antibodies are added to the system. Finally Streptavidin-coated magnetic particles are applied for tagging the biotinylated antibodies.
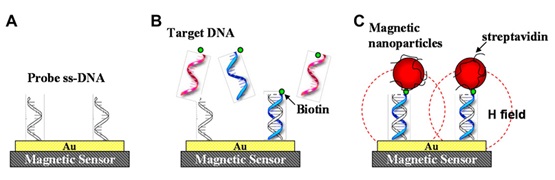
MNP-based Giant Magnetoresistance (GMR), Spin Valve (SV), Magnetic Tunnel Junction (MTJ), Hall biosensor, and Giant Magneto Impedance (GMI) sensor [52-54] have been successfully developed for biosensing. Steven M Hira et al., has shown the successful detection of a 35-base pathogenic DNA target by Hall-based magnetic transduction. The detection platform has low background noise, large signal amplification ratio following target binding, and can discriminate low concentration of DNA labeled by 350 nm superparamagnetic bead [55]. Another example is the combination of magnetoresistive (spin valve) sensors and magnetic labeling of bioanalytes. By using this technique, a powerful quantitative immunological assays is attained. By reading out the magnetic signals of bio-functionalized magnetic nanoparticles through spin valves sensor, it is shown that the detection sensitivity of human chorionic gonadotropin hormone was well below 5.5 ng/mL (the visual inspection limit) [53].
Drug delivery
Targeted drug delivery of molecules with MNPs can improve the drug specificity and reduce this side-effect [57]. Therapeutic agents are attached to, or encapsulated within MNPs to form the MNPs/therapeutic agent co-complex. These magnetic carriers are injected into the blood-stream and guided to focus over the tumor location through external applied inhomogeneous magnetic fields [58]. The therapeutic agents packed in the NMPs are then released to destroy the tumor cell effectively (Figure 5). Targeted delivery of drug molecules with MNPs can improve the biodistribution and protect the drugs from the microenvironment, exhibiting higher internalization by cancer cells than healthy cells and permitting the use of the therapeutic agents at low enough doses to reduce the toxicity of chemotherapy [24].
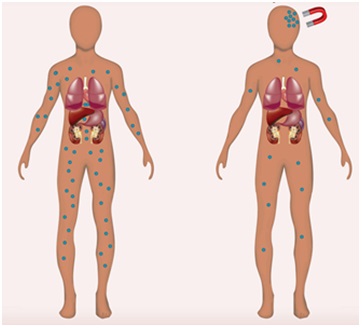
Figure 5: Magnetic targeting. No accumulation of Magnetic Nanoparticles (MNPs) occurs in the absence of a magnetic field, whereas under the influence of this field, MNPs alone or in combination with therapeutic cargo accumulate at a destined site [59].
A number of studies have demonstrated the advantages of using MNPs for drug delivery. Jon Dobson demonstrated that targeted delivery technique using magnetic nanoparticles was a major breakthrough to the treatment of many diseases in the clinical practice in recent years. Therapeutic compounds are attached to biocompatible magnetic nanoparticles and the applied magnetic fields are focused on specific targets in-vivo. The fields capture the particle complex and result in enhanced delivery to the target site [8].
WK Oh et al., reports that the Carbonized Polypyrrole (CPyNs) nanoparticles show low toxicity at low concentrations via cell viability test. The magnetic property of CPyNs provides selective separation and CPyNs sustains in-vitro drug release properties. A new platform for the drug delivery using carbonized polypyrrole nanoparticles was established [60].
