
Microbial Fuel Cell (MFC) Application for Generation of Electricity from Dumping Rubbish and Identification of Potential Electrogenic Bacteria
*Corresponding Author(s):
Abu HashemPrincipal Scientific Officer, Microbial Biotechnology Division, National Institute Of Biotechnology, Ganakbari, Ashulia, Savar, Dhaka-1349, Bangladesh
Email:hashemnib04@yahoo.com
Abstract
Microbial Fuel Cell (MFC) is a device in which microorganisms consume organic compounds as nutrient source and discharge electrons to the electrode, thereby generating electricity. In this study, double chamber MFCs and multiple chambers MFCs were constructed for the generation of electricity from microorganisms present in organic waste samples. Samples were collected from organic wastes from local garbage dumping area in wetland and electricity was generated by the oxidation of endogenous microbes present in samples. Electricity production was gradually increased with growth of organisms, which was decreased after time interval due to depletion of organic matter. A steady state for electricity generation was maintained by adding external glucose. In total, 44 bacteria were isolated from the anodic biofilm. The electrogenic activity of each isolate was observed using artificial wastewater (without organic matter) as substrate. A significant generation of electricity (Maximum 5.78 V and 5.03 mA in multiple chambers MFC) was attained connecting multiple chambers containing MFCs and able to lid light. Microbial diversity on anodic biofilm was observed by scanning electron microscope (SEM) image analysis. Characterization of anodic biofilm bacterial community suggested that 54.54% of electrogenic bacterial community belonged to Enterobacteriaceae family. In addition, the non-fermentative genera Pseudomonas, Moraxella, Vibrio, Burkholderia, Escherichia, Enterobacter, Photobacterium, Obesumbacterium, Sphingomonas and Raoultella also played an important role. MFC, a renewable method for electricity generation from biodegradable compounds without emission of carbon dioxide, is crucial for sustainable in electricity production in countries like Bangladesh as an environment friendly approach.
Keywords
Biofilm; Electrogenic Bacteria; Electricity Generation; Microbial Diversity; Microbial Fuel Cell (MFC); Scanning Electron Micrograph (SEM)
INTRODUCTION
Now a day the world is observing energy crisis due to huge energy demand and limited resources. Non-renewable energy sources are tremendously depleting, and renewable energy sources are not properly utilized. Combustion of non-renewable energy emits a lot of greenhouse gas like carbon dioxide, which has shown alarming consequences to the environment. There is an immediate need for search of alternate routes for energy generation [1,2]. Microbial Fuel Cells (MFCs) are one of the solutions to mitigate this problem. MFC technology, which generates energy especially from the oxidation of organics [3,4] by the metabolic activity of microorganisms seems to be attractive to warrant power generation [5-7]. The microbial fuel cells might be come to light for a wide range of uses, including serving as domestic electrical generators and powering items for example small portable electronic devices like robotics [8], automobiles [9], electronics in space [10] and self-feeding robots [11]. Electricity production by using microbial cultures was first observed over 90 years ago by Potter [12,13].
Mostly organic compounds act as bases of chemical energy and these are electron sources for value added product like electricity generation in MFCs. Acetate, glucose, wastewater and petroleum compounds have been studied as substrates in MFCs for electricity generation [3,14]. Mediator plays an important role to generate electricity from different substrates by the catalytic activity of microbes. Sometime external mediator is not necessary because some bacteria are capable to synthesize its internal mediators [15,16] or even to pass electrons to the anode through direct contact [17,18] when Biofilms are formed on the anodes of MFCs. The bacterial conductive pili are necessary for the development of these dense biofilms and higher level of current production [19]. Not all bacteria can have direct contact with the electrode within the biofilm [5]. The bacterial community and predominant species vary depending on operational status for example inoculum, substrates nature and electrode materials [5,20,21]. When MFCs operated with mixed cultures of organisms, current generation observed with greater power densities than those with pure cultures [6,16]. Several two-compartment MFC systems or Single-compartment MFC systems sometime connected in series or in parallel to investigation of performances [22]. MFC is a reliable, clean and alternative source for power generation, which utilizes renewable methods and does not emit any toxic by-product [1]. Many rural areas of country like Bangladesh still deprived of electricity and there is no effective waste management system. People dump their wastes nearby water bodies that cause environmental pollutions.
