
Mouldability of the Body Core in Adaptive Form
*Corresponding Author(s):
Edwin Chau Leung YuHong Kong Institute Of Integrative Medicine, The Chinese University Of Hong Kong, Hong Kong
Email:yuchauleung@gmail.com
Abstract
The current structural makeup of body form and structure of the axial and appendicular system is contributed by evolutionary precedents and developmental paths. Adapting to terrestrial life, particular body regions are specialized for certain functions. The rigidity of the thorax is advantageous for both protection inside and support of skilful hand movements outside. Its hexagonal profile is a dominant constraint, described here for the first time. With the axial system being appendicular based, the pelvic hind-limb girdle stabilizes the retractile components between upper and lower body for core stability and control in the diverse locomotor mechanics to overcome surrounding terrains. Forces would impact up the rigid mid-trunk region, where mouldabilty is observed. Adaptive mouldabilty facilitates functional stability and activity as a dynamic process. The six meridians in hexagonal coordinates mediate the fascia tensile systems up the lower limbs to the trunk. In this interactive musculoskeletal framework, the constituting back and pelvic musculature of the Zang Kidney support for cultivated actions with firmness and agility. The Zang Kidney is a self-vitality subsystem that tunes the body to environment adaptively. Strengthening its associated neurohumoral mechanisms and reconditioning the fascia tensile systems are ways to tune up and maintain stability of the axial-appendicular system.
Keywords
Axial-appendicular system; Core stability and mouldabilty; Hexagonal coordinates; Six meridial fascia tensile systems
Introduction
The body form, the body state, the body disposition are areas to assess for making out a good therapy [1]. Understanding life dynamics is involved. Without a full picture, a clinician would at best be a technical expert over a part. This article is an abbreviated attempt to describe the structure wherein the whole and the parts interactively relate.
The Endowed Body Formation
The body's rigid framework is provided by the skeletal system including cartilage, tendons, and ligaments. The whole musculoskeletal system is anchored together with the integumentary system to enable the body to move about as this support and is supported by the body operational organ systems – cardiovascular-lymphatic system, respiratory system, digestive system, nervous system, endocrine system, urinary system, and reproductive systems. Working in the in Neural Perfusional Interconnective (NPI) dimensions for adaptation [2], the fascial sheets underneath would encase, separate and stabilize various parts (Figure 1).
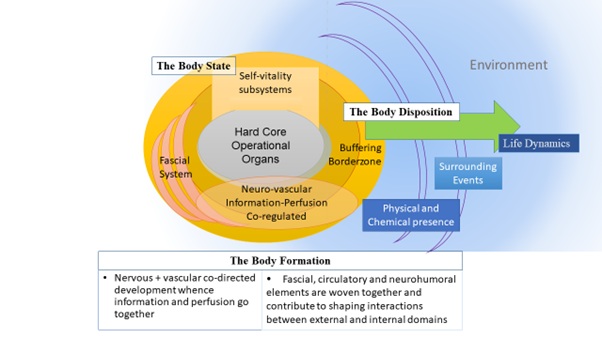 Figure 1: The body formation with its hard core and borderzone made up in Neural Perfusional Interconnective fascial (NPI) dimensions for adaptation
Figure 1: The body formation with its hard core and borderzone made up in Neural Perfusional Interconnective fascial (NPI) dimensions for adaptation
As the proper individual being develops, these organ systems are installed early. When the endowed biological robustness declines through life, the individual’s resilience would be sustained through well-patterned dynamics [3]. For a snug and fit living, the whole body would pattern and remodel itself through self-vitality systems [4] which tune the body up to the environment adaptively for life dynamics, with the operational organ systems as the base. Befittingly, an integral person may still have wholistic gaps yet not filled but the person remains could positive as a whole [1]. All these functions for life are embodied as one body formation.
As function and structure are related, the makeup or arrangement into forms and organization would determine and be determined by the functions served and interactions inside-out. This close relationship is seen from cellular level to whole body levels, and structure-property and structure-function relationships have long been considered important interactive concepts. As structure and function are requisite for each other for the body to work out meaningfully, evolutionary precedents and developmental paths contribute to the current structural makeup. The axial skeleton (head and trunk) and the limbs are described here for this matter.