Magnetic resonance imaging
Many efforts have been undertaken to improve the current contrast agents, aiming to increase the circulation time and to reduce toxicity. Manuel Pernia Leal et al., showed that water soluble MNPs display a high dense Polyethylene Glycol (PEG) core shell and allow long blood circulation times up to 24 h. The combination of low toxicity, excellent T2 relaxation times, as well as excellent r2/r1 values under a low magnetic field, together with the long circulation times, makes these nanomaterials very promising contrast agents for MRI-based molecular imaging in clinical practice (Figure 6) [63]. Ji-Ho Park et al., demonstrated that the geometry of the nanoparticles can enhance their magnetic relaxivity in MRI. The elongated shape of the magnetic iron oxide nanoparticle, with its larger surface area, multiple attachment points, and long blood half-life, improves the T2 relaxivity for MR imaging [64].
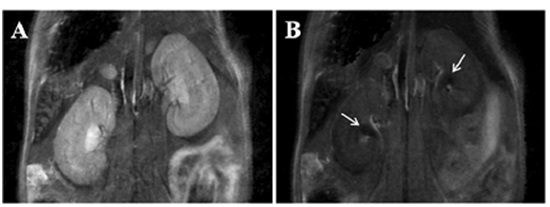
Figure 6: Renal clearance of MNP-GA-PEG-OH. Kidneys before injection of the MNPs (A) and 12 minutes after injection (B), where accumulation of MNPs can be observed in the renal pelvis (white arrows) [63].
In a study by Hyeon et al., Polyethylene Glycol (PEG) coated MNPs with a diameter of 3 nm have been explored. These MR-active nanoparticles enable clear observation of various blood vessels. Thus they can be used for imaging the vasculature, liver and other organs, as well as molecular imaging, cell tracking and theranostics [65]. Yan Wang et al., indicated a system combination of Poly(Lactide-co-Glycolide)-methoxy poly(Ethylene Glycol) (PLGA-mPEG) nanoparticles and MNPs for Magnetic Resonance Imaging (MRI) can improve the imaging with reduced side effects [66]. KG Neoh and ET Kang also have the similar work about MNPs application in MRI [67,68].
HYPERTHERMIA
In the MNPs-based hyperthermia treatment, ferromagnetic or superparamagnetic particles are employed to generate heat into the tumor tissue, followed by irradiation using an Alternating Current (AC) magnetic field [72]. Compared with conventional hyperthermia treatment, MNPs can offer several advantages: 1) Increasing the effectiveness of hyperthermia. MNPs used for hyperthermia are in the range of few tens of nanometer in size, thus allowing easy reach into tumors after intravenous injection with the aid of the applied magnetic field [73]; 2) Improving the specificity of hyperthermia. The MNPs tailored with cancer-specific binding agents allow them being targeted toward specific tumor tissues [74-76]; 3) Ensuring the safety of hyperthermia. The heating of MNPs by the externally alternating magnetic field allows the heat action only killing the tumor cells with minimum damage to normal tissue [77]. Figure 7, illustrates the hyperthermia experimental scheme, where the mouse is placed inside the solenoid coil (blue color) with the tumor positioned at the coil center in order to expose the tumor to the strongest magnetic field generated by the coil [78].
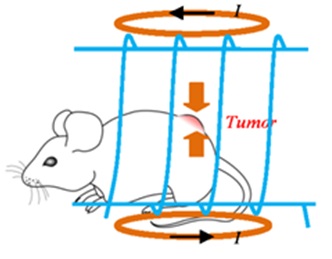
Figure 7: Illustration of the hyperthermia experimental scheme [78].
Chun-Han Hou et al., investigated the effect of magnetic hydroxyapatite nanoparticles injected around the tumor of mice. The mice were placed into an inductive heater with high frequency and alternating magnetic field. Laboratory results showed that the tumor volume of mice was reduced with injected magnetic hydroxyapatite nanoparticles, which demonstrated its therapeutic effect in the mice [79]. In the study by Y Krupskaya, the potential of iron containing Carbon Nanotubes (Fe-CNT) for contactless magnetic heating in hyperthermia cancer treatment was presented. In this paper, the heating mechanism also has been studied [80].