Therefore, aims of this study is to develop a low-cost domestic electricity production system using dumping rubbish which will simultaneously solve environmental pollution as well as electricity crisis. In this study, first power generation from dumping rubbish using MFCs was investigated, followed by connection of generation chambers in series to combine produced electricity in single device and characterization of individual organism responsible for electricity production.
MATERIALS AND METHODS
Sample collection
Waste samples were collected in sterile bottles and sterile sampling bags from three locations in Dhaka city of Bangladesh. Then the samples were carried to Microbial Biotechnology Division lab of National Institute of Biotechnology (NIB) within four hours and kept into refrigerator at 2-4°C for further investigation.
Construction and operation of mediatorless double chamber MFC
The MFC consisted two chambers (anode and cathode) were separated by a salt bridge. The opening of the salt bridge was block with cotton so that the solution of anode and cathode could not mix with each other. Zinc plate was used as anode and copper plate as cathode. This mediatorless double chamber MFCs were constructed where anode chamber contained garbage sample containing rotten organic matters in arrested water and growth medium (Composition (g/L): Glucose 3, NH 4Cl 0.5, NaHPO4 0.25, Na2HPO4 0.25, MgCl2 0.3, CaCl2 0.005, ZnCl2 0.015, CuCl2 0.0105, MnCl2 0.015 and final pH 7.5) in the ratio 6:4. The growth medium was used as nutrient suppliment for electrogenic bacteria which played roles to enhance the growth and oxidation power of electrogenic bacteria. The cathode chamber contained distilled water and 0.1M phosphate buffer in same ratio as anode chamber. Electricity generation (volt and milli-amp unit) was measured with a multi-meter (SUOER Model: SD 830L) from 1st day to 20th day with regular interval compared to control. Every 5 days interval 100 mL of 3% glucose solution per liter was added in anode chamber after discarding same amount of solution from the anode cell. Oxidation capacity of electrogenic bacteria was maintained applying continuous mode operation (discard 100 mL of previous solution from MFC and added same amount of 3% glucose solution in each chamber). Electricity generation (volt and milli-amp unit) was measured by using electric multi-meter as well as connecting blub to observe direct illumination.
Construction and operation of mediatorless multiple chambers MFC
To combine the electricity generation, a mediatorless multiple chambers MFC was constructed by making connection among ten single chamber MFC in series [23]. In this type of design each chamber was contained both anode and cathode electrodes and each anode were connected with the cathode of just next chamber in series. Cathode of first chamber and anode of last chamber were connected with each other to measures total electricity generation. Electricity generation (volt and milli-amp unit) was measured by using electric multimeter as well as connecting blub to observe illumination.
Anode sample collection and culture
In microbial fuel cell, Zinc plate used as anode was the source of Electrogenic Bacteria (EB). After 30 days, the anode plates were collected from MFC aseptically. Sterile distrilled water was rinsed over the biofilm containing anode surface and rubbed the surface with sterile toothpick for collection of biofilm. Liquid bacterial samples were collected in sterile beaker. Samples were then cultured in nutrient agar media using serial dilution technique and spread plate method for isolation of pure colonies. All petriplates were tranfered into the incubator at 37°C and incubated for 24 hours. Each plate was observed for colony morphology. Good characterized colonies were picked randomly from nutrient agar media and transferred to new nutrient agar plates by streaking for pure culture by sterile loop. The plates were then incubated for 24 hours at 37°C.
Preparation of inoculum
For the proof of electricity generation by our isolated microorganisms, all the chambers were autoclaved and reconnected. Isolated 44 bacterial pure (from anodes) cultures were inoculated in sterile nutrient broth separately and incubate for 18 hours at 37°C and 150 rpm to prepare culture inoculum. Each sterile nutrient broth was inoculated with isolated bacterial culture. After the incubation period the cultures inoculum were distributed in the separate chambers of MFC and electricity generation was measured as stated in as section 2.3 & 2.4.
