
Metabolism, Microbiota-Host Interactions, Aging and Stress Response: Diagnostic and Therapeutic Applications of the Physiological Fitness Landscape
*Corresponding Author(s):
Deepak ChopraThe Chopra Foundation, Orlando, United States
Tel:+1 9174149188,
Email:frank@deepakchopra.com
Abstract
The principles of the Physiological Fitness Landscape are presented here both in general terms and with specific applications.When they are applied to physiology and medicine,their main manifestations can be fundamentally reduced to an individual’s level of homeostasis as maintained over time. The foundation of most chronic disease stems from loss of metabolic coordination to resource availability and circadian physiology that parallels and is largely precipitated by mitochondrial dysfunction. This is the central thesis of what we propose defines the metabolic inflexibility of pathogenic non-circadian insulin resistance, which in turn leads not only to diabetes, obesity, hypertension and dyslipidemia, but also to the chronic disease states of aging, including CVD, cancer and Alzheimer’s disease. Using methods first developed in the physical sciences and adapting them to medicine and physiology, as is proposed here regarding the Physiological Fitness Landscape, can be a powerful tool in the management of disease and in the maintenance of long health span.
Keywords
Aging and Stress Response; Diagnostic and Therapeutic Applications; Metabolism; Microbiota-Host Interactions; Physiological Fitness Landscape
Metabolism in Health and Disease
The word metabolism (Greek: “change”) refers to the balance of energy as the sum of all chemical reactions that occur in living systems. Energy is extracted from nutrients obtained from the external environment and is utilized within the organism for the biosynthesis of molecules and other functions. The biochemical reactions of metabolism are organized into functional pathways whereby the product of one reaction becomes the substrate for the next. Every product or substrate is a metabolite. Metabolism is carried out by thousands of enzyme-catalyzed reactions occurring simultaneously in cells and the inter-conversion of metabolic fuels in either anabolic or catabolic pathways. Metabolism is a continuous flux of molecules in these pathways as the organism seeks the balance of energy and remains remarkably constant. Maintaining this stability or steady state of metabolic fluxes is called homeostasis. Stability and survival are achieved through plasticity or flexibility by networks of biochemical pathways of the living systems. All of the metabolic pathways are organized to achieve two main goals. The first is to extract energy from food into ATP and other molecules with reducing power, especially NADPH and carriers of energy-rich electrons. The second goal of metabolism is to provide precursor molecules that can be converted to larger ones, namely proteins, nucleic acids, polysaccharides and lipid molecules.
Metabolic processes can be viewed as fueling the biological engine of a living system. This metaphorical biological engine includes the mitochondria (combustion chamber), the ATP (and its hydrolysis), molecular motors and all the moving component parts of formed at micro and macroscopic scales. For example, the work done using the potential energy of ATP by muscle contraction, mediated by molecular motors. In fact, the totality of the human body may be considered a biological engine. Biological motors are central force generation elements to the machinery of living systems just as mechanical motors are to a car engine [1]. In both cases the motor is responsible for converting one form of energy into a force that generates motion involving translational or rotational kinetic energy. A biological system as an organism may be considered not only a biological engine but a complex structure comprised of several types of biological engines as well as motors intricately coordinated into a single larger system. This intricate coordination is achieved by metabolic networks running across the living system such as the human body.
Metabolic networks are best understood as the conduits for the flow of energy through the living system. Cellular networks send signals in terms of biochemical reactions such as phosphorylation of proteins in a signaling pathway. Energy requiring activities, for example the “fight of flight” response, or the chronic inflammatory process of disease, reduce the amount of available energy within these networks for other life-sustaining functions. Accordingly, a compromised number of pathways due to the diversion of energy from the network leads to the loss of redundancy (or reduced resilience) and often ultimately results in pathology and senescence. As the pathological process progresses, vastly reduced levels of metabolic redundancy eventually become a single metabolic tendril, which represents life support for the most important biological function, survival. Hence, disturbed metabolism is implicit as the cause, a consequence, or both of any disease state. The metabolic network has a greater capacity for metabolic flexibility and fitness accommodating the allostatic hallmark of health [2,3].
Metabolic pathways may be categorized as either catabolic or anabolic. The former degrade large molecules into simpler ones by cleaving the chemical bonds. There, simple cellular building block organic molecules with high-energy covalent bonds are oxidatively catabolized in pathways including glycolysis, the TCA cycle and Pentose Phosphate Pathways for energy extraction, conserved in the form of ATP and the reduced forms of FADH2, NADPH and NADH. Conversely, anabolic pathways coalesce smaller molecules into larger ones with the associated creation of chemical bonds using the energy captured by catabolic pathways [4].
The metabolic cycle is the most fundamental and shortest of all living system cycles. It underlies and regulates all the other cycles including the transcription of biological clocks in cells and is responsible for synchronizing the behavior of the organism as a whole. Time and metabolism are closely linked since in metabolism, time defines the rate of conversion of substrates into products. Metabolic rate and metabolic efficiency are terms referring to the amount of energy production per unit substrate from diet or energy stores per unit time. The metabolic rate typically parallels VO2maxor sub max, that is, the maximum or sub-maximum volume of oxygen consumption per kilogram of body weight per minute. This oxidative metabolism mode of energy production is carried out through the process known as beta phosphorylation. The alternative way of producing energy is the cytosolic process of glycolysis, also called substrate level phosphorylation, whereby a phosphate group is transferred from substrates to an ADP molecule forming an ATP molecule, which is a quantum of biological energy. The amount of energy produced per unit substrate in oxidative phosphorylation is 16-to-19-fold greater (32 to 38 molecules of ATP produced from one glucose molecule) than in glycolysis (only 2 net molecules of ATP produced) [5]. Hence, mitochondrial oxidative phosphorylation is so much more efficient than glycolysis bioenergetically, its contribution to metabolic rate, generally approaching 95%, and hence it correlates to the volume of oxygen consumption. In athletes this is measured commonly as VO2max. Metabolic rate however does not always correlate with VO2max rates, which are quite divergent in disease states. Clinicians equate high metabolic rate with high oxidative stress (e.g. hyperthyroidism). Typically, parameters such as VO2maxor VO2submaxliters of O2consumed per kg body weight per minute are used as a measure of fitness. They can be introduced as the fundamental order and control parameters to quantify susceptibility of disease states within the physiological fitness landscape model. The reciprocally related insulin resistance and mitochondrial dysfunction can be connected with impaired VO2max, which is premised on the loss of metabolic efficiency as fundamental to senescence and chronic disease and since metabolic efficiency is a measure of physical fitness.
Classical And Quantum Metabolism
Metabolism can manifest itself in two main forms: classical and quantum, but is typically a hybrid of both forms. The highest and most efficient level of metabolic functioning occurs in the quantum mode of energy production. In the case of a 100% quantum mode, the biological system would be in a virtually timeless state due to no entropy production. Hence, with no heat lost to the surroundings and the maximum amount of energy from nutrient substrate transformed into the useful work of maintaining homeostasis the system would be unchanged over time as the process is perfectly cyclical. In this hypothetical ideal case aging would not occur because aging itself is a function of energy lost as heat, which is unable to do useful work. On the other end of the metabolic spectrum is the classical mode of energy production whereby the transformation of energy from food nutrient substrate is highly inefficient due to lack of synchronization between parts of the whole. Accordingly, the entropy production rate, proportional to the heat lost and unavailable to do useful work, is maximal. In this case aging is maximally accelerated, coinciding with the loss of the synchronization in metabolic pathways.
Metabolic rate of living species can be expressed by an allometric scaling law that highlights the mathematical relationship between Metabolic Rate (MR) to body weight, W. Each species has its own proportionality constant, α, but most species have the same scaling exponent β, typically 3/4, such that MR =∼αWβ. The 3/4 scaling exponent is characteristic of the quantum mode of metabolism while the classical mode is linked to isometry where MR and W are linearly proportional to each other, with a scaling exponent 1 [6]. Both the aging processes and metabolic diseases result in a tendency toward isometry as a result of the loss of synchronization of metabolic processes across scales, poor transport of oxygen and nutrient as a result of blockages and inefficiencies. Consequently, the scaling exponent may shift gradually from the value of 3/4 to 1 indicating metabolic disease state development and can be used diagnostically. It is also expected that normal aging processes cause a gradual shift toward isometry. In both cases, efficiency of metabolic energy production is compromised and it results in a gradual loss of quantum coherence with concomitant entropy and heat production. Hence, longevity is thought to correlate with slow MR. This is underpinned by the proper timing, quality and quantity of activity/exercise, diet and stress coupled to genetic factors.