CONCLUSION AND PERSPECTIVES
Although, there are many promising and exciting accomplishment of the magnetic nanoparticles in biological applications, as described in this review, researches on these magnetic nanoparticles for biomedicine application is just on the beginning of the road with vast challenges and issues remaining to be resolved. For instance, the clinical trials are very restricted since toxicity and biocompatibility are of major concerns for biomedical use. Although so many magnetic nanoparticles documented in the references have shown the potential to be applied in biomedicine systems, in-vitro and in-vivo studies are still very rare at the moment. Another challenge is that, though the functional molecules attached on the surface of the magnetic nanoparticles, such as antibodies, aptamers, DNA or RNA, offer the required high sensitivity, these biomolecules are easily denatured by the surrounding environment. New strategy is thus necessary to increase their environmental stability. Fortunately, Young-Wook Choi et al., showed good long-term stability of particles and a narrow particle distribution using poly-electrolytes with a negatively charged functional group [81]. In addition, developing and designing multifunctional magnetic nanoparticles which, for example, is capable to integrate optical imaging with magnetic resonance imaging or to deliver the drug while performing the hyperthermia treatment, is of great interest in the current research activity and is considered as the one of future directions of designing smart materials. In these concerns, the development of multifunctional magnetic nanoparticles with the distinguished features of broad versatility, biocompatibility and unique multifunctional properties will play a key role in the future biomedicine areas.
REFERENCES
- Nhiem Tran, Thomas J Webster (2010) Magnetic nanoparticles: biomedical applications and challenges. Journal of Materials Chemistry 20: 8760-8767.
- Catherine C Berry, Adam SG Curtis (2003) Functionalisation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D Applied Physics 36: 198-206.
- Gupta AK, Naregalkar RR, Vaidya VD, Gupta M (2007) Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine (Lond) 2: 23-39.
- Frimpong RA, Hilt JZ (2010) Magnetic nanoparticles in biomedicine: synthesis, functionalization and applications. Nanomedicine (Lond) 5: 1401-1414.
- Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26: 3995-4021.
- Sonvico F, Dubernet C, Colombo P, Couvreur P (2005) Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr Pharm Des 11: 2095-2105.
- Tobias Neubergera, Bernhard Schöpfa, Heinrich Hofmannb, Margarete Hofmannc, Brigitte von Rechenberga (2005) Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. Journal of Magnetism and Magnetic Materials 293: 483-496.
- Dobson J (2006) Magnetic nanoparticles for drug delivery. Drug Development Research 67: 55-60.
- Christopher J Sunderland, Matthias Steiert, James E Talmadge, Austin M Derfus, Stephen E Barry (2006) Targeted nanoparticles for detecting and treating cancer. Drug Development Research 67: 70-93.
- Ling Lia, Maohong Fana, Robert C Browna, J (Hans) Van Leeuwena, Jianji Wang, et al. (2006) Synthesis, properties, and environmental applications of nanoscale iron-based materials: A review. Critical Reviews in Environmental Science and Technology 36: 405-431.
- Lee CS, Lee H, Westervelt RM (2001) Microelectromagnets for the control of magnetic nanoparticles, Applied Physics Letters 79: 3308-3310.
- Gupta AK, Curtis AS (2004) Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials 25: 3029-3040.
- Gupta AK, Wells S (2004) Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Transactions on Nanobioscience 3: 66-73.
- Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl 46: 1222-1244.
- Kim DK, Zhang, Y, Voit W, Rao KV, Muhammed M (2001) Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles, Journal of Magnetism and Magnetic Materials 225: 30-36.
- Ang Bee Chin, Iskandar Idris Yaacob (2007) Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart’s procedure. Journal of Materials Processing Technology 191: 235-237.
- Cecilia Albornoz, Silvia E Jacobo (2006) Preparation of a biocompatible magnetic film from an aqueous ferrofluid. Journal of Magnetism and Magnetic Materials 305: 12-15.
- Eun Hee Kima, Hyo Sook Leea, Byung Kook Kwakb, Byung-Kee Kimc (2005) Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. Journal of Magnetism and Magnetic Materials 289: 328-330.