Selection of potential Bacteria through monoculture
Single chamber MFC was designed to search potential electrogenic bacteria where both anode and cathode plate were inserted in a chamber. Each of isolated 44 bacterial pure culture was inoculated separately in 100 ml sterile nutrient broth and incubated overnight at 37°C and 150 rpm. After incubation, OD of each pure culture was measured and re-incubated to make same cell density where applicable. Then each single chamber MFC was filled with artificial wastewater (composition (mg/L): Urea 91.74, NH4Cl 12.75, Na-acetate 79.37, Peptone 17.41, KH2PO4 23.4, Glucose 3000, FeSO4.7H2O 5.80, Starch 122, Milk powder 116.19, Yeast 52.24, Soy oil 29.02, CuCl2. 2H2O 0.536, MnSO4.H2O 0.108, ZnCl2 0.208) and pure culture suspension (900 mL artificial wastewater and 100 mL culture solution). Individual electricity generating capacity was recorded as before up-to 5th day in regular interval from 1st day.
SEM analysis of anode
MFC was kept for 55 days to produce biofilm over the anode plate. For SEM analysis, the anode plates were collected, and the samples were sputter coated in a Polaron E-5100 Sputter Coater (2 min at 2.2 kV) with argon at 13 Pa by using a palladium target and observed in a JEOL JSM 6490 Scanning Electron Microscope (SEM) at Centre for Advanced Research in Sciences (CARS) in University of Dhaka, Bangladesh. The SEM was operated at 20 kV and images were captured digitally [17].
Characterization of bacterial community
Morphological and biochemical characterization
Morphological characteristics, gram straining and 15 different types of biochemical tests and online software (ABIS) based approach was used for identification of potential electrogenic bacteria.
Molecular characterization
Molecular identification approach was used for identification of the most potential bacterial species. Phenol-chloroform chemical lysis method was used for extraction of genomic DNA. PCR was performed using 16S rRNA bacterial universal primer set of 27f (5'-AGAGTTTGATC CTGGCTGAG-3') and 1492r (5'-GCCTACCTTGTTACGACTT-3'). Molecular identification of the most potential bacterial isolates HWWA-1 C17 and HWWA-1 C19 were performed by 16s rDNA sequence analysis. Then the obtained sequences were compared with those of 16S rDNA deposited at GenBank by using BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and closely related sequences were multiple aligned using ClustalW to identify organism with maximum similarity. A phylogenetic tree was constructed using MEGA 7.0 software.
RESULTS
Electricity generation by double chamber MFC
Anode and cathode were connected with salt bridge and electricity generation was assessed with a multi-meter, from 1st day to 20th day. After 4th day, electricity generation started to decrease due to depletion of nourishment. After 5th day when 100 mL 3% glucose solution was added to the anode chamber, then the value of electricity generation was started to rise as previous and followed the same pattern over the time (Figure 1).
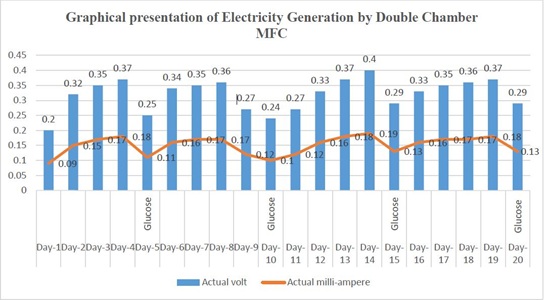 Figure 1: Trend of Electricity Generation by double chamber MFC at time interval.
Figure 1: Trend of Electricity Generation by double chamber MFC at time interval.
Eletricity generation through multiple chamber connected in series
In double chamber MFC, the generation of electricity was not enough for illumination of light. It was increased by designing multiple chambers MFC. Ten single chambers MFC were bridge together in series and it provided optimal result than double chamber MFC. Electricity generation was combined for higher energy for practical application regular life (Figures 2&3).