Optimum physiologic health may be described as having a low metabolic rate in conventional classical terms, or a high metabolic efficiency in the quantum sense. A relative oversupply of nutrition leads to a prolongation of the minimum cycle and hence precludes the quantum mechanical scaling from taking place. Instead, it is characterized in classical terms as being linear rather than quantum mechanical, given by a 3/4 exponent, which causes the reduction in metabolic rate efficiency and metabolic health. In other words, for a given mass unit of the body, more energy is required to maintain its metabolic activity. This represents a reduced efficiency in the hierarchical organization of the biological system. The takeover threshold is a point at which oxidative phosphorylation mediated by the electron transport chain breaks down and the production of ATP occurs predominantly in the classical regime and the metabolic efficiency is reduced. This threshold pertains to the energetic overload of mitochondria from excess calorie intake. The high rate of nutrient supply corresponds to classical regime of metabolism in contrast to a low rate of nutrient supply that corresponds to the quantum regime. A shift from quantum to classical metabolism turns oxidation of foods away from the mitochondria to the cytoplasm, a process referred to as aerobic glycolysis [7].
The slowest possible rate of aging is associated with a minimum amount of redox disturbance, inflammation and entropy per unit time, i.e. Entropy Production Rate (EPR). This is tantamount to the pace of biological aging most closely approximating the chronological age. Conversely, heat is lost from the biochemical bonds as part of an inflammatory process with an accompanying increased EPR. The latter effect shifts the metabolic mode of energy production from quantum to classical with an associated inefficiencies provoked by chronic external circumstances of poor nutrient quality and/or timing, disruption of other circadian behaviors, and ensuing prolonged exaggerated stress. It follows that the quantum mode of energy production results in a reduction in the total body ATP production to less than the 1021molecules of ATP per second required for optimal physiology. Organizational complexity is lost in a process that, if prolonged, may represent metabolic disease and a susceptibility to premature chronic diseases of aging.
The concept of quantum metabolism introduced by L. Demetrius [6] and later applied to cancer by L. Demetrius and J. Tuszynski [7], integrates the Einstein-Debye models of the thermal properties of solids with the Mitchell theory of chemiosmosis [8]. Chemiosmosis describes the process of ion movement from regions of higher concentration to lower concentration across semi-permeable membranes. Mitochondria are remarkable quantum mechanical transducers. The human body requires conversion of ADP into ATP, which is equivalent to its weight in ATP every day in order to function. This translates into 1021 molecules of ATP per second. Mitochondria occupy up to 25% of the volume of cells and each cell has several thousand mitochondria, which are literally its power plants. It is within these organelles that the majority of caloric fuel is oxidized, using the energy to produce ATP. Since more than 95% of the oxygen consumed by humans is used for this purpose, breathing ultimately occurs at the cell level in the form of cellular respiration. Importantly, the electron transport chains of mitochondria are essentially conveyor belts for transferring electrons from the extracted energy originally contained in food to the high-energy phosphate bonds in ATP. The transfer of electrons through each of the electron transport chain complexes provides the energy required to pump hydrogen protons to the outside of the inner mitochondrial membrane. Therefore, the bio-energetics of living organisms is driven by the useful energy in food stored in pairs of electrons that make carbon-carbon and carbon-hydrogen chemical bonds. Electron transport occurs along the components of the electron transport chain embedded within the inner mitochondrial membrane. Movement of the protons at the end of the electron transport chain back across the membrane depolarizes the electrochemical gradient and captures the energy with the synthesis of ATP. Concomitantly, pairs of hydrogen protons and electrons reduce molecular oxygen to water, which taken together describes the process of cellular respiration. Although heat production is a measure of metabolic inefficiency, it nonetheless is necessary to maintain physiologic body temperature.
Cellular metabolism involves the movement or molecular oscillations of enzymes embedded within the energy transducing organelles due to changes in redox potential of their reciprocal substrates. Metabolic enzymes engage in periodic activity, which is correlated across mitochondria in a cell, many cells in a tissue and all tissues and organs across an organism. The oscillation frequency of these processes parallels the metabolic rate. The metabolic rate and associated enzymatic oscillation frequency is considerably more rapid and more efficient for mitochondrial oxidative phosphorylation versus cytosolic glycolysis. Metabolic efficiency in the quantum mode of energy production per quanta of ATP per unit mass is greater than in the classical mode. In the former case, we can say that time is maximally dilated and aging is relatively slow in contrast to the case of declining physiological health. In the latter case metabolic cycles of energy production are in the classical regime, which means the metabolic time runs faster but with a slower frequency cycle time per quanta of ATP per unit mass per unit time, and hence with a lower metabolic efficiency. The maximum efficiency in the quantum regime applies across the scales of biological organization due to intra- and inter-cellular synchronization of the energy production processes. There is a continuum of outcomes whereby the greater the percentage of mitochondrial electron transport chains operating in the quantum mode, the less heated the metaphorical biological engine becomes with the least wear and tear and the greatest efficiency. Conversely, inherent in the classical mode of energy production is a degree of inefficiency in this process manifesting itself in the release of heat, and the inseparable biological processes of inflammation and redox modifications of molecular cell structures. Although the classical regime of mitochondrial oxidative phosphorylation has intrinsic inefficiency, it should not be construed as necessarily physiologically harmful. Indeed there is an optimal adaptive level that is consistent with the notion of hormesis whereby low levels of oxidative stress and structural injury induces antioxidant effects and cell repair. A hybrid state of quantum and classical metabolism at some optimal ratio that is biologically feasible underpins maximal fitness function. Such an optimal ratio may translate into prolonged human health and life spans. The ratio of the quantum to classical metabolic regimes of energy production parallels the rate of biological time and aging relative to physical cycles of time. Time runs slowest in the context of maximum physiological health in the sense that metabolic rate is lower when in the quantum mode of energy production. The greater the relative contribution to energy production in the quantum regime, the slower the aging process. Conversely, the greater the relative contribution of the classical mode, the more rapid the process of aging.
Metabolic Dysfunction, Aging and Insulin Resistance
In the case of dietary overconsumption that overloads the electron transferring potential of mitochondria, electron slippage and formation of superoxide not only oxidatively modifies molecular components of mitochondria, but additionally, it uncouples the mitochondrial membrane gradient potential from the production of ATP. Hence, it releases heat, and the loss of structure is tightly linked to the loss of function. This becomes a self-escalating process of redox stress that promotes a progressive deterioration of mitochondrial structure and function accompanying the development of insulin resistance. The final stage of bioenergetic decline may be understood as the compensation of classical oxidative phosphorylation mode of ATP production by glycolytic ATP production. Mitochondrial dysfunction is the root cause of the transition from a healthy to an unhealthy metabolic state and a trajectory to premature metabolic and chronic diseases of aging. The interwoven relationship of insulin resistance and mitochondrial dysfunction is at the genesis of abnormal or disease states including obesity, sarcopenia, hypertension, dyslipidemia, glucose intolerance, type 2 diabetes, cardiovascular disease and dementias. In particular, advancing deterioration of mitochondrial function is accompanied by increasing inflammation, EPR, and redox stress that promote mutagenesis and increasing vulnerability to carcinogenesis.
Nutrient excess provokes the fundamental pathological web of insulin resistance and mitochondrial dysfunction, whereas calorie restriction enhances mitochondrial function and insulin sensitivity. Molecular sensors of low energy status, especially AMP Activated Protein Kinase (AMPK) and Sirtuin1(SIRT1), for example generated by the external control parameters fasting, calorie restriction or exercise, in turn signal through peroxisome proliferator gamma co-activator 1alpha (PGC1alpha), which in concert with various transcriptional activators promote mitochondrial biogenesis. The setting of low available energy provides selective pressure to maximize the efficiency of energy production through mitochondrial oxidative phosphorylation. However, in the setting of excess energy from diet fed into mitochondria, its function is compromised by the pathological accumulation of reactive oxygen species causing redox modifications of mitochondrial and other cell structures. Redox stress impairs mitochondrial function, which further worsens the redox stress in a positive feedback loop. With each cycle the entropy production rate accelerates in parallel with a decline in free energy available to do the work of physiology. In the natural course of chronic pathophysiology this acceleration is not linear but rather interrupted by states of stability. In contrast to low energy states that promote mitochondrial biogenesis, high-energy states degrade mitochondrial function. There are other external control parameters of mitochondria biogenesis beyond fasting, calorie restriction, and endurance exercise, such as the Peroxisome Proliferator Activated Receptor (PPAR). Declining mitochondrial efficiency can be seen in a strict classical sense in the context of hyperglycemia promoting microvascular disease and insulin resistance induced macrovascular disease. The interdependent relationship between obesity, inflammation and insulin resistance is also linked to the calorie restriction mimetic, NAD+ dependent deacetylase SIRT1. SIRT1 activity is inhibited by oxidative stress, a state that is promoted by inflammation, whether generated by obesity or otherwise. SIRT1 appears to exert powerful effects on the health of all living systems including humans, and its activity is upregulated by low-energy states, such as resulting from endurance exercise and calorie restriction. Furthermore, the positive regulation of mitochondrial structure and function reciprocally enhances insulin sensitivity generating a feed-forward loop. Consistent with the connection of mitochondrial dysfunction and insulin resistance to cancer, SIRT1 provides a mechanistic link for plausibly effective therapeutic strategies to include calorie restriction, intermittent fasting and endurance exercise and high-dose resveratrol.