- Junxi Wan, Xiangying Chen, Zhenghua Wang, Xiaogang Yang, Yitai Qian (2005) A soft-template-assisted hydrothermal approach to single-crystal Fe3O4 nanorods. Journal of Crystal Growth 276: 571-576.
- Mitsumasa Kimata, Daiki Nakagawa, Masahiro Hasegawa (2003) Preparation of monodisperse magnetic particles by hydrolysis of iron alkoxide. Powder Technology 132: 112-118.
- German Salazar-Alvarez, Mamoun Muhammed, Andrei A Zagorodni (2006) Novel flow injection synthesis of iron oxide nanoparticles with narrow size distribution. Chemical Engineering Science 6: 4625-4633.
- Soubir Basak, Da-Ren Chen, Pratim Biswas (2007) Electrospray of ionic precursor solutions to synthesize iron oxide nanoparticles: Modified scaling law. Chemical Engineering Science 62: 1263-1268.
- LI Ya-Jing, Sun Xiao-Feng, YE Qing, Liu Bai-Chen, Wu Yao-Guo (2014) Preparation and Properties of a Novel Hemicellulose-Based Magnetic Hydrogel. Acta Physio-Chimica Sinicia 30: 111-120.
- QA Pankhurst, J Connolly, SK Jones, J Dobson (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36: 167-181.
- Kohler N, Fryxell GE, Zhang MQ (2004) A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J Am Chem Soc 126: 7206-7211.
- Bourlinos AB, Bakandritsos A, Georgakilas V, Petridis D (2002) Surface modification of ultrafine magnetic iron oxide particles. Chem Mater 14: 3226- 3228.
- Sun C, Sze R, Zhang M (2006) Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J Biomed Mater Res A 78: 550-557.
- Sahoo Y, Goodarzi A, Swihart MT, Ohulchanskyy TY, Kaur N, et al. (2005) Aqueous ferrofluid of magnetite nanoparticles: Fluorescence labeling and magnetophoretic control. J Phys Chem B 109: 3879-3885.
- Ji X, Shao R, Elliott AM, Stafford RJ, Esparza-Coss E, et al. (2007) Bifunctional Gold Nanoshells with a Superparamagnetic Iron Oxide-Silica Core Suitable for Both MR Imaging and Photothermal Therapy. J Phys Chem C Nanomater Interfaces 111: 6245-6251.
- Hamley IW (2003) Nanotechnology with soft materials. Angew Chem Int Ed Engl 42: 1692-1712.
- Tan C, Saurabh S, Bruchez MP, Schwartz R, Leduc P (2013) Molecular crowding shapes gene expression in synthetic cellular nanosystems. Nat Nanotechnol 8: 602-608.
- 32. Schladt TD, Schneider K, Schild H, Tremel W (2011) Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalton Trans 40: 6315-6343.
- Gao J, Gu H, Xu B (2009) Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc Chem Res 42: 1097-1107.
- Safarík I, Safaríková M (1999) Use of magnetic techniques for the isolation of cells. J Chromatogr B Biomed Sci Appl 722: 33-53.
- Gu H, Xu K, Xu C, Xu B (2006) Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun (Camb): 941-949.
- Liane M Rossi, Natalia JS Costa, Fernanda P Silvaa, Robert Wojcieszaka (2014) Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem 16: 2906-2933.
- Colombo M, Carregal-Romero S, Casula MF, Gutiérrez L, Morales MP, et al. (2012) Biological applications of magnetic nanoparticles. Chem Soc Rev 41: 4306-4334.
- Christopher M Earhart, Casey E Hughes, Richard S Gaster, Chin Chun Ooi, Robert J Wilson, et al. (2014) Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip 14: 78-88.
- Zhang L, Zhu X, Jiao D, Sun Y, Sun H (2013) Efficient purification of His-tagged protein by superparamagnetic Fe3O4/Au-ANTA-Co2+ nanoparticles. Mater Sci Eng C Mater Biol Appl 33: 1989-1992.