The axial skeleton
The craniates evolutionarily developed the skull and then vertebrates developed the spine. The spine provides the capability of supporting the body weight as well as facilitating activity from support for upright walking to flexibility for speedy running. Genetic control of axial patterning is now understood. Homeotic genes carry DNA sequences encoding protein transcription factors that determine animal structure. The head-tail anterior-posterior polarity of the embryo and adult has its origin in the anterior-posterior polarity of the egg. The body cranial-caudal axis may be influenced by maternal genes in chordates [5] and Hox genes with transcription factors maintain positional information in vertebrates [6,7]. Hox genes mediate the number of body segments, the number and placement of appendages, and head-tail directionality.
Related fundamental changes in body movement planes and associated differences in the moments acting on the trunk reorganized the spine into cervical, truncal, sacral, and tail regions in tetrapods. In mammals, the truncal vertebral column further subdivided into anatomically distinct regions of thoracic and lumbar region. The human axial configuration with the cervical:thoracic:lumbar: sacral vertebral formulae of 7:13:4:6 is an evolutionary descent. The number of cervical vertebrae in nearly all mammals is constant at seven [8,9] regardless long necks in giraffes or nul necks in whales.
The Neck
The human neck with seven cervical vertebrae has its evolutionary advantage as a cantelever for the skull, catering for demands associated with different head movements and postures (foraging, drinking, locomotion, gaze stabilization during locomotion, grooming and mating). Flexibility of neck movements allows and maximises necessary positioning for sensory organs at the front for exploration, orientation and function. As the brain increase in size and complexity in human beings, the neck works as a major conduit for neural perfusional and interconnectivestructures [2] between the head and the body.
Functional demands led to morphological specialization [10]. The cervical vertebrae C1 and C2 are shaped to cater for skull pivot rotation at its base insertion and C6 and C7 have unique morphologies while C3 to C5 shapes are more uniform across species [11]. This position-specific modularity (upper, middle, lower cervical spine), being highly conserved across mammals [12], becomes morphologically tripartite in close relationship to the underlying Hox gene head-tail pattern [13].
Biomechanical considerations, neck:
- The massive head is cantilevered by the neck. With overly flexed postures, more vertebral body loading results
- The flexibility of the neck has lent strength to extraneous need of present-day human catering activities different from other animals (e.g. computer use) as these have posed a different load and tension system
- The muscular coupling to the forelimb poses extra biomechanical demands on the neck. Though > 25 pairs of muscles act over several joints with multiple degrees of freedom, some muscles are not even attaching to the cervical vertebrae
- The lack of data on the characteristics and shear-under-load behaviour of the cervical functional spinal unit [14] imposes many gaps to understand the model biomechanics [15], as studies were mostly done for cervical injuries, while others ignored force-length and force-velocity relationships
For neural control, humans cannot voluntarily activate isolated neck muscles. Neural control of neck muscles is not activating individual muscle for biomechanical function. Besides, the function of a neck muscle is not based solely on its underlying biomechanics [16]. Essentially, it is both the complementary neural controls and biomechanics of muscles that act together to achieve actuated movement.
In terms of axial stability, the body has righting reflexes, including righting reflex, positive support reflex, asymmetrical tonic neck reflex and symmetrical tonic neck reflex, all born autonomously to keep the poise by responses to return the individual to the righty stabilizable frame [17]. This is the body’s adaptive strategy and no specific large neck muscle but rather many predominantly vertical muscles are used to generate axial moments [18-20]. For example, the right splenius capitis SPL and left sternocleidomastoid muscle SCM would act agonistically to generate right axial rotation, noting associated stray moments generated in other directions that need to be cancelled by other muscles. As some muscles are not even attaching to the cervical vertebrae, the head can stay at righty positions while the neck muscles are asymmetrically twisted [21].