Metabolic Origins of Cancer
Otto Warburg hypothesized in the 1920’s that cancer arises as a result of impaired aerobic oxidative metabolism [9]. While he did not use the term mitochondrial dysfunction, essentially he proposed that as a result of the inability of native host cells to efficiently utilize available oxygen for bio-energetic demands, subclinical cancer cells, via primarily compensatory glycolytic metabolism, outcompete normal cells for available resources. Thus, in an environment of abundant glucose availability the Warburg effect serves the bio-energetic requirements of rapidly proliferating tumor cells. The Warburg hypothesis has opened the curtain to a metabolic perspective to dietary and pharmacologic approaches to cancer prevention and treatment. For example, ketogenic diets originally were hypothesized to be useful for brain cancers noting that normal but not malignant brain cells readily use ketone bodies bio-energetically. Competition between cancer and non-cancer cells is such that the metabolic circuitry of the cell best matched to the environmental conditions that prevail. While the enzymatic network turnover frequency relying predominantly on cytoplasmic glycolytic pathway for energy production is rapid, cancer cells do not cooperate with one another making energy production inefficient across the tumor that leads to isometric scaling. The energy-inefficient nature of this pathway typically precludes the likelihood of nutrient oversupply of the rapid cell replicating process of cancer. In fact, the opposite is often the case whereby the voracious nature of cancer cells’ metabolism being very inefficient leads to an overall energy undersupply resulting in cachexia. The theory of quantum metabolism provides a molecular basis relating metabolic rate with cell size and body size. It also explains differences in the metabolic rates of normal cells and cancer cells.
Alzheimer’s Disease and Insulin Resistance
Alzheimer’s disease provides another example of a chronic disease state characterized by insulin resistance, mitochondrial dysfunction and an over-reliance on glycolytic metabolism. Metabolic compromise with reduced glucose metabolism is present often for many years prior to the onset of clinical dementia. Alzheimer’s disease invokes an understanding of the Reverse and Inverse Warburg effects [10] as well as insulin resistance in the pathogenesis of the disease. Alzheimer’s disease evolves due to neuronal cells’ inability to upregulate glycolysis. The healthy brain prefers glucose as a metabolic fuel over fatty acid oxidation. While in the healthy state brain neurons have the capacity to rapidly produce ATP via glycolysis to adapt to cognitive demands, this glycolytic upregulation is limited. Overall, aerobic glycolysis accounts for about 10-15% of glucose metabolism in the normal brain. In the case of crisis, brain neurons rely on upregulated oxidative metabolism whereby they are provided additional fuel as lactate from surrounding astrocytes. Neurons of the brain have a lower capacity to handle oxidant stress via antioxidant systems, which becomes a dilemma when faced with compensatory mitochondrial oxidative bioenergetic demands. The brain exhibits metabolic flexibility in the sense that it utilizes different metabolic fuels to adapt over the various stages of Alzheimer’s disease. Initially, increased aerobic glycolysis and the reverse Warburg effect are capable of generating a hypermetabolic response, compensating for subclinical disease rooted in mitochondrial dysfunction. However, progressive loss of mitochondrial function accompanies worsening oxidative stress, amyloidogenesis and insulin resistance. Bioenergetic capacity of diminishing mitochondrial functional reserve eventually is unable to compensate, and deteriorates into a hypometabolic state. The bidirectional mechanistic cause-effect relationships exist between insulin resistance and mitochondrial dysfunction that promote and self-amplify one another
Inflammation, Oxidative Stress and Metabolic Diseases
The flames of all metabolic diseases are propelled by the inter-convertible processes of inflammation and oxidative stress. Every oxidation reaction is coupled to a reduction reaction, their combination referred to as a redox reaction. Unregulated transfer of electrons is known as disturbed redox that creates free radicals and loss of an uneven number of electrons and a feed-forward propagation that permeates neighboring tissue molecules refers to oxidative stress. Metabolic disease is best understood by looking at the physiological sources of energy in its different forms in the body, the efficiency of how these forms are converted, especially electrochemical conversion in the mitochondria, and the utilization of this energy to create biological structure and function across the networks of systems. Insufficient or excessive calorie sources of energy, poisoning or interference of electrochemical conversion within mitochondria are sources of oxidative stress, disturbed redox and the inextricable processes of inflammation.
With the natural process of aging the maximal entropy reduction and metabolic stability becomes gradually degraded by the inevitable accumulation of reactive oxygen species and inflammation resulting from less than 100% efficient metabolic processes. These destructive processes are inextricably linked. Reactive oxygen species and reactive nitrogen species bind to DNA, protein and lipid components that contain biological information disrupting its structural and functional integrity, and in the case of DNA, potentially causing mutations. Oxidative stress elicits an inflammation response by up-regulating the expression of NF-KB, a central hub transcription factor whose role is to amplify open processes of inflammation and reactive oxygen species. Hence, the “regulation” is actually a feed-forward dysregulation. Mitochondrial structure and function decline as a hallmark of the reduction of metabolic stability and loss of quantum metabolism as well as other likely integrated and synchronized quantum biological processes. The orchestration of circadian rhythm by molecular clocks [11] and apparent quantum biological processes are responsible for this synchronized organization. It follows that disruption of the central and essential organelle energy transformer lies at the core of both accelerated senescence and its associated chronic disease states. Further, the central axis of the biological equivalent to the second law of thermodynamics is the desynchronization and decoupling of this temporal organizing fabric. The uncoupling of bioenergetic pathways of oxidative phosphorylation to glycolysis is fundamental. This uncoupling is the corollary to metabolic inflexibility that is the hallmark of insulin resistance [12], the precursor to type 2 diabetes.
Finally, it is important to stress that an overlooked and underappreciated reality is that the required energy necessary to mount the immune system is extraordinary. Therefore, maintenance of energy balance requires the body to shut down energy requiring biosynthetic processes in the settings of immune system activation and inflammation. As a growth-promoting hormone, the actions of insulin are adaptively disrupted by the energy supplanting requirements of the immune system.
Monitoring the metabolic rates of individuals as functions of weight over time could be a diagnostic tool for the onset and progression of diseases and conversely, a return toward a quantum scaling exponent of 3/4 could be prognostic of healing processes taking hold.
Physiological Fitness Landscape
The concepts of the Physiological Fitness Landscape [3,4] can be applied to clinical practices concerning metabolism, microbiota-host interactions as well as microbial composition, which can directly affect states of health and disease in the host. Numerous factors influence both positive and negative trajectories across this landscape including psychological, social, lifestyle and nutritional components. Extrinsic control parameters such as circadian rhythms, stress, and importantly the timing, quantity and quality of diet are other primary drivers of shifts within the fitness landscape. Collectively, these factors combine to determine the overall state of health and disease; their exquisite regulation maintains homeostasis, while poor lifestyle choices or disease facilitate allostatic overload.
The everchanging topography of the Physiological Fitness Landscape can be depicted as a series of valleys and peaks, which represent zones of stability and instability of the state of health as measured by various performance indicators.
When an organism is at its most optimally healthy condition, the system is in a state of equilibrium, maintained by allostasis. If unfavorable changes in external control parameters occur, such as poor dietary choices or excess dietary consumption, the system is shifted out of the metaphorically homeostatic valley and up toward an adjacent peak. These peaks represent zones of instability as they are more metabolically demanding and require greater free energy consumption, which may cause metabolic inefficiency such as a shift from oxidative phosphorylation to glycolysis. If a correction in adverse external parameters is made before the system transitions to the neighboring valley, we may fall back into our original valley and regain a state of metabolic efficiency and allostasis. However, if negative changes in these parameters persist, the system will reach a state of allostatic overload, propelling us down the slope into a neighboring valley, representing pathology. Disease progression is shown by the continual push to the top of a peak and subsequent fall down into a less stable valley at a lower amplitude within the physiological fitness terrain, until ultimately the system falls into a free energy of zero at ground level, which is thermodynamic equilibrium representing death as we explain below. Before we discuss it, it’s worth mentioning again hormesis, which involves a favorable change in the fitness function following exposure to small or moderate amount of stress.
Applications of the Physiological Fitness Landscape to Health and Disease
The application of the Physiological Fitness Landscape to physiology and medicine is essential for the development of precision personalized medical care and treatment. It is aninterpretive roadmap we can utilize to guide our understanding of patient symptoms of disease and how to treat disease progression over time most effectively and efficiently. Thus, we can employ this approach oftargeting control parameters to steer the trajectory of order parameters away from disease advancement and toward a more stable state, in efforts to most adequately maintain health and homeostatic control.