- Intorasoot S, Srirung R, Intorasoot A, Ngamratanapaiboon S (2009) Application of gelatin-coated magnetic particles for isolation of genomic DNA from bacterial cells. Anal Biochem 386: 291-292.
- Cui YR, Hong C, Zhou YL, Li Y, Gao XM, et al. (2011) Synthesis of orientedly bioconjugated core/shell Fe3O4@Au magnetic nanoparticles for cell separation. Talanta 85: 1246-1252.
- Liu Z, Li M, Yang X, Yin M, Ren J, et al. (2011) The use of multifunctional magnetic mesoporous core/shell heteronanostructures in a biomolecule separation system. Biomaterials 32: 4683-4690.
- Ying Jing, Lee R Moore, P Stephen Williams, Jeffrey J Chalmers, Sherif S Farag, et al. (2007) Blood progenitor cell separation from clinical leukapheresis product by magnetic nanoparticle binding and magnetophoresis. Biotechnology and Bioengineering 96: 1139-1154.
- Mizukoshi Y, Seino S, Okitsu K, Kinoshita T, Otome Y, et al. (2005) Sonochemical preparation of composite nanoparticles of Au/gamma-Fe2O3 and magnetic separation of glutathione, Ultrason Sonochem 12: 191-195.
- Wang C, Irudayaraj J (2010) Multifunctional magnetic-optical nanoparticle probes for simultaneous detection, separation, and thermal ablation of multiple pathogens. Small 6: 283-289.
- Lunov O, Syrovets T, Röcker C, Tron K, Nienhaus GU, et al. (2010) Lysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytes. Biomaterials 31: 9015-9022.
- Lin Tang, Mengshi Wu, Guangming Zeng, Juan Yin, Yuanyuan Liu, et al. (2012) Magnetic separation and detection of a cellulase gene using core-shell nanoparticle probes towards a Q-PCR assay. Anal Methods 4: 2914-2921.
- Koh I, Josephson L (2009) Magnetic nanoparticle sensors. Sensors (Basel) 9: 8130-8145.
- Chenjie Xu, Shouheng Sun (2007) Monodisperse magnetic nanoparticles for biomedical applications. Polymer International 56: 821-826.
- Liyuan Maa, Chaoming Wanga, Minghui Zhang (2011) Detecting protein adsorption and binding using magnetic nanoparticle probes. Sensors and Actuators B: Chemical 160: 650-655.
- Devkota J, Wang C, Ruiz A, Mohapatra S, Mukherjee P, et al. (2013) Detection of low-concentration superparamagnetic nanoparticles using an integrated radio frequency magnetic biosensor. Journal of Applied Physics 113: 104701-104705.
- Marquina C, De Teresa JM, Serrate D, Marzo J, Cardoso F A, et al. (2012) GMR sensors and magnetic nanoparticles for immuno-chromatographic assays. Journal of Magnetism and Magnetic Materials 324: 3495-3498.
- Serrate D, De Teresa JM, Marquina C, Marzo J, Saurel D, et al. (2012) Quantitative biomolecular sensing station based on magnetoresistive patterned arrays. Biosens Bioelectron 35: 206-212.
- Shu-Jen Han, Shan Wang (2010) Magnetic Nanotechnology for Biodetection. Journal of Laboratory Automation 15: 93-98.
- Hira SM, Aledealat K, Chen KS, Field M, Sullivan GJ, et al. (2012) Detection of target ssDNA using a microfabricated Hall magnetometer with correlated optical readout. J Biomed Biotechnol 2012: 492730.
- Bao G, Mitragotri S, Tong S (2013) Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng 15: 253-282.
- Jain RK (2001) Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev 46: 149-168.
- McBain SC, Yiu HH, Dobson J (2008) Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine 3: 169-180.