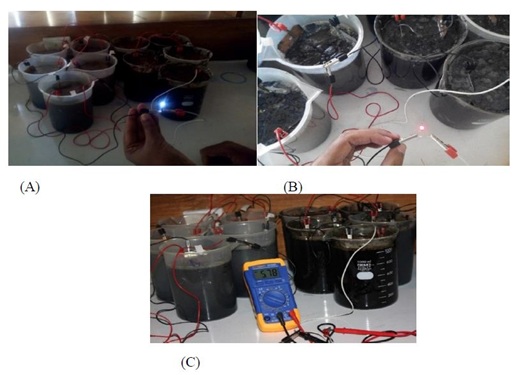 Figure 2: Eletricity Generation by Multiple Chamber Connected in Series: (A) Illumination of 4 V bulb by MFC, (B) Illumination of 1.5 V blub by MFC, and (C) Multi-meter volt reading of MFC in 6th day.
Figure 2: Eletricity Generation by Multiple Chamber Connected in Series: (A) Illumination of 4 V bulb by MFC, (B) Illumination of 1.5 V blub by MFC, and (C) Multi-meter volt reading of MFC in 6th day.
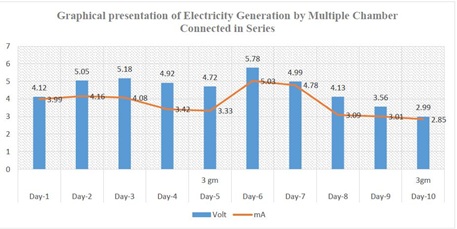 Figure 3: Pattern of electricity generation by multiple chamber MFC with time. Bar graph representing electricity production in voltage and line graph representing electricity production in milliampere.
Figure 3: Pattern of electricity generation by multiple chamber MFC with time. Bar graph representing electricity production in voltage and line graph representing electricity production in milliampere.
Confirmation test results to determine electricity generated by bacterial metabolism
The lights were illuminated when the bacteria contaminated wastewater applied in anode chambers. However, the lights are not illuminated when autoclaved wastewater were used in anode chambers indicating that the electricity generation was due to the action of electrogenic microbes present in the sludge. Without bacteria the value of multi-meter was dramatically down (from 3.31mA to 0.19mA). After addition of all 44 isolates, electricity generation was observed as a pattern like raw sludge and the light was illuminated again.
Potential electrogenic bacteria determination
Artificial wastewater was used to find out potential electrogenic bacteria. Individual isolate was tested by using single chamber MFC for determination of its ability on electricity generation. Among 44 isolates 22 isolates (50%) have the capability of electricity generation as electrogenic bacteria and the remaining 50% have no capacity for electricity generation. Based on experiments electricity production by each bacterium was depicted in Figure 4 and most potential bacteria were also distinguished.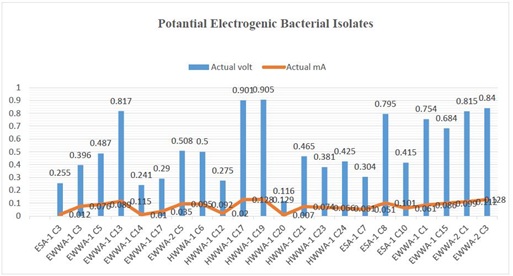 Figure 4: Electricity production by individual electrogenic bacterial isolate. Bar graph representing electricity production in voltage and line graph representing electricity production in milliampere.
Figure 4: Electricity production by individual electrogenic bacterial isolate. Bar graph representing electricity production in voltage and line graph representing electricity production in milliampere.
SEM analysis results
Anodes of MFCs were an excelent source for electrogenic bacterial biofilm. The bacterial community created a biofilm over the anode which was evidently ensured under Scanning Electron Microscopy (SEM) (Figure 5). The anode sample contains a maximum of 3.0 × 108 cfu per ml and a minimum of 1.16 × 107 cfu per ml.
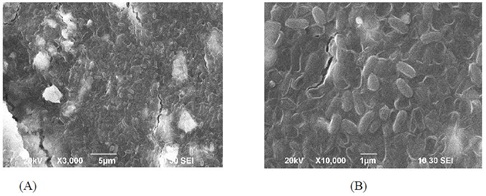 Figure 5: Scanning Electron Micrograph: Bacterial biofilm observed at (A) × 3000 resolution and (B) × 10000 resolution.
Figure 5: Scanning Electron Micrograph: Bacterial biofilm observed at (A) × 3000 resolution and (B) × 10000 resolution.