An important aspect affecting the Physiological Fitness Landscape is the tight coupling between an external control parameter related the diurnal cycles and the internal order parameters of the healthy human physiology. The Earth’s rotation around its own axis creates the light/dark cycle in its relationship with the Sun. Inhabiting this planet from the dawn of time, human beings as biological systems have evolved the astonishing molecular internalization of the Earth’s rotation around its axis in the form of circadian cell clocks and transcriptional-translational feedback loop systems. Consequently, sleep/wake, feed/fast and rest/active extrinsic behavioral cycles correspond to many intrinsic biological systems coordinated as cycles integrated and synchronized across many hierarchical scales of temporal cycles within cycles. Examples include circadian cycles of autonomic, hormonal (cortisol, GH, TH, androgens and estrogens) activity, and immune system activation. These systems regulate the cycles of insulin secretion and sensitivity, mitochondrial bioenergetic function and biogenesis, which in turn regulate the healthy circadian cycles of redox and inflammation. Each of these systems of cycles is involved in interlocking relationships whereby they feedback and regulate the systems that regulate them.
Comprising the gestalt of these many systems in the total manifold of a human biological system are a virtually infinite number of physiological parameters. Each system is comprised of a handful of key parameters, which we understand to varying degrees. However, these systems are comprised of many more parameters that we do not understand in terms of their interactions with other components of the system. Still, there are undoubtedly many interacting parts in each or most of these systems yet to be discovered.
The exquisite and organizational perfection exemplified by the state of optimal health is a manifestation of the temporal and spatial synchronization of these systems of cycles. In this state, the most fundamental parameters of homeostasis, redox, acid base and the flow of free energy are in lockstep within very narrow physiological ranges. Fitness is optimal, free energy flow as the support network is maximal and evenly distributed across the organism to provide stress reduction, resilience and stability in each of these systems of parameters. This is due to tight connectivity and synchronization of the subsystems in terms of their metabolism.
There are two overarching forces that shape the topological terrain of the Physiological Fitness Landscape as a model to depict a biological system. They are both, in a strong sense, rooted in the 1st and 2nd laws of thermodynamics [13]. Biological thermodynamics involves the ineluctable increase in redox and loss of efficiency in free energy flows within the system of systems resulting in the generation of unusable heat and entropy. Accordingly, there are insidious phase transitions over the course of a lifetime and the aging process, a deteriorating progression from health (synchronized systems of biological cycles) to disease (desynchronization of the systems of cycles), to the ultimate transition from the biological cycles of time to the physical arrow of time. Ultimately, the energy that created us is returned to the Universe, hence obeying the 2nd law of thermodynamics and contributing to the rising entropy of the Universe.
The other major force that shapes the Physiological Fitness Landscape is rooted in the perfect organizational connectedness of the system of systems that under perfect health conditions allows for an optimized stress response of the whole system with respect to a plethora of physical, chemical, psychogenic and other stresses that are a part and parcel of being alive. It is the stress distribution mechanism over the organism but also, very importantly, over a social support network that enables this efficiency in dealing with stress. This is the energy flow dynamics across biological chemistry that structurally transforms the system, promoting functional adaptation to stress. This unique adaptability of biological systems to adversity is at the core of their anti-fragility.This adaptation forms a stability zone on the Physiological Fitness Landscape, the metaphorical valley; the steeper the slope of the valley the greater the energetic support for robustness of the system with respect to the confronting stress.
Over the course of the biological aging process as a function of physical time, the trajectory of the composite parameters of the human system of systems and cycles of cycles that form the topological Landscape, must show a progressive overall decline in amplitude, or fitness as the system degrades due to the gradual (or not) generation of inefficiencies associated with the generation of entropy and hence heat.
Stress Response
The stress response itself, underpinned by a requirement for energy expenditure [14], contributes to the heat loss and entropy (inflammatory and redox stress), which is at the core of the aging process. Each acute stress response causes an insignificant acceleration of the aging process but this is an accumulating process so we can unfortunately never get younger.
Teleologically the stress response provides energetic support needed to meet the challenges of the stress. In other words, it distributes the support network of available energy to where it is most needed to handle the stress. For example, it pulls it from energy stores in adipose tissue and the liver for gluconeogenesis and glycogenolysis, to subsequently furnish glucose substrate that can rapidly be transformed to ATP via the glycolytic pathway. However, while this adaptive behavior, the metaphorical incline that maintains a metabolic stability zone, resists stress from pushing a system of parameters onto an instability zone of a hilltop, nonetheless the inherent inefficiency of the energy it produces contributes to the aging process.
However, if the stress response is transient and effectively provides the energy support to the relevant systems to meet the physiological challenges of the stress, a pathological acceleration of the aging process can be prevented. Alternatively, often the stress overpowers the resilience of the system, pushing it through the energy barrier onto an instability zone of the next hilltop and subsequently to a new trough represented by re-equilibration of parameter interactions. In this event, there is a punctuated acceleration of biological aging with a corresponding decline in overall fitness.
The totality of free energy available to the aggregate of systems comprising the organism is continually lowered, which is represented by a decline in the altitude of the Physiological Fitness Landscape, and a reduction in the slope of the valley leading to the next hilltop. This metaphorically parallels reduced metabolic efficiency.
Metabolic inefficiency is a function of mitochondrial dysfunction and of the uncoupling of metabolic pathways of energy production, fundamentally caused by redox stress. It results in the loss of physiological adaptive functioning. Each new valley represents a minimization of metabolic inefficiency for a system of interacting parameters. Accordingly, a phase transition with a decline in the altitude of a new valley on the PFL, is hallmarked by a re-equilibration of interacting parameters guided by the optimal available free energy and lowest exposure to redox stress. The number and complexity of the changing landscape of interactions between parameters declines, largely as a result of a decrease in the intricacies of adaptive coordinated synchronization and the associated organizational perfection.
The interactions involving parameters characterizingthe organism’s subsystems change as these parameters themselves change over time or in the course of a disease. Consider the reciprocal regulation of insulin and glucose as a system that includes each of their signaling components within and between tissues. The interactions between the parameters of this system change, and consequently the parameters that define the system change in the context of prediabetes or insulin treatment of diabetes. The endogenous and exogenous hyperinsulinemia, respectively, attenuate the degree of hyperglycemia. However, relative responsiveness of the parameters of glucose and insulin on one another diminish with the degree of insulin resistance and hyperinsulinemia, while each of these parameters forms new interacting relationships with other elements, forming new systems.
With the progression of pathogenicity, redox stress and inflammatory incineration of adaptive structure and function, there is a search for stability points in the Physiological Fitness Landscape through changing interactions. Among the available options, the new state of the system is selected based on one in which the least redox stress is imposed, and thus the least metabolic inefficiency.
Although a given parameter within this system may have relatively robust inherent stability, for example glucose, there is always a cost associated with the physiological or iatrogenic euglycemia. Similarly, a narrowly defined system would be portrayed on a PFL as a valley with a steep slope or possibly even at a higher altitude than its previous position on the topological terrain. However, this places greater stress on the other parameters of a more broadly defined system, or on its interactions with other subsystems. For example, there is a metabolic cost of the high insulin levels as well as the positive feedback exacerbation of insulin resistance due to the hyperinsulinemia. Another metabolic cost is represented by impaired bioenergetics due to reduced glucose uptake in insulin responsive tissues. For example there appears to be a differential greater degree of insulin resistance in satiety and cognitive areas of the brain versus adipose tissue, accounting for phenotypic manifestations of obesity and accelerated cognitive decline or AD. There is also an apparent differentially greater severity of insulin resistance in muscle versus adipose tissue, resulting in the characteristic comorbidities of sarcopenia and obesity. Moreover, hyperinsulinemia has toxic inflammatory effects in the brain impairing cognition, and promoting tumor cell proliferation in a number of epithelial tissues.
Nevertheless, it is critically important to understand that the gestalt of the system of systems represented as a PFL topological trajectory of consecutive hilltops and valleys must be lower than the preceding one. The trajectory of individual systems that follow different courses of altitude and slope steepness, some with greater resilience to subsequent stressors than others, is itself a manifestation of temporal and spatial desynchronization.This is due to the selective degradation of the molecular fidelity of biological chemistry; oxidative modification of structures such as core clock components in a certain tissue or tissues results in circadian dissonance. Disruption of the synchronized metabolic cycle of ATP production due to mitochondrial dysfunction in both a quantum and classical sense is central to the aging and chronic inflammatory disease process. While we cannot reverse these processes, the main objective of the physician and patient alike should be to reduce the rate of decline of the overall fitness level over time.
