Journal of Non Invasive Vascular Investigation Category: Clinical
Type: Research Article
Microcirculation Evaluation of Leg Ulcers - An Innovative Diagnostic Approach
*Corresponding Author(s):
Palumbo FPFu Surgery, Casa Di Cura “Villa Fiorita”, Prato, Italy
Tel:+39 349 7524568,
Email:Drpalumbo.studio@gmail.com
Received Date: Nov 08, 2018
Accepted Date: Feb 08, 2019
Published Date: Feb 22, 2019
Abstract
Recent researches in the field of lower limb ulcers have focused on the persistent state of chronic inflammation the probable cause of delayed healing. The oximetry studies have also highlighted the hypoxia condition in the periwound territories. Using the LASCA method (Laser Speckle Contrast Analysis), which makes possible remote study of the ulcer bed, and transcutaneous oximetry was evaluated in 21 patients with lesions of the lower limbs of different origins the state of the microcirculation both of the bed that in the surrounding area the ulcerative lesion. The preliminary study allowed to highlight very low O2 values (similar, if not worse than those found in subjects suffering from PAD) in the context of the ulcer and gradually decreasing values in the periwound site, suggesting the need to deepen the role of the microcirculation in the various evolutionary stages of the ulcerative lesion.
Keywords
Chronic inflammation; Laser Speckle Contrast Analysis (LASCA); Leg ulcers; Microcirculation; Perfusion Unit (PU); Region of Interest (ROI)
INTRODUCTION
In recent years, research into ulcers of the lower limbs has underlined the importance of chronic inflammation as the main cause of tissue repair delays [1,2]. Pathogenesis is still unclear, and many hypotheses have been proposed [3,4]. The data emerging from biochemical research have identified different molecules and enzymatic systems able to perpetuate the Chronicity of inflammation, and further acquisitions on cellularity (chemo-taxis, macrophages and monocytes) demonstrate their complex interconnection that makes it still difficult to univocally explain the processes involved respectively at the cellular level, intercellular and with the extracellular matrix [5-7]. Biofilms, ROS / NOS systems [8-13], microvesicles [14,15], metalloproteases [3-5] and other factors have been examined and are sometimes held responsible for the transformation of the acute inflammatory state into chronic [14-20]. Only the presence of the infection in bio film form and the ability of the latter to modify the host’s reaction appear to be able to provide a plausible explanation, but not all ulcers are infected and not all are found of biofilm [21,22]. Moreover, the research on the microcirculation has shown that at the level of the area surrounding the ulcer there is a hypoxia state that can cause suffering in the endothelial glycol-calyx [23-26]. Recent studies have demonstrated in vivo and in computational models that this structure is able to dialogue with endothelial cells and to release factors that can modify leukocyte populations (macrophages) inducing the development of pro-inflammatory populations [27-32].
Because of difficulty to obtain effective data in the bottom of the ulcer, Laser Speckle Contrast Analysis (LASCA) has been used to study capillary perfusion in this side [33-35]. LASCA is based on the concept that when a coherent light (as laser light) hits an object the scattered light will form a random interference pattern consisting in dark and brightness areas or speckle pattern. If the object is stationary the pattern will be clear, but with movement (as blood flow) speckle pattern will changes over time. By CCD camera is possible to capture these changes and depending on the degree of movement the level of blurring. The level of blurring is quantified by the speckle contrast and could be correlate with blood flow and blood perfusion measurement. Usually a helium-neon laser (633 nm) and CCD camera are employed for source and detector, respectively (Perimed AB) [36-40]. Aim of this research is the evaluation of skin circulation in vivo in patients affected of leg ulcers.