The Mid-Trunk
The axial skeleton serves multiple mechanical functions including bending the body, storing elastic energy, transmitting forces from limbs, and ventilating the lungs. Evolutionary development of separate thoracic and abdominal cavities [22] was associated with regionalization of axial column for specialized functions in mammals [23]. Regionalization of axial skeleton during evolutionarily adaptation to terrestrial life allows particular body regions to be morphologically and physiologically specialized for certain functions. Mammals exhibit a strong differentiation between the thoracic and the lumbar spine: the thoracic stiff while the lumbar flexible and adapted to locomotion patterns [23,24]. The human thoracic cage provides rigidity for protection as well as the required flexibility to cyclic breathing. Movements in the thoracic spine are relatively limited compared to cervical or lumbar regions. Rigidity is provided by the bony rib and sternum, and flexible mobility is related to the costal cartilages and their adjoining sternal and vertebral joints.
To provide for the precision of spatiotemporal movements for coordinated reach to grasp, the stiff thoracic region is an advantage. Precise spatiotemporal synchrony between proximal forelimb muscles is required during coordinated movements from reach to grasp [25]. In the complex process for performance of a specific movement or skill, a high-level of body stability facilitates fine adjustments and achievement of skilful hand movements for craftsmanship.
Biomechanics, thorax: With the thoracic region constrained by the setup of costvertebral and costtransverse articulations, direction and shape of the articular facets, the relatively thin discs, and the tension of the ligamentum flava, the functional clustering around the three-dimensional mid-truncal series as a whole could be taken as one formation. Rotation and lateral flexion are less complicated, while movement in any other direction are constrained. To understand the relationships between, and within multiple regions of the whole thoracic region of the axial skeleton, the following are salient points.
- Known from early researches and clinical observations [26-28], the whole costo-thoracic-vertebral complex and not just the thoracic spine accounts for necessary basic understanding in biomechanics. The thoracolumbar spine is unstable without a rib cage. Emphasized recently, any investigations without intact ribs should be interpreted cautiously. Relatively little research so far have been conducted to investigate function of the thoracic cage with the human spine under dynamic loading [29]
- Axial rotation is dependent on the whole cage with ribs. One side rotation of the superior vertebra, would push the superior aspect of the head of the contralateral rib of the other side forward at the costvertebral joint inducing its posterior rotation and anterior rotation of the contralateral rib. Ligaments of the costtransverse joints are tightened consequently
- Thoracic cage stability during flexion/extension is mainly attributed to the sternum and anterior rib cage, while during lateral bending, stability is mostly contributed by the posterior rib cage [30]
- The integrated thorax region will involve the arms. When one arm is raised, the thorax rotates and laterally flexes towards that side [31,32], with a variability of coupling which reflects variations in motor control strategies for performance of this task [33]. The upper thoracic spine shows extension, side flexion, and axial rotation. As the 11th and 12th ribs are not connected to the sternum, when both arms are elevated in the sagittal plane, thoracic spine shows extension but no axial rotation and no side flexion [33,34]
The “thoracic complex”: The human thorax is a complex body region with musculoskeletal structures strengthened by the spine and ligaments at the back, as well as soft tissue organs in the thoracic cavity. The ribcage consists of 12 thoracic vertebrae, 12 pairs of ribs, costal cartilages, sternum, scapulae, and clavicles. The whole makeup of the thorax consists of these skeletal elements and the thorax cavity with lungs, heart and thymus gland, vessels and connective fascia (Figure 2). While the thoracic system allows motion in all planes, it is stabilized by the spine and rib cage together. The arms often become part of this integrated system.