Physiological Fitness Landscape and the Future of Medicine
The concept of Physiological Fitness Landscape as a potentially powerful predictive model, we hope will play a major role in the future of medicine, is also a relevant construct for making sense of seemingly counterintuitive observations. Below we provide a concrete example. When/why would successfully targeting a therapeutic goal for blood glucoses in a diabetic to the normal physiological range be detrimental? Normal blood sugar levels attained interventionally by intravenous insulin continuous infusion, in hospitalized critically ill patients have been associated with unexplained increased mortality. For this reason, this common clinical biomarker as a parameter of the Physiological Fitness Landscape is chosen to exemplify an explanation of this model. Metabolic memory, or hyperglycemic memory, is an intriguing phenomenon with many unanswered questions. A number of landmark studies [15-24] have demonstrated that early tight glucose control immediately, but not if delayed by several months, following the diagnosis of type 1 diabetes is critical for long-term prevention of target organ small vessel disease of the eyes and kidneys. Similarly, vigorous metabolic control of glucose, lipids and hypertension immediately following diagnosis of diabetes type 2, but not if delayed, prevented mortality and other endpoints of CVD. Furthermore, there is ongoing active research interest in metabolic memory in diabetic cancer patients. A theoretical basis and experimental evidence for this phenomenon point to AGE’s and other mediators of inflammation, and redox stress. Metabolic memory has also been called the “legacy effect” to highlight the enduring effect of metabolic disease, and it may be understood in the context of the proposed Physiological Fitness Landscape model. Furthermore, this model is fundamentally premised on the “fitness” of a biological system, which correlates positively with the free energy flow available to the system and inversely with the totality of inflammatory and redox stress in the system.
Within this methodology, each patient should be viewed as a complex, multi-dimensional dynamical system presenting with symptoms, which have some overlap with other patients suffering from the same condition but also with individual characteristics, hence there is a need for personalized precision medicine. In this context, every medical specialty or specialty physician interrogates a limited trajectory in a complex phase space of dynamical behaviors exhibited by the patient. This could be seen as the analysis of a single dimension (parameter), such as glucose regulation, in the context of many other dimensions or parameters in the overall physiology or disease of an individual. Restoring one parameter to a range of normal values does not necessarily signal a return to health.
Glycemia as a dimension in the Physiological Fitness Landscape represents the energy flow available to the system and its capacity to connect with other intersecting subsystems, for example lipids, insulin secretion, sensitivity and satiety, vascular endothelial, and epithelial cell, in the total fitness manifold of the individual. These are the subsystems that control the entire physiological system’s regulation and those bio-energetically regulated by it. When things go awry, the result is metabolic disease (e.g. hypertension, dyslipidemia, central obesity, glucose intolerance, diabetes, low grade or “meta” inflammation), with manifestations in, for example, lipids, insulin secretion, sensitivity and satiety, and those systems regulated by glucose (neural and neuroglial brain cells), vascular endothelial, and epithelial cells. This is followed by chronic disease manifestations of aging (often prematurely) including CVD, cancers, accelerated cognitive decline and Alzheimer’s disease.
Stress Response And Allostatic Overload
What mediates things going awry? The short answer is stress. Stress responses, from the psychogenic perception of stress to the molecular scale mechanisms that carry it out, are teleologically adaptive. However, it is the chronicity of the stress response, not necessarily the external stress per se, which are the pathogenic underpinnings of disease. Allostatic responses [25] chiefly involve primary (catecholamines, HPA axis hormones, cytokines) and secondary mediators ((a) insulin resistance markers of the metabolic and hemodynamic response (e.g. hyperglycemia, hyperinsulinemia, hyper/dyslipidemia, hypertension, subcutaneous adiposity/obesity/ diabetes); (b) other hormones, NHR’s and transcriptional regulators)). Together, these mediators are purposed to respond to extrinsic and intrinsic stressors and govern bio-energetic, redox and acid base demands of homeostatic stability. This orchestration of the stress response is the central organizational design that is responsible for human health and disease. Furthermore, this theme is captured by the Physiological Fitness Landscape which this author proposes as a mathematical and clinical model that offers the significant potential to improve the power and predictive capacity of medicine.
Allostatic overload defines states whereby allostatic response to stressors are unable to effectually maintain parameters of homeostasis within their narrow optimally healthy physiological ranges. Accordingly, following the removal of the extrinsic stress the homeostatic parameters are outside their optimal range and hence the allostatic mediators do not return to their normal baseline state. Consequently, the chronically activated primary allostatic parameters search for interactions with secondary metabolic and hemodynamic parameters of allostasis to find the next most stabile zone of “fitness”(i.e. free energy and other parameters of homeostasis).
This describes the induction and pathogenesis of noncyclical insulin resistance, in turn responsible for the hyperglycemic and hyper/dyslipidemic environment, impaired satiety and obese state, endothelial cell dysfunction, hypertension and atherogenesis. The hyperinsulinemia response to (or trigger of) insulin resistance is an important driver of Alzheimer’s disease as well as of cancer proliferation of many non-classical insulin responsive metabolic tissues (e.g. gastrointestinal, reproductive and urogenital systems). The cortisol surplus sympathetic dominant and pro inflammatory insulin resistant state appears to significantly promote hippocampal atrophy and accelerated cognitive decline of aging. The pro-inflammatory redox disturbed state of allostatic overload causes mutagenesis and cell transformation, i.e. cancer cell initiation. Insulin resistance and redox is a fundamental driver of mitochondrial dysfunction, which is essential and core to the progression of all the chronic disease mentioned above. Figure 1 shows schematically the effects of stress leading to allostatic overload.
 Figure 1: An Illustration of the Allostatic Overload Effect on the Physiological System.
Figure 1: An Illustration of the Allostatic Overload Effect on the Physiological System.
When conditions are good and systems are healthy, biological function is exquisite. However, when things go awry in these systems the result is waste at the bio-energetic transformation centers, i.e. mitochondria, and redox stress that degrades biological structure and function resulting in premature chronic diseases such as neurodegenerative pathologies, cancers and CVD, respectively, reducing the total fitness of the individual. These are all critical parameters contributory to pathways, systems and networks that become building blocks of larger scales of systems and networks that interact in complex, non-linear and unpredictable fashion, i.e., local redox in the endothelial cell leading to CVD, in the epithelial cells leading to a GI tract malignancy or brain neuron leading to AD.
These are all critical parameters contributory to pathways, systems and networks that become building blocks of larger scales of systems and networks that interact in complex, non-linear and unpredictable fashion. Taken together, the energy flow forms a composite trajectory across a metaphoric physiological fitness landscape, is the representation of health and disease in terms of the totality of useable energy in the living system. This total energy is the amplitude along the Y axis over time (X axis). Separate Physiological Fitness Landscapes may be constructed for the various component composite organ systems critical for sustaining life, such as the cardiovascular or pulmonary respiratory. If the altitude over time of the Physiological Fitness Landscape falls to zero, that equates to attaining the thermodynamic equilibrium of the system. If this occurs in a vital organ system it amounts to the mortality of the individual. Therefore, constructing such profiles of the Physiological Fitness Landscape for individual physiological systems, while limited in scope, is still valuable in gaining insights into the patient’s disease state. Moving the system out of equilibrium by both pharmacological and non-pharmacological means, for example by a cardiological stress test, provides an important quantitative assessment of this system in the form of its sensitivity (or conversely stability) to external perturbations. The system’s ability to restore its balance (equilibrium) is a measure of its homeostasis.
When a physiological system is moved out of homeostasis by an external perturbation, this equates to allostatic overload, whereby allostasis is unable to maintain homeostasis. The robustness of the system is manifested by its ability to return to equilibrium following exposure to perturbation.When the system lacks sufficient resilience relative to the stress it is exposed to, its measures of flexibility are markedly altered.This applied to the autonomic nervous system as well as hormonal and immune system responses capable of maintaining free energy and redox parameters within optimal narrow physiological ranges. When this occurs, in terms of the stress challenge it provides sufficient energy to overcome the energy barrier represented by the slope and height of the adjacent physiological fitness landscape “mountain”.Consequently, the bioenergetic demands of the system responding to the stress require the insulin resistance response to make glucose, fatty acids, ketones and other energy substrates available to peripheral tissues.Here, the classical insulin responsive metabolic tissues are the unselfish providers for the sake of survival of the larger system whole (the human individual).The initial sensory input triggers stress resilience programs at the most basic and foundational scales of energy fuel gauges, particularly AMPK, but interwoven SIRT-1. These gauges in turn govern the autonomic, hormonal and NHR, as well as immune system allostatic responses purposed to maintain stability of the parameters (free energy and redox) that are sine qua non for vital organ system function.