Because of difficulty to obtain effective data in the bottom of the ulcer, Laser Speckle Contrast Analysis (LASCA) has been used to study capillary perfusion in this side [33-35]. LASCA is based on the concept that when a coherent light (as laser light) hits an object the scattered light will form a random interference pattern consisting in dark and brightness areas or speckle pattern. If the object is stationary the pattern will be clear, but with movement (as blood flow) speckle pattern will changes over time. By CCD camera is possible to capture these changes and depending on the degree of movement the level of blurring. The level of blurring is quantified by the speckle contrast and could be correlate with blood flow and blood perfusion measurement. Usually a helium-neon laser (633 nm) and CCD camera are employed for source and detector, respectively (Perimed AB) [36-40]. Aim of this research is the evaluation of skin circulation in vivo in patients affected of leg ulcers.
METHODS
21 patients (13 males and 8 female average age 59, 6 ± 1, 4 yy) affected of leg ulcers were enrolled at Operative Unit of Angiology in Vittorio Emanuel Hospital Catania, Italy from September 2017 to January 2018 (Table 1).
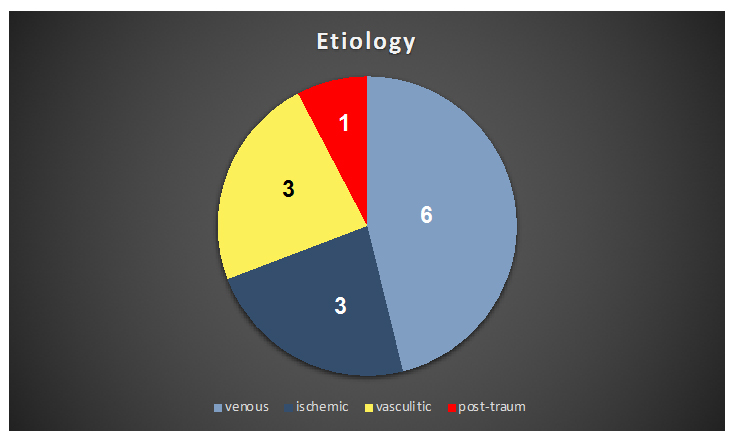
Table 1: Etiology.
All patients underwent to clinical, instrumental and serological examination and during admittance period routinary anti-platelet therapy was administered. In venous patients affected of post-thrombotic syndrome antithrombotic therapy was administered (Acenocumarol or NAO) plus compressive therapy (high stiffness bandages) and topic application of silver sulphadiazine surfactant dressing directly on the wound [29-31]. Vasculitis patients were assessed by arterial and venous Eco Color Doppler and Serological Immunoassays. In post-traumaticul cerno-arterial lesions were detected. Therapy was carried out with steroids, analgesics and local advanced dressings in vasculitic ulcers.
In patients affected of PAD iloprostev. Infusion was administered daily for a week. In each patient pO2, pCO2, Laser doppler and Laser Speckle Contrast Analysis (LASCA) (Peri Cam PSI System, Perimed Italy) on three Regions Of Interest (ROI) corresponding to the bottom of the wound, periwound area and peripheral area of skin as healthy skin were studied.
RESULTS
Data show increase of Perfusion Units (PU) in periwound areas in allulcers. In venous and vasculitic PU were greater respectively 52, 3 and 26% than healthy control. Also ischemic ulcers, even poorly, showed the same trend (Table 2).
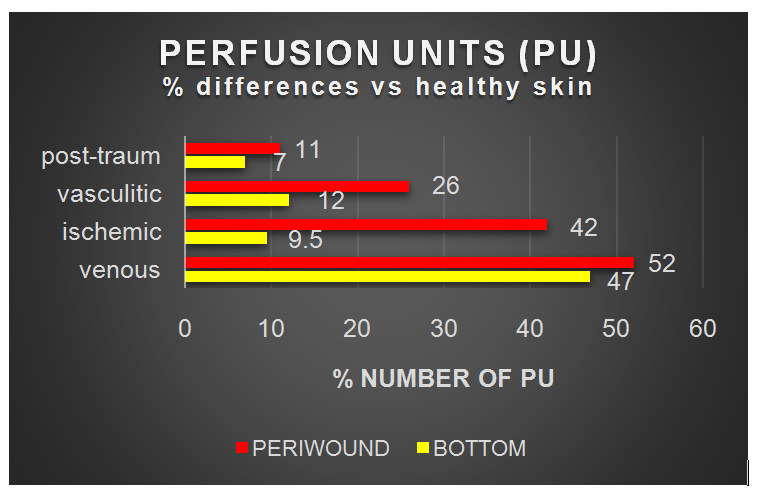
Table 2: Perfusion unit’s differences in Region of Interest (ROI).