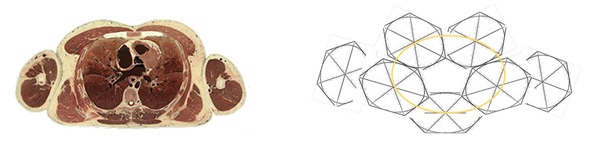 Figure 2: The thoracic complex with skeletal elements and the thorax cavity together with the arms are biomechanically integrated and can be depicted as a system
Figure 2: The thoracic complex with skeletal elements and the thorax cavity together with the arms are biomechanically integrated and can be depicted as a system
This thoracic system is relatively stiff by evolutionary descent, as it protects the vital thoracic organs. The rib cage acts as an auxiliary support to improve the stability of the whole spine in reducing the amplitude of all-direction-deviations [35,36]. The rib cage facilitates respiration, protects thoracic organs, and provides stability of the spine while sharing the load [37-39]. The presence of ribs forming the rib cage provides rigidity for the thorax. The costvertebral complex serves as scaffolding for the musculature of the thoracic spine and shoulder, while the costvertebral joints and ligaments determine thoracic stabilization, load bearing, mobility, protection and chest wall movement for respiratory effort. The strong stabilizing costvertebral and costtransverse ligaments also contribute some flexibility [40] while they stay constantly taut with their uniform bidirectional spread [41].
As the upper limbs are attached through the shoulder to the thoracic system, the upper thoracic cage provides the necessary tensile forces according to its shape which can be diagrammatically depicted according to circles and tangents of a pentagon complex (Figure 3). This pentagon profile is particularly pertinent when the arms act on the thorax complex so that the back is tightened.
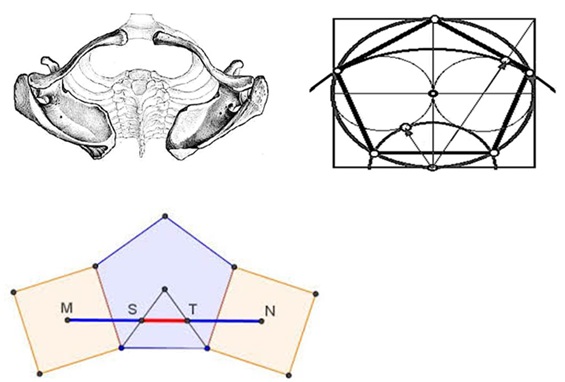 Figure 3: The Thoracic Cage from above providing the necessary tensile forces according to it shape, diagrammatically depicted according to circles and tangents of a pentagon complex when arms (not depicted) are acting.
Figure 3: The Thoracic Cage from above providing the necessary tensile forces according to it shape, diagrammatically depicted according to circles and tangents of a pentagon complex when arms (not depicted) are acting.
The thoracic region allows movements in the horizontal and transverse planes but less flexion and extension, reflected by more or less horizontally oriented zygapophyses, in contrast to lumbar region with vertical zygapophyses where sagittal plane movements are facilitated. In general in this transverse plane, the thorax is typically hexagonal in profile (Figure 4). Explanation of this established hexagonal profile is reserved to a later section for more details.
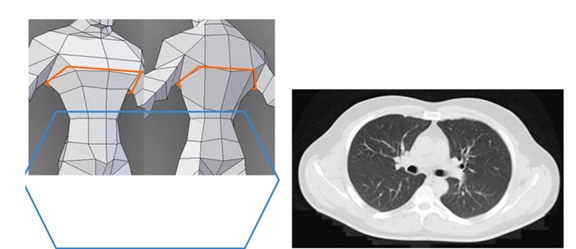 Figure 4: Thoracic transverse plane generally hexagonal in profile shown in ordinary computerized scans and clinically. Thoracic region movements notably mainly in horizontal plane.
Figure 4: Thoracic transverse plane generally hexagonal in profile shown in ordinary computerized scans and clinically. Thoracic region movements notably mainly in horizontal plane.
The thoracic cage acts as a flexibility limiter for the spine preventing damage caused by high bending moments during body movements and provides strength and energy-absorbing capacity to increase the overall rigidity of the spine [39]. The thoracic system may be altered during the long process of growth and development when prolonged, heavy or biased use of each of the elements cause shape deviations. The cylindrical shape of the rib cage and the spinal alignments are important factors to preserve. In another perspective, the thoracic profile is developed for males and females in promoting structural aesthetics of human beings.