Identification of electrogenic bacteria
On the basis on morphological and biochemical tests electrogenic bacteria were identified using online software. ABIS online bacterial identification regnum prokaryote software (https://www.tgw1916.net/bacteria_logare_desktop.html) was used for identification of potential electrogenic bacteria. The results are shown in Table 1.
|
Isolates ID |
Closest taxon’s |
Probability of taxon |
Isolates ID |
Closest taxon’s |
Probability of taxon |
|
ESA-1 C3 |
Moraxella canis |
99% |
HWWA-1 C20 |
Moraxella cuniculi |
91% |
|
EWWA-1 C3 |
Enterobacter aerogenes |
91% |
HWWA-1 C21 |
Pseudomonas fluorescens |
90% |
|
EWWA-1 C5 |
Pseudomonas acidovorans |
90% |
HWWA-1 C23 |
Aeromonas hydrophila |
82% |
|
EWWA-1 C13 |
Escherichia fergusonii |
92% |
HWWA-1 C24 |
Aeromonas salmonicida |
83% |
|
EWWA-1 C14 |
Burholderia caryophylli |
99% |
ESA-1 C7 |
Photobacterium damselae |
84% |
|
EWWA-1 C17 |
Aeromonas salmonicida |
95% |
ESA-1 C8 |
Burkholderia mallei |
88% |
|
EWWA-2 C5 |
Obesumbacterium proteus |
86% |
ESA-1 C10 |
Sphingomonas paucimobilis |
86% |
|
HWWA-1 C6 |
Pseudomonas fuscovaginae |
81% |
EWWA-1 C1 |
Pseudomonas flavescens |
99% |
|
HWWA-1 C12 |
Moraxella bovoculi |
99% |
EWWA-1 C15 |
Moraxella atlantae |
81% |
|
HWWA-1 C17 |
Bacillus siamensis |
80% |
EWWA-2 C1 |
Raoultella terrigena |
85% |
|
HWWA-1 C19 |
Bacillus tequilensis |
82% |
EWWA-2 C3 |
Burkholderia mallei |
88% |
Table 1: Biochemical Identification of electrogenic bacteria with the help of online software.
Molecular characterization of most potential electrogenic bacteria
For molecular identification, two most potent electrogenic bacteria having higher electricity generation capacity (based on Volt and mA) were selected. After biochemically identification, those isolates were subjected to sequence-based detection. PCR was performed using 16S rRNA bacterial universal primer. The DNA bands were visualized by agarose gel electrophoresis and purified PCR product was sequenced with DNA sequencer. Finally, Phylogenetic tree (Figure 6) was constructed for identification of electrogenic bacteria based on DNA sequences. Phylogenetic tree indicated that HWWA-1 C17 (Sample-1) and HWWA-1 C19 (Sample-2) were closely related to Bacillus paralicheniformis strain SBP14 and Bacillus subterraneus strain COOI3B respectively.
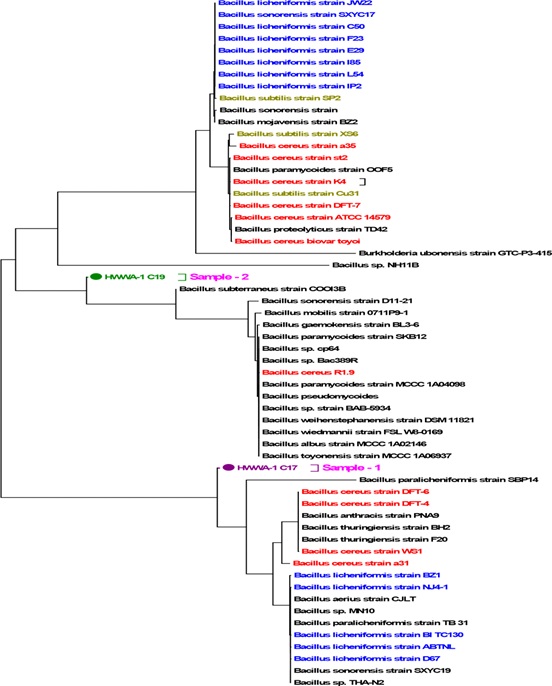 Figure 6: Phylogenetic tree constructed with the sequences of most potential electrogenic bacteria.
Figure 6: Phylogenetic tree constructed with the sequences of most potential electrogenic bacteria.