In this last group iloprost infusion have reduced PU in perilesional areas and increased PU in the bottom of ulcer. No significative changes were present in vasculitic ulcers after iloprost administration (Figure 1). Local administration of SSD (Surfactant Silver Sulphadiazine) Gel-Dressing was followed by reduction of PUs both in the bottom and periwound areas. Data analysis at basal time high lights a greater amount of PU sin periwound areas (ROI) than in the bottom and check areas.
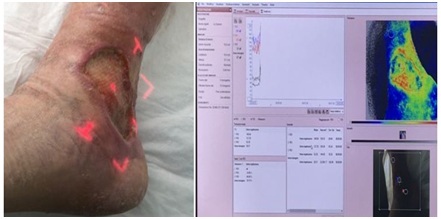
Figure 1: Laser Speckle of a leg ulcer showing high perfusion (chronic inflammation?) in periwound area.
After SSD-dressing administration a decreased number of PU sin periwound and an increase in bottom area have been observed (Figure 2). Despite the limited number of cases examined, the preliminary data review implies a number of considerations:
a. Imaging by means of laser speckle allows the observation of the ulcer bed in real time and the changes that occur after the administration of vaso active substances.
b. There is a correlation between PUs and oximethric data.
c. Especially in vasculitis patients, even the presence of chronic inflammation of the skin, oxymethry values tend to be hypoxic.
d. After the administration of drugs, achronic inflammation in periwound area persists.
e. Data of Laser-doppler show the presence of vasomotion before and after the administration of SSD-dressing and of iloprost.

Figure 2: Laser speckle examination of a venous ulcer and oxymethric data showing low pO2 in the border of the lesion.
This could be interpreted as the primary cause of the chronicity of the lesion and the presence of elevated levels of MMPs in the exudates and ulcer tissue confirm the state of chronic inflammation.
Even the administration of the SSD-Gel dressing (a transparent gel and which does not alter their fraction of the laser light) LASCA is able to determine (especially in the bottom of the ulcer) the same PUs alterations observed after infusion of iloprost, but it is not yet clear whether this information is related to anti-inflammatory and antibacterial properties of the dressing or its own vasoactive properties.
CONCLUSION
Data analysis high lights confirm the presence of a chronic inflammation in all the ulcers. The alteration of skin microcirculation is more evident in periwound areas, while in the bottom number of PU is poor. After the local application of topical SSD dressing, PU numbers in the bottom are increased. These data suggest a direct correlation between iloprost infusion and capillary bed vasodilatation. The effect of SSD dressing could be explained by its direct anti inflammatory and antiseptic properties they could be responsible of the micro-environment changes in the bottom of ulcer. The persistence of a ring area surrounding the ulcer (presence of chronic inflammation) could be explained as there all cause of delay in healing. These data are not significant from a statistical point of view, but open up new diagnostic horizons in the field of wound care. This new method together with O2 oxymetry and Laser-doppler, allows evaluating in vivo and in real time the microcirculation directly in the bottom of ulcer helping to verify the effectiveness of both systemic and local-therapy in this area.
REFERENCES
- Wolcott RD, Cox SB, Dowd SE (2010) Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. J Wound Care 19: 272-281.
- Raffetto JD (2013) Inflammation in chronic venous ulcer. Phlebology 28: 61-67.