Ultimately, allostatic overload defines stability overload states that are not healthy homeostasis (free energy and redox states are stable following the cessation of the extrinsic stress but not in the physiologically optimal range). In this setting the allostatic parameters are chronically active, inducing pathogenic (noncyclical) insulin resistance, which is responsible for the hyperglycemic and hyper/dyslipidemic environment, impaired satiety and obese state, endothelial cell dysfunction, hypertension and atherogenesis. The hyperinsulinemia response to (or trigger of) insulin resistance is an important driver of Alzheimer’s disease as well as of cancer proliferation of many non-classical insulin responsive metabolic tissues (e.g. gastrointestinal, reproductive and urogenital systems). The cortisol surplus sympathetic dominant and pro inflammatory insulin resistant state appears to significantly promote hippocampal atrophy and accelerated cognitive decline of aging. Pro-inflammatory redox disturbed state of allostatic overload causes mutagenesis and cell transformation, i.e. cancer cell initiation. Insulin resistance and redox is a fundamental driver of mitochondrial dysfunction, which is essential and core to the progression of all the chronic disease mentioned above.
On a multidimensional Physiological Fitness Landscape, an extraordinary quantity and complexity of parameters defines this synchronized and orchestrated biological design.Local interactions of these parameters include: glucose production by the insulin resistance-triggered glycogenolyic and gluconeogenic enzymes; fatty acid release promoted by the lack of insulin inhibition of hormone sensitive lipase in lipid storing adipocytes; lack of insulin stimulation of adipose tissue lipoprotein lipase that keeps VLDL lipoprotein lipid containing substrates in the circulation available for uptake in peripheral tissues.
Other more central factors involved (relative to the initial responses of the sensory stimulus) are locally intersecting parameters that include the hormones, NHR’s and other transcriptional regulators that integrally coordinate the bioenergetic and redox resistance programs that maintain homeostasis. Figure 2 represents a simplified network of stress responses involved in the key physiological systems.
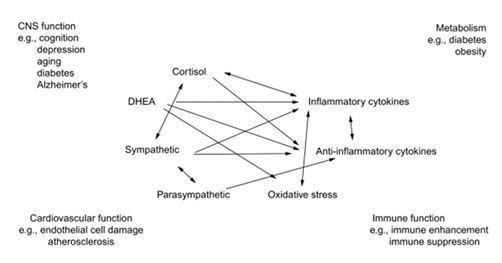 Figure 2: A graphical representation of the interconnected network of stresses and physiological responses.
Figure 2: A graphical representation of the interconnected network of stresses and physiological responses.
Each of the individual system parameters (e.g. immune function suppression) is usually taken separately but in reality these parameters are tightly connected and changing one affects the others. For the sake of simplicity, making a projection along a single control parameter axis and a single order parameter dependence of the physiological fitness landscape, one can see how the response of the system relates to its physiological state. A stable healthy system is not easily perturbed since its equilibrium state (a valley) is surrounded by steep slopes of the surrounding metaphorical mountains, which can only be traversed when the amount of stress exceeds a critical value (allostatic overload). When this happens, the system is thrown out of its previous equilibrium and into a new stability zone with a lower amount of stability (less steep slopes and a lower energy level. This signifies a transition to a chronically pathological state of health. This situation can get progressively worse both with the passage of time (another parameter axis which represents aging) and the addition of more of the same type of stress (e.g. financial) or another types of stress (e.g. physical exertion). The latter should be represented by another control parameter axis. When eventually the landscape becomes flat and featureless, this corresponds to an unresponsive system, i.e. death.
In Figure 3 we schematically depict both the physiological fitness landscape and the stress response behavior corresponding to traversing through this terrain. In these figures we illustrate graphically how stress leads to the loss of fitness, which can be measured in terms of metabolic efficiency, for example. We then show the difference between healthy aging in terms of the fitness landscape with optimal health dropping gradually as a function of time and compare it to pathological aging with sudden drops in the fitness level as the patient progresses through the subsequent stages of the chronic disease he/she is suffering from. We also show the function of the response of the physiological systems to stress as the stress increases resulting in the terminal stage of the disease. Finally, a susceptibility function is plotted in relation to stress as the patient progresses through the disease stages. It should be noted that stress represents any control parameter that affects human physiology while response to stress is the corresponding reaction of the body to this type of stress, which may be cardiovascular, immune response or metabolic function response. As noted above, these systems are interconnected, so responses to a given type of stress will be seen across the physiological systems as they form networks.
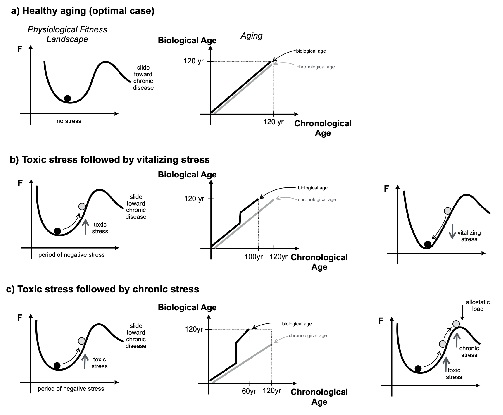 Figure 3: The effects of stress on the physiological fitness and aging.
Figure 3: The effects of stress on the physiological fitness and aging.
The most fundamental parameters of health homeostasis and disease (loss of homeostasis) are the inextricably interwoven fabric of free energy, redox and acid base. As energy is lost to heat (systemic inflammation (the classical tumor, rubor, calor, dolor localized)) and is thus unable to do the work of maintaining homeostasis, it is coupled to entropy (the redox stress of a biological system) that breaks down the organizational construction of the biological system. Therefore, as the amplitude of the Physiological Fitness Landscape falls in the setting of critical illness for example, over a period of days to weeks, the interactions between physiological parameters (e.g. glycemia, lipids, insulin sensitivity, endothelial,epithelial, neuronal, neuroglial and immune cell functions, cardiovascular, renal, pulmonary, cognitive and neurological systems) across cascading hierarchical scales search for new stability zones to accommodate and compensate for energy being dissipated and irreversibly lost and consequently entropy being created. That is, increasing inflammatory processes and redox disturbances promote the destructive incineration of organizational eloquence and complexity of biological structure and function necessary for health and ultimately life itself. Moreover, as argued throughout this book, entropy generation is tantamount to the gradual deterioration of perfect cyclicity of the biochemical reactions for many scales of cycles. These include the metabolic cycles of ATP production, the many circadian hormonal cycles, the monthly reproductive cycle and so on; these all cycle within the life-death cycle. The rate at which redox stress disrupts the molecular fidelity of these cycles, with an increasing and exponential divergence from returning to the original starting pointtaking place in the body. This ultimately is a measure of the accelerated rate of aging.
In the setting of critical illness, why is a normal blood sugar in a person with a historical early period of prolonged poor glucose control a marker for susceptibility for mortality despite the demonstrated absence of hypoglycemia? According to this Physiological Fitness Landscape model it has to do with the free energy previously lost from the plexiform of systems reliant on glucose pathways that have been degraded (such as cell mitochondria) from redox modifications. This scenario is well known in the context of cancer where a hypoxia-related shift from oxidative phosphorylation mode of energy production to the inefficient and pro-inflammatory glycolytic mode of metabolism is a measure of the severity of malignancy and is a hallmark of virtually all cancers. Recent publications indicate that this effect, also known as the Warburg effect, may be at play in the context of diabetes. It is known that the Warburg effect is irreversible even when normoxic conditions are restore in the environment of the tumor cells. Perhaps this irreversible loss of mitochondrial function is also true for diabetic patients, which would explain the above-mentioned paradoxical demise of patients with normal glucose levels.
This observation might point to inherently limited options tin regard to how these systems interact on a broader scale upon which vital organ systems and ultimate survival are dependent. There is an unpredictable point of criticality, beyond which energy lost from the system cannot be recovered; this prompts the notion of search.The many systems of interacting parameters search for a new stability zone lower in altitude on the fitness, or free energy, landscape.The key is to find a new trough within the terrain that avoids falling too far, that is without losing too much altitude of free energy flow or fitness, required for maintaining another “far-from-equilibrium” state of stability.However, the lower in altitude the system falls, the more vulnerable it becomes to a stress overcoming its energy barrier.The energy barrier may be in an otherwise healthy person attempting to lift more weight than he or she is conditioned for. Analogously, it may be a caloric consumption that exceeds the mitochondrial capacity to burn clean or the resilience to handle the dose of a psychogenic stress. Moreover, it can be a critical illness imposing energetic demands of immune system activation and other allostatic responses that exceed the bioenergetic capacity of mitochondria. Accordingly, there becomes greater reliance on the much less efficient glycolysis mode of energy production. This explains fundamentally why establishing a euglycemic “normal glucose” environment in the intensive care setting of critically ill diabetics with historically poor glycemic control, sometimes triggers unexpected mortality.