The Lower Body
The lower body with the lower trunk and limbs often functions as a whole dynamic unit. Trunk control and core stability is built for being snug gained especially during sitting, standing and lying, while limb locomotion and skill formation are built for being fit to the surrounding environment where ecology factors are influential [23]. Snug and fit dynamics are important for living and for life [1,3]. In the meanwhile, the abdominal and pelvic organs are anchored in place, well protected. Starting evolutionarily with axial-based locomotion of aquatic craniates, tetrapods have integrated action of trunk and limbs to facilitate body propulsion, which becomes increasingly dependent on limb action [24]. The axial skeleton provides the coordination basis for the locomotor engine of the vertebrate body, with the pelvis bearing most stresses while transferring truncal loads to the lower extremities. Muscles for core stability and control include many muscles supporting the lumbo-pelvic-hip complex, having positive effects on daily life activities [42-44]. The foundation of human movements are key locomotor skills including walking, running, jumping, hopping, crawling, marching, climbing, galloping, sliding, leaping, hopping, and skipping.
Form-structure correlates, though well studied for each muscle and muscle group, cannot be fully understood without relating them to their evolutionary descent. This is especially pertinent in form-structural studies of the lower body. The change from quadripeds to a dorsal kyphotic posture from starting the use of lumbar lordotic stance and shift of centre of moments and action for changed ecological habitats have resulted in a whole different setting of bony structures and muscles insertion and usage with consequently a dissociation of serial and functional homologues. Some muscles grouping which were serving important functions in ancestors would now seem superfluously inefficient by its own. In fact, the preferred activation direction often is not aligned with assumed biomechanical function based on musculoskeletal anatomy [45,46]. This is particularly so as multiple functions are served by the same set of musculoskeletal components: muscles generally fulfil different functions during different behaviours or even the course of one behaviour. Thus, the evolutionary progressively appendage-based axial system during adaptation to a terrestrial life is associated with marked anatomical and functional reorganization of the axial musculature, including subvertebral and epaxial musculature [47-53] (erector spinae, the transverse spinal muscles (including the multifidus, semispinalis and rotatores), the splenius and suboccipital muscles (as dorsal muscles associated with the vertebrae, ribs, and base of the skull), and the vertebral column. On the side ventral to the horizontal septum of the vertebrae, starting from folds of the pleural and peritoneal membranes in reptiles [22], the diaphragm evolved. The diaphragm has muscularization unique in mammals as it freed most axial muscles from a ventilatory function during locomotion [54,55], probably related to thoraco-lumbal differentiation and an increase in locomotor and breathing efficiency [56,57].
In mammalian evolution, the subvertebral musculature was drawn in to assist the abdominal wall muscles as an antagonist of the epaxial musculature. Parts of the hindlimb musculature were shifted onto the trunk (i.e., puboischiofemoralis as iliopsoas muscle), and an axial part of the subvertebralis muscle were freed to become the psoas minor muscle [47-49] to act as hindlimb protractors and flexors of the vertebral column [50-53].
The pelvis, a hind-limb girdle, stabilizes against the retractile components between the spine and lower limbs. As the whole body is anchored upon the axial skeleton, and moving around with the lower appendages, a diversity of locomotor dynamical mechanics is supported by the pelvis. The whole setup would enhance core stability and internal strength for the development of greater power to overcome surroundings and terrains.
This increasing differentiation of muscular, neural, and skeletal elements of the lower body is certainly related to enhancing internal strength to support for diversity of locomotor mechanics. Simply, the locomotor forces produced by the limbs and the inertia of the body, result in an increased need for muscular dynamic power for sagittal stability [58]. Sagittal-plane truncal motions noticeably have developed after mammalian evolution [24]. The decoupling of the thoracic and pelvic with a greater number of lumbar vertebrae regions in humans also opens up for greater mobility in all body planes, allowing more adaptive coverage of a wide range of biomechanical capabilities. A finer muscular spinal control and an increased need for postural feedback would consequently be required for maintaining an upright posture [59], and related mechanoreceptors are present in spinal ligaments [60-62].
Biomechanics, lumbar: To understand the relationships the lumbar region and the limbs in the whole axial skeletal system, the following salient points will be first described.