- Ligi D, Croce L, Mannello F (2018) Chronic Venous Disorders: The Dangerous, the Good and the Diverse. Int J Mol Sci 19: 2544.
- Ligi D, Croce L, Mosti G, Raffetto JD, Mannello F (2017) Chronic Venous Insufficiency: Transforming Grow Factor-β Isoforms and Soluble Endoglin Concentration in Different States of Wound Healing Int J Mol Sci 18: 2206.
- Serralheiro P, Novais A , Cairrã E, Maia C ,Costa Almeida CM et al. (2018) Variability of MMP/TIMP and TGF-β 1 Receptors throughout the Clinical Progression of Chronic Venous Disease. Int J Mol Sci 19: 6.
- Chen Y, Peng W, Raffetto JD, Khalil RA (2017) Matrix Metalloproteinases in Remodeling of Lower Extremity Veins and Chronic Venous Disease. Prog Mol Biol Transl Sci 147: 267-299.
- Schmidt A, Bekeschus S (2018) Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants (Basel) 19: 7, Atta HM (2012) Varicose Veins: Role of Mechanotransduction of Venous Hypertension. Int J of Vascular Medicine Article ID: 538627.
- Kunkemoeller B, Kyriakides TR (2017) Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Signal 27: 823-838.
- André-Lévigne D, Modarressi A, Pepper MS, Pittet-Cuénod B (2017) Reactive oxygen species and nox enzymes are emerging as key players in cutaneous wound repair. Int J Mol Sci 18: 2149.
- Dwivedi G, Gran MA, Bagchi P, Kemp ML (2015) Dynamic redox regulation of il-4 signaling. PLOS Comput Biol 11: 1004582.
- Sies H (2015) Oxidative stress: A concept in redox biology and medicine. Redox Biol 4: 180-183.
- Roberts DD (2017) Extracellular matrix and redox signaling in cellular responses to stress. Antioxid Redox Signal 27: 771-773.
- Roberts DD, Kaur S, Isenberg JS (2017) Regulation of Cellular Redox signaling by matricellular proteins in vascular biology, immunology, and cancer. Antioxid Redox Signal 27: 874-911.
- Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, et al. (2017) Microvesicles in vascular homeostasis and diseases. Position paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost 117: 1296-1316.
- Tricarico C, Clancy J, D’Souza-Schorey C (2017) Biology and biogenesis of shed microvesicles. Small GTPases 8: 220-232.
- Yamazaki T, Mukouyama YS (2018) Tissue specific origin, development, and pathological perspectives of pericytes. Front Cardiovasc Med 5: 78.
- Thomas H, Cowin AJ, Mills SJ (2017) The Importance of Pericytes in Healing: Wounds and other Pathologies. Int J Mol Sci 24: 18.
- Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T (2016) What is a pericyte? J Cereb Blood Flow Metab 36: 451-455.
- Schött U, Solomon C, Fries D, Bentzer P (2016) The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med 24: 48.
- LeBlanc AJ, Kelm NQ (2017) Thrombospondin-1, Free Radicals, and the Coronary Microcirculation: The Aging Conundrum. Antioxid Redox Signal 27: 785-801.
- Martin L, Koczera P, Zechendorf E, Schuerholz T (2016) The Endothelial Glycocalyx: New Diagnostic and Therapeutic Approaches in Sepsis. Biomed Res Int 2016: 3758278.
- Liu C, Wu C, Yang Q, Gao J, Li L, et al. (2016) Macrophages Mediate the Repair of Brain Vascular Rupture through Direct Physical Adhesion and Mechanical Traction. Immunity 44: 1162-1176.
- Cruz-Chu ER, Malafeev A, Pajarskas T, Pivkin IV, Koumoutsakos P (2014) Structure and Response to Flow of the Glycocalyx Layer. BiophyS J 106: 232-243.
- Mehta D, Ravindran K, Kuebler WM (2014) Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 307: 924-935.