DISCUSSION
In this study the main focusing subject is to isolate and enrich a microbial consortium for electricity generation from organic waste samples. An MFC is basically an anaerobic treatment process because the bacteria grow in the absence of oxygen in a chamber on an electrode. To generate electricity, the bacteria oxidize organic matter in the wastewater and pass the electrons to an anode. Electricity is only generated when electrons are passed to the electrode. Protons are also created to maintain a charge balance and these protons must be able to migrate to the counter electrode through the salt bridge so that they can combine with electrons and oxygen to form water. The generation of current and the potential difference between the cathode and the anode chambers, creates the basis of the MFC. There is a maximum potential of 0.9 V. This low voltage was transformed to produce a higher voltage by connecting the reactors in series which was capable to lid light.
There are many promising future research avenues in electromicrobiology. The understanding of how microorganisms donate electrons to electrodes is still rather super?cial and even less is known about electron transfer from electrodes to cells. As, the electricity generation is directly proportional to how much electron release from organic waste, there were a lower amount of electricity (highest 0.40 V and 0.19 mA) in double chamber MFC while the level of electricity generation was better (highest 5.78 V and 5.03 mA) when used multiple chamber MFCs for the oxidation of organic wastes by endogenous microbes of wastewater samples. In this study, the potential bacterial strains (HWWA-1 C17 and HWWA-1 C19) are capable for producing ~0.90V and ~0.50 mA respectively which was better than previous studies [24-27].
The biochemical profile of suspected bacteria represented that isolates were belonged Enterobacteriaceae family (54.54%) and non-fermentative family (10 bacterial species). The dominant bacterial genus were Pseudomonas and Moraxella (18.18%). The second dominant bacterial genus were Vibrios and Burkholderia (9.09%). Escherichia (4.54%), Enterobacter, Photobacterium, Obesumbacterium, Sphingomonas and Raoultella were also present and involvement of different microorganisms in electricity generation supported by others [24,26]. For further identification four most potential electrogenic bacteria were tested for 16s ribosomal RNA gene amplification by using 16s 27f & 1492r primers. Biochemical tests are not reliable enough for identification of bacteria up to species level, but sometimes it may be possible for the common well-known bacterial species using commonly available test systems. We have done a several biochemical tests (e.g.15 tests), that amount may be not good enough for identification of bacteria up to species level and used a software basis analysis to know the genus of bacterial communities. This software predicts the bacterial genus by ensuring different percentage (e.g. Bacillus siamensis 80%) of possibilities. That is why, biochemical prediction of Bacillus siamensis and Bacillus tequilensis might be replaced by with Bacillus paralicheniformis and Bacillus subterraneus respectively. These bacteria showed significant potential for the harvesting of energy from organic compounds in the form of electricity. If these potential microorganisms can be inoculated onto wastewater, it can serve a dual role of both electricity generation and waste treatment. In near future, renewable energy source may the only solution of energy and waste management.
CONCLUSION
The microbial power technology is still in an initial stage of development but showed great promise as a new method to accomplish both wastewater treatment and electricity generation. We have specified the electrogenic bacteria. Further optimization of growth condition, elucidation of electron transport mechanism and identification of potential gene for electron transfer may open for new arena for its proper management practical application. Besides, the effect of mediator in electricity generation should be evaluated. Moreover, the use recombinant DNA technology could bring the scope of industrial application MFC. In developing countries like Bangladesh where energy crisis is prominent and waste materials are burden for environment, with the application of this MFC technology may solve both problems. A key factor in future implementation of the technology will be the feasible method development and cost to build & operate the system.
ACKNOWLEDGEMENT
The study was conducted at National Institute of Biotechnology, Bangladesh under the Government of the people’s Republic of Bangladesh. The authors are grateful to Authority for their support. Abstract of this research was submitted to Conference (September 17-18, 2018) of Annual Industrial Biotechnology and Bioprocessing Congress 2018.
REFERENCES
- Logan B (2004) Biologically extracting energy from wastewater: Biohydrogen production and microbial fuel cells. J Environ Sci Technol 38: 160-167.