A physiological fitness model not only helps to understand this and many other phenomena conceptually, but it also provides a computerized and mathematical high-powered framework for selecting on a precision personalized scale of medicine, optimal strategies and targeted goals of therapy.The model increases the armament of tools by a vast potential for ingesting data of control parameters, for example drug strategies or blood glucose ranges, and intrinsic order parameters, for example/ VO2max or VO2submax (as proxy to mitochondrial capacity), redox markers, pH, measures of primary and secondary allostatic mediators, as well as of susceptibility and disease states.The integration of hundreds of thousands of such parameters by this model enriches the predictability of the trajectory of a disease or susceptibility state, and the responses to alternative interventions or targeted goals of therapy. Therefore, the model employs the notion of search to find the optimal solution, that is the trough highest in altitude in the terrain of the Fitness Landscape with the steepest slope (energy barrier of resilience) to an adjacent mountain.
A crucial concept is the irreversibility of free energy lost from a living system. Energy, and hence fitness, can only be restored by extrinsically applying work to the system via cognitive or physical exercise. However, this seems only possible, with potential exceptions, in two types of tissues, cognitive regions of the brain and skeletal muscle, respectively. The increased level of fitness in these tissues is mediated by mitochondrial bioenergetic capacity for efficient ATP production in skeletal muscle, and by neural and synaptic plasticity in the case of cognitive regions of the brain. Free energy” should be defined as the potential for a system to make energy, bio-energetically in the currency of ATP, or for enhanced flow of energy for adaptive functioning. Mitochondrial oxidative metabolism is the most efficient means of doing this, and hence mitochondrial dysfunction and a decline of VO2max or VO2submax (an underutilized tool as a proxy) parallels the process of fitness decline, aging, disease and the loss of altitude within the terrain of the fitness landscape.
In the case of brain bio-energetics, neural plasticity includes increased mitochondrial biogenesis, and synaptic plasticity promotes greater flow of energy between neurons in the form of neurotransmission. The energy barrier of the mountain to the left from which the system fell following a stress pushing it from a higher to a lower altitude trough, amounts to the work required, which is impossible by the physiological system to mobilize from its diminished energy machinery. Furthermore, any true reversibility of the decline in free energy or fitness would amount to the reversal of time.Typically the best that can be done is to find a new stability zone, or trough, at an equal altitude with a reshuffling of interactions between parameters that may be expressed as a reversal of disease manifestations (such as normalizing blood glucose levels, blood pressure or lipids following weight loss). However, these are metastable states attainable through exercise, or calorie restriction approaches by dietary strategies (e.g. ketogenic, time restricted, intermittent fasting), pharmacologically or bariatric surgically induced. These practices lower energy balance and activate the sensors of low energy states, especially AMPK and SIRT-1. These energy fuel gauge sensors in turn stimulate nuclear hormone receptors and other transcriptional regulators that increase mitochondrial biogenesis, upregulate fatty acid oxidation, and promote redox cell stress resistance programs such as DNA and cell repair enzyme systems, and the biosynthesis of antioxidant programs. Nonetheless, the increase in free energy or fitness that parallels attenuation of pathological hyperactivity of primary and secondary allostatic mediators, including improvement of insulin resistance, is offset by the baseline redox damage and destruction of biological organization that is not reversible. Furthermore, the feed-forward self-amplifying and destabilizing loops such as redox and inflammatory stress, mitochondrial dysfunction and redox stress, as well as mitochondrial dysfunction and insulin resistance, together push the free energy trajectory down.
This may also explain phenomena such as weight set points and metabolic compensation, which is responsible for “slowing down metabolism” following weight loss via diet and/or exercise requiring increasing amounts of exercise or dietary restriction to avoid weight regain. There is also increasing hunger that likely is a consequence of the lower bio-energetic potential because of pre-existing mitochondrial dysfunction. Accordingly, a significant proportion of dietary consumption gets deposited in tissues widespread as ectopic fat, which is not useable or not easily useable for energetic demands. Indeed, the likelihood of sustaining 10% weight loss is unusual over a 2-4 year period of time. Only in the setting of continued exercise and dietary restriction is weight regain not accompanied by the return of metabolic syndrome features such as hypertension and dyslipidemia. Thus, despite the weight regain from metabolic compensation, the favorable lifestyle practices influence insulin sensitivity effects including PPAR gamma nuclear hormone receptor mediated subcutaneous adipogenesis recruited from mesenchymal stem cells. In this context, body weight does not have a punitive effect on the trajectory of fitness (or free energy) within the Physiological Fitness Landscape. Pharmacological weight loss often changes weight set points, thus explaining weight regain upon discontinuation of drug therapy. Bariatric surgery appears to favorably affect a triumvirate of gut microbiota composition, bile acid metabolism and hormone metabolism in the wall of the GI tract. In these cases there may also be GLP-1 mediated nesidioblastosis, i.e. pancreatic beta cell regeneration. Accordingly, successful bariatric surgery may offer the greatest potential for a more durable new stability zone within the fitness landscape, best conceptualized as a deeper trough and a steeper slope. What these successful strategies all have the potential to achieve is to slow the pace of biological deterioration and aging, and its non-linear divergence from chronological aging.
Nevertheless, energy that has been lost as heat from a biological system, equating to an incinerating inflammatory process cannot be used to do the useful work of biological construction, including the reconstruction of the parallel process of redox stress induced structural entropy. Moreover, these quasi-stability zones will inevitably decline in altitude to lower troughs as a function of natural aging. Over such time, the state of fitness becomes increasingly susceptible to being pushed over the free energy barrier of a mountaintop to fall to a lower altitude in the trough of an adjacent mountain, by control parameters of stress (including dietary, dysynchronous circadian behaviors, psychogenic and microbiota). The result is an ineluctable movement towards absolute thermodynamic equilibrium, which is death. That criticality point of elevated blood glucose levels in the example of a critically ill diabetic with a history of poor glucose control, in retrospect was a final metabolic tendril, which was life support.
Microbiota And Physiological Fitness Landscape
Special consideration should be given to the implications of the Physiological Fitness Landscape in the context of the microbiome and microbial function. A multitude of factors can alter these sensitive intestinal communities of microbes in addition to dietary intake, such as recreational and prescription drugs, stress, exercise, circadian disruptions, disease conditions, and inflammation to name a few. In this context, a zone of stability and homeostasis is maintained by an increased level of microbe diversity as well as careful regulation of optimal ratios of specific microbes within the gut that supports immune system function. Changes in the abovementioned factors that cause alterations in microbial composition and create intestinal dysbiosis, such as decreased diversity, an increase in proinflammatory microbes or a decrease in anti-inflammatory microbes, are responsible for pushing the system up a nearby peak into a zone of instability and out of equilibrium.
Of great clinical relevance is the role microbiota play in the biotransformation of foods, drugs, xenobiotics, and bile acids. Microbial composition is critical for the absorption and metabolism of and physiological response to these compounds [26,27]. For example, certain bacteria in the gut are responsible for the metabolism of phosphatidylcholine, which is found in the biologic membranes of foods such as red meat, dairy, and shellfish, into Trimethylamine N-Oxide (TMAO), betaine and choline. Metabolomics studies have recently shown these three metabolites to be predictive of cardiovascular disease in humans. Further, individuals in this study provided with antibiotic treatment showed a suppression in plasma concentrations of TMAO, indicating that a healthy microbial milieu is required to metabolize phosphatidylcholine [28]. Intestinal microbiota can additionally regulate absorption of drugs, such as acetaminophen, levodopa, digitalis, and sulfonamides, and predispose or protect individuals to the effects of toxic drug interactions. Another important clinical consideration is the interaction of microbiota and xenobiotics. A wide range of bacterial biotransformation can occur in numerous chemicals; however, not all have pronounced toxic effects on the host. Since our microbiota is located predominantly in the large intestine, and drugs are absorbed in the small intestine, it is likely that deleterious interactions mostly occur with poorly absorbed or delayed release drugs. Of final note, is the role of bile acids in the regulation of microbial composition? Bile acids have been known to have antimicrobial properties and are thus toxic to intestinal bacteria. Rodent studies have revealed that the composition of bile acid can alter microbiota composition and shift the quantity of Firmicutes and Bacteroidetes in the intestine to an unfavorable ratio, leading to pathology [26,27]. These studies highlight the significance of maintaining a healthy microbiota environment and some of the consequences of microbial disturbance [29]. Figure 4 shows a graphical representation of the effects of microbiota on the physiological fitness landscape, disease development of the host and aging.
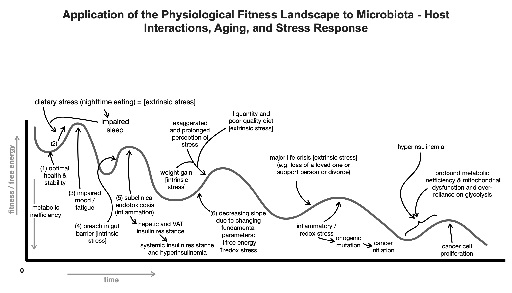 Figure 4: Schematic representation of the microbiota-host interactions and their effects on the physiological fitness landscape, emergence of disease and aging.