- Jaipersad AS, Lip GY, Silverman S, Shantsila E (2014) The Role of Monocytes in Angiogenesis and Atherosclerosis. J Am Coll of Cardiol 63: 1-11.
- Van Ierssel SH, Jorens PG, Van Craenenbroeck EM, Conraads VM (2014) The endothelium, a protagonist in the pathophysiology of critical illness: Focus on cellular markers. BioMed Research International Article ID: 985813.
- Kotschy D, Kotschy M , Socha P, Mas?owski L, Kwapisz J, et al. (2015) [Selected endothelial hemostatic markers in patients with peripheral arterial disease after endovascular revascularization and restenosis formation]. Postepy Hig Med Dosw (online) 69: 905-912.
- Magri D, Vasilas P, Muto A, Fitzgerald T, Fancher T, et al. (2011) Elevated monocytes in patients with critical limb ischemia diminish after bypass surgery. J Surg Res 167: 140-150.
- AM Salisbury, Marc Mullin, Rui Chen, SL Percival (2017) Control of biofilms using a concentrated surfactant based wound dressing.
- Nastase MV, Janicova A, Wygrecka M, Schaefer L (2017) Signaling at the crossroads: Matrix-Derived proteoglycan and reactive oxygen species signaling. Antioxid Redox Signal 27: 855-873.
- Devi DR, Sandhya P, Hari BNV (2013) Poloxamer: A novel functional molecule for drug delivery and gene therapy. J Pharm Sci & Res 5: 159-165.
- Ghatak PD, Mathew-Steiner SS, Pandey P, Roy S, Sen CK (2018) A surfactant polymer dressing potentiates antimicrobial efficacy in biofilm disruption. Scientific Reports 8: 873.
- Varaki SE , Gargiulo GD, Penkala S, Breen PP (2018) Peripheral vascular disease assessment in the lower limb: a review of current and emerging non?invasive diagnostic methods. BioMed Eng OnLine 17: 61.
- Stewart CJ, Frank R, Forrester KR, Tulip J, Lindsay R, et al. (2005) A comparison of two laser-based methods for determination of burn scar perfusion: Laser Doppler versus laser speckle imaging. Burns 31: 744-752.
- Jayachandran M, Rodriguez S, Solis E, Lei J, Godavarty A (2016) Critical review of noninvasive optical technologies for wound imaging. Adv Wound Care (New Rochelle) 5: 349-359.
- Failla G, Palumbo FP, Majani A, Di Salvo MM (2013) Transcutaneous oxymethry and measurementof pain as predictors of wound healing response. Acta Phlebologica 14: 93-97.
- Palumbo FP, Failla G, Serantoni S, Abbrittj F, Gazzabin L (2016) Transcutaneous oxymetry as predictive index for homologous skin graft in the treatment of leg ulcers. EWMA.
- Failla G, Adamo G, Palumbo FP, Antignani PL (2016) Laser Speckle evaluation of microcirculation in leg ulcers. Acta Phlebologica 17: 86-90.
- Failla G, Palumbo FP (2016) The modern approach to venous ulcer Cap 43. In: Allegra C, Antignani PL (Eds.). Tips and tricks in Angiology, Minerva Med, Torino, Italy.
- Failla G, Palumbo FP, Abbritti F (2016) Le lesioni cutanee vascolari. Lesioni cutanee vascolari degli arti inferiori Cap XV In: Aragona SE, Marazzi M (Eds.). Medicina Rigeneratica-Nuove metodologie di Cura. Istituto Europeo di Scienze Forensi e Biomediche, Gallarate, Italy.
Citation: Palumbo F P, Failla G, Serantoni S, Ardita G, Gazzabin L, et al. (2019) Microcirculation Evaluation of Leg Ulcers - An Innovative Diagnostic Approach. J Non Invasive Vasc Invest 4: 013.
Copyright: © 2019 Palumbo FP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!