- Singh D, McMillan JM, Kabanov AV, Sokolsky-Papkov M, Gendelman HE (2014) Bench-to-bedside translation of magnetic nanoparticles. Nanomedicine (Lond) 9: 501-516.
- Oh WK, Yoon H, Jang J (2010) Size control of magnetic carbon nanoparticles for drug delivery. Biomaterials 31: 1342-1348.
- Fang C, Zhang M (2009) Multifunctional Magnetic Nanoparticles for Medical Imaging Applications. J Mater Chem 19: 6258-6266.
- Liu Y, Hughes TC, Muir BW, Waddington LJ, Gengenbach TR, et al. (2014) Water-dispersible magnetic carbon nanotubes as T2-weighted MRI contrast agents. Biomaterials 35: 378-386.
- Pernia Leal M, Rivera-Fernández S, Franco JM, Pozo D, de la Fuente JM, et al. (2015) Long-circulating PEGylated manganese ferrite nanoparticles for MRI-based molecular imaging. Nanoscale 7: 2050-2059.
- Park JH, von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, et al. (2008) Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv Mater 20: 1630-1635.
- Hu F, Zhao YS (2012) Inorganic nanoparticle-based T1 and T1/T2 magnetic resonance contrast probes. Nanoscale 4: 6235-6243.
- Y Wang, YW Ng, Y Chen, B Shuter, J Yi, et al. (2008) Formulation of superparamagnetic iron oxides by nanoparticles of biodegradable polymers for magnetic resonance Imaging. Advanced Functional Materials 18: 308-318.
- Donglu Shi, Hoon Sung Cho, Yan Chen, Hong Xu, Hongchen Gu, et al. (2009) Fluorescent Polystyrene-Fe3O4 Composite Nanospheres for In Vivo Imaging and Hyperthermia. Advanced Materials. 21: 2170-2173.
- Liang Wang, KG Neoh, ET Kang, Borys Shuter, Shih-Chang Wang (2009) Superparamagnetic Hyperbranched Polyglycerol-Grafted Fe3O4 Nanoparticles as a Novel Magnetic Resonance Imaging Contrast Agent: An In Vitro Assessment. Advanced Functional Materials 19: 2615-2622.
- van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, et al. (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ 9: 1031-1042.
- Goldstein LS, Dewhirst MW, Repacholi M, Kheifets L (2003) Summary, conclusions and recommendations: adverse temperature levels in the human body. Int J Hyperthermia 19: 373-384.
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldöfner N. et al. (2005) Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperthermia 21: 637-647.
- Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, et al. (2012) water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 6: 3080-3091.
- Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 54: 631-651.
- Ito A, Shinkai M, Honda H, Kobayashi T (2001) Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther 8: 649-654.
- Ito A, Ino K, Kobayashi T, Honda H (2005) The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials 26: 6185-6193.
- D Bahadur, Jyotsnendu Giri (2003) Biomaterials and magnetism. Sadhana 28: 639-656.
- Kumar CS, Mohammad F (2011) Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev 63: 789-808.
- Zhao Q, Wang L, Cheng R, Mao L, Arnold RD, et al. (2012) Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2: 113-121.
- Hou CH, Hou SM, Hsueh YS, Lin J, Wu HC, et al. (2009) The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 30: 3956-3960.
- Y Krupskayaa, C Mahna, A Parameswarana, A Taylorb, K Krämer, et al. (2009) Magnetic study of iron-containing carbon nanotubes: Feasibility for magnetic hyperthermia. Journal of Magnetism and Magnetic Materials 321: 4067-4071.
- Choi YW, Lee H, Song Y, Sohn D (2015) Colloidal stability of iron oxide nanoparticles with multivalent polymer surfactants. J Colloid Interface Sci 443: 8-12.
Citation: Wu Y, Yang X, Yi X, Liu Y, Chen Y, et al. (2014) Magnetic Nanoparticle for Biomedicine Applications. J Nanotechnol Nanomed Nanobiotechnol 2: 003
Copyright: © 2015 Gang Liu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.