Figure 4: Schematic representation of the microbiota-host interactions and their effects on the physiological fitness landscape, emergence of disease and aging.
Physiological Fitness Landscape And Medical Practice
Here, we summarize the main aspects of the adaptation of the physiological fitness landscape to medical practice. A disease state may be any that involves a vital organ system e.g. CVD, all types of cancers, Alzheimer’s disease and other neurodegenerative diseases. Mathematically, each time point for a patient’s disease state may be a composite of 100’s of 1000’ of parameters characterizing this patient’s physiology at this point in time.
The most proximal control parameters (1,3,4,5 all bidirectional feed-forward loop interrelated relationships; 1-5 are all stressors) are:
1) diet-quality/quantity (extrinsic)
2) stress-psychogenic/physical (extrinsic)
3) stress response (intrinsic)
4) circadian behaviors-sleep/wake, fasting/feeding, others (extrinsic)
5) Microbiota composition(may be considered intrinsic or extrinsic-See text)
Proximal intrinsic control parameters elicited by the upstream extrinsic control parameters and the stress response are:
1) primary allostatic listed (note redox is also a homeostatic parameter to which only subtle perturbations elicit inflammatory cytokine responses)
2) secondary allostatic parameters:
a) insulin resistance markers (e.g. hyperglycemia, hyperinsulinemia, hyper/dyslipidemia, hypertension, subcutaneous adiposity/obesity/ diabetes (?))
b) other hormones, NHR’s and transcriptional regulators that govern bioenergetic and redox homeostasis
3) Homeostatic (inextricably interwoven) parameters:
a) Gibbs free energy
b) redox and
c) acid base status
4) allostatic overload states (visceral adiposity/obesity, diabetes(?), CVD, Cancers, AD, accelerated cognitive decline , accelerated biological aging and other chronic diseases of aging)- emerge when allostatic responses are unable to maintain homeostatic parameters in physiological range
When all free energy flow in the biological system is lost as heat, free energy and biological fitness level are zero, and the system is inanimate at thermodynamic equilibrium. However, it is also essential to recognize that in each valley the system being described attains a state of local free energy minimum. This is a state of the lowest metabolic inefficiency, which minimizes energy being lost as heat due to inflammatory and redox stress or excess uncoupling proteins along the ETC unable to be harnessed for useful coordinated structure and function needed to maintain physiological stability. Moreover, minimum free energy within each parameter subsystem being illustrated corresponds to a maximum number of synchronized interactions with other parameter systems in the total manifold of the individual. This equates to maximum total free energy flow and hence fitness of the systems biology (the organismic system comprised of numerous subsystems). Heat lost from the subsystems parallels the desynchronization and compartmentalization of pathophysiology. The various stresses push individual parameters onto instability zones represented by hilltops and ultimately into new valleys. These suboptimal valleys are characterized by less total organism-wide fitness and less minimization of free energy within subsystem parameter interactions. This is because there is greater intrinsic stress on these parameter interactions and because there is less energy distribution across a support network of the total living system. This less equitable stress distribution creates weak links and bottlenecks in the metabolic energy flows leading to overloads on specific organs and ultimately organ failure. When a vital system, such as glycemia becomes detached from interacting parameters, and death occurs, that means there was not enough energy in the whole system as a result of one or both of the following causes. 1) The “metabolic memory” of poor glycemic control during the initial weeks/months following diagnosis (so-called a critical period), when a significant amount of energy was lost relative to the total energy of the system. This can be seen as analogous to the initial air lost from a totally inflated tire prior to the slowing down of air loss due to air less pressure inside the tire pushing it out. 2) The current critical illness context in the ICU, which is a debilitating energy wasting state. The latter case is exemplified by the unexpected finding that intravenous insulin given in the critical care setting to normalize blood glucose, while dramatically reducing mortality overall in half (20%—>10%), in a significant number of patients this practice seemingly paradoxically increases mortality.
Conclusion
The single greatest challenge to the profession of medicine is predictability of sudden death, the onset and trajectory of disease, and its response to interventions. The Physiological Fitness Landscape model addresses this head on. While the concept is relatively simple, its implementation in the clinical setting will require introduction of algorithms whose parameters may need to be estimated “on the fly”. For chronic diseases this can be done in the course of routine examinations and tests. In the case of acute emergencies, computational models may need to be built using massive amounts of big data analytics. Irrespective of the various complications facing the early adopters of the model, this author is strongly convinced that it’s only a matter of time before the practice of medicine will undergo as massive a revolution as the one that swept through the area of telecommunications and information technology. Here, the stakes are even higher because there is no price high enough to preserve one’s health and extend out life spans.
It is extraordinary that this concept of a Fitness Landscape has never been invoked in Medicine before. A widespread extension of the prototypical Cardiac Stress test to the practice of Medicine in all specialties is a critical complement to Metabolic or Physiologic Fitness Landscape Moreover; it is in fact the composite of stress tests from multi-specialties from an interdisciplinary perspective. In this chapter we provided an overview of this concept with illustrative examples from medicine such as diabetes and cancer. A comprehensive monograph on this topic will be published shortly.
References
- Howard J (2001) Mechanics of motor proteins and the cytoskeleton. Sinauer Associates, Massachusetts, USA.
- Fertig B (2022) Metabolism and Medicine: The Physics of Biological Engines (Volume 1). CRC Press, Florida, USA.
- Fertig B (2022). Metabolism and Medicine: The Metabolic Landscape of Health and Disease (Volume 2). CRC Press, Florida, USA.
- Stryer L (1995) Biochemistry, WH Freeman and Company, New York, USA.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al. (2007) Molecular biology of the cell. Garland Science, USA.
- Demetrius L (2006) The origin of allometric scaling laws in biology. Journal of theoretical biology 243: 455-467.
- Demetrius L, Tuszynski JA (2009) Quantum metabolism explains the allometric scaling of metabolic rates. Journal of the Royal Society Interface 7: 507-514.
- Mitchell P (1976) Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochemical Society Transactions 4: 399-430.
- Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134: 703-707.
- Demetrius LA, Simon DK (2012) An inverse-Warburg effect and the origin of Alzheimer’s disease. Biogerontology 13: 583-594.
- Kumar S (2005) Molecular clocks: four decades of evolution. Nature Reviews Genetics 6: 654-662.
- Zeyda M, Stulnig TM (2009) Obesity, inflammation, and insulin resistance–a mini-review. Gerontology 55: 379-386.
- Naim AYB (2013) Statistical thermodynamics for chemists and biochemists. Springer Science & Business Media. Springer, Switzerland.
- Sapolsky RM (2002) Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM (Eds.). Behavioral endocrinology MIT Press, 409-450.
- Testa R, Bonfigli AR, Prattichizzo F, Sala LL, Nigris D, et al. (2017) The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients 9: 437.
- Nathan DM, DCCT/Edic Research Group (2014) The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes care 37: 9-16.
- Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, et al. (2003) Intensive diabetes therapy and carotid intima–media thickness in type 1 diabetes mellitus. New England Journal of Medicine 348: 2294-2303.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. New England journal of medicine 359: 1577-1589.
- Engerman RL, Kern TS (1987) Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 36: 808-812.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. New England journal of medicine 359: 1577-1589.
- Gæde P, Vedel P, Larsen N, Jensen GV, Parving HH, et al. (2003). Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 348: 383-393.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, et al. (2009) Glucose control and vascular complications in veterans with type 2 diabetes. New England journal of medicine 360: 129-139.
- Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, et al. (2019). Intensive glucose control in patients with type 2 diabetes—15-year follow-up. New England Journal of Medicine 380: 2215-2224.
- Tarnopolsky MA, Rennie CD, Robertshaw HA, Tarnopolsky SNF, Devries MC, et al. (2007) Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 292: 1271-1278.
- McEwen BS (1998) Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York academy of sciences 840: 33-44.
- Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, et al. (2011) Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773-1781.
- Klaassen CD, Cui JY (2015) Review: Mechanisms of How the Intestinal Microbiota Alters the Effects of Drugs and Bile Acids. Drug Metab Dispos 43: 1505-1521.
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, et al. (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57-63.
- Sherwin E, Dinan TG, Cryan JF (2018) Recent developments in understanding the role of the gut microbiota in brain health and disease. Annals of the New York Academy of Sciences 1420: 5-25.
Citation: Fertig BJ, Chopra D, Tuszynski JA (2021) Metabolism, Microbiota-Host Interactions, Aging and Stress Response: Diagnostic and Therapeutic Applications of the Physiological Fitness Landscape. J Altern Complement Integr Med 7: 211.
Copyright: © 2021 Brian J Fertig, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