- Barua E, Hossain MS, Shaha M, Islam E, Zohora FT, et al., (2018) Generation of Electricity Using Microbial Fuel Cell (MFC) from Sludge. Bangladesh J Microbiol 35: 23-26.
- Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in Microbial Fuel Cells (MFCs) for sustainable energy production. J Bioresource technology 101: 1533-1543.
- Catal T, Li K, Bermek H, Liu H (2008) Electricity production from twelve monosaccharides using microbial fuel cells. Journal of Power Sources 175: 196-200.
- Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. J TRENDS in Microbiology 14: 512-518.
- Rabaey K, Lissens G, Siciliano SD, Verstraete W (2003) A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. J Biotechnology letters 25: 1531-1535.
- Mohan SV, Saravanan R, Raghavulu SV, Mohanakrishna G, Sarma P (2008) Bioelectricity production from wastewater treatment in dual chambered Microbial Fuel Cell (MFC) using selectively enriched mixed microflora: Effect of catholyte. J Bioresource Technology 99: 596-603.
- Mathuriya AS, Yakhmi J (2016) Microbial fuel cells-Applications for generation of electrical power and beyond. J Critical reviews in microbiology 42: 127-143.
- Shukla A, Suresh P, Berchmans S, Rajendran AJCS (2004) Biological fuel cells and their applications. J Curr Sci 87: 455-468.
- Konikoff J, Reynolds L, Harris E (1963) Electrical energy from biological systems. J Aerospace medicine 34: 1129-1133.
- Wilkinson S (2000) “Gastrobots”-benefits and challenges of microbial fuel cells in food powered robot applications. J Autonomous Robots 9: 99-111.
- Potter M (1910) On the difference of potential due to the vital activity of microorganisms. Proc Univ Durham Phil Soc.
- Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. J Proc R Soc Lond B 84: 260-276.
- Jiang J, Zhao Q, Zhang J, Zhang G, Lee DJ (2009) Electricity generation from bio-treatment of sewage sludge with microbial fuel cell. Bioresource Technology 100: 5808-5812.
- Bond DR, Lovley DRJA (2005) Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol 71: 2186-2189.
- Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. J Applied and environmental microbiology 70: 5373-5382.
- Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69: 1548-1555.
- Kim BH, Kim HJ, Hyun MS, Park DH (1999) Direct electrode reaction of Fe (III)-reducing bacterium, Shewanella putrefaciens. Journal of Microbiology and Biotechnology 9: 127-131.
- Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, et al., (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72: 7345-7348.
- Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7: 375.
- Sun Y, Wei J, Liang P, Huang X (2012) Microbial community analysis in biocathode microbial fuel cells packed with different materials. AMB Express 2: 21.
- Aelterman P, Rabaey K, Pham HT, Boon N, Verstraete W (2006) Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ Sci Technol 40: 3388-3394.
- Daniel DK, Mankidy BD, Ambarish K, Manogari R (2009) Construction and operation of a microbial fuel cell for electricity generation from wastewater. International Journal of Hydrogen Energy 34: 7555-7560.
- Ismail ZZ, Jaeel AJ (2013) Sustainable power generation in continuous flow microbial fuel cell treating actual wastewater: Influence of biocatalyst type on electricity production. ScientificWorldJournal 2013: 713515.
- W?odarczyk PP, W?odarczyk B (2019) Wastewater treatment and electricity production in a microbial fuel cell with Cu–B alloy as the cathode catalyst. Catalysts 9: 572.
- Idris SA, Esat FN, Rahim AAA, Rizzqi WZ, Ruzlee W, et al., (2016) Electricity generation from the mud by using microbial fuel cell. MATEC Web of Conferences 2016: EDP Sciences 69: 4.
- Parkash A, Aziz S, Soomro SA (2015) Utilization of sewage sludge for production of electricity using mediated salt bridge based dual chamber microbial fuel cell. J Bioprocess Biotech 5: 251.
Citation: Saha TC, Protity AT, Zohora FT, Shaha M, Ahmed I, et al., (2019) Microbial Fuel Cell (MFC) Application for Generation of Electricity from Dumping Rubbish and Identification of Potential Electrogenic Bacteria. Adv Ind Biotechnol 2: 010.
Copyright: © 2019 Titon Chandra Saha, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