- Flexed postures result in more vertebral body loading and a vertical spine is mostly loaded in compression [63]. Extended postures create more neural arch loading [64-66] as compressive load follows the curvature of the spine [67-69]
- The individual dynamic functional stability is a process between the appendicular system and the whole axial skeleton. One of the most common measures involving dynamic stability is postural stability [70,71]
- Core stability and control in the diversity of locomotor mechanics with internal strength together build up the dynamic muscular stabilization in the sagittal plane
- The mechanics, morphometry, and geometry of joints, segments, and muscles are fundamental biomechanical properties intrinsic to human neural control. The biomechanical goal of postural stability maintenance is to make fine adjustments to the centre of mass in relation to the base of support [72]
- Axial muscles during different locomotor functions may act to mobilize the axial skeleton or stabilize the trunk to counteract, control, or restrict movements induced by gravitational or inertial forces.Stabilizing actions may involve long periods of activation, by tonic local stabilization, or briefer muscle action for quick responses against rapid loading for dynamic, global stabilization [24]
Supported by increased postural feedbacks, body propulsion results from integrated action of trunk and limbs.
An Integrated Whole Body Actuational Form
Shaped by Form and Functional Endowment
Static and dynamic control of body posture and the integration of coordinated body and limb actions provide the foundation for living. Emerging fitness is attributed first to genetic endowment for the human being. Then epigenetic regulations influence the setup. Mechanical forces or biochemical cues around or phenomenological memory from precedent forms of ancestors would have influenced the functional-structure tie itself.
Fit to survive, snug to be alive; both affect survival and life quality [1]. The whole system of body form would have been subjected to basic underlying principles like stability, control, flexibility and cost-economy. For the integrated whole body in actuation and endeavors, axial core strength and stability as well as appendicular locomotor power to command domains and terrains are essential.
Core stability has been first defined as the ability to stabilize the spine as a result of local muscle activity [73]. The “core”muscles include many muscles supporting the lumbo-pelvic-hip complex. With the body tuned at snug states, little extrancous muscle activity would be necessary to maintain the erect posture if the weight-bearing structures are in good alignment and the ligaments are healthy. The interconnectivity array of differently differentiated Connective Tissues (CT) including cartilage, bone, tendon and associated bi-directional muscle-CT communication is a continuum of fascia tensile system in the interactive musculoskeletal framework [2]. The passive characteristics of the osteoligamentous spine are connected with the active neuromuscular system to order to maintain mechanical stability [74,75]. In this underlying CT-muscular-ligamentous continuum, each part merely develops its special role and usage [2], wherefore muscles along the axial-appendicular system may not be built by the direct biomechanical interactional specification. As described earlier, they may have been recruited members into the action group during evolution. Thus, for the human appendicular muscles on the spine, the preferred activation direction may not align with assumed biomechanical function based on musculoskeletal anatomy [45,46,76-78] and the direction of action of a neck muscle does not accord solely with the muscle’s assumed biomechanical function [17,79,80]. The voluntary preferred directions of SCM and SPL were more aligned with axial rotation than the electrically stimulated directions [19,20,81]. When tuned to fit the surrounding terrains, the repertoire of each of the parts as well as the whole head and spine would be effectuated and recruited from other parts where necessary.
The body form has developed and altered as it met up with other living demands. The apomorphic mammalian characteristics of sagittal bending, as a behaviour related to locomotive sagittal mobility [82-84], has probably been evolutionarily incorporated into the locomotor repertoire [85,86]. The axial-appendicular system, apart from serving for locomotor and skill dynamics, also contain vital organ systems for functioning. For example, the diaphragm could have evolved from multi-origins of nearby axial and trunk-abdominal musculature, after folds of the pleural and peritoneal membranes partitioned the unitary coelomic cavity into separate thoracic and abdominal cavities for biological advantage: generating both a negative intrathoracic pressure for ventilation of the lungs and a positive intraabdominal pressure for venous return of blood to the heart and expulsive behaviours as well as straining behaviours including defecation, vomiting, coughing sneezing and parturition [22]. The costal ribs are recruited to provide a stable truss for the diaphragm to leverage the pumping while the oblique muscles in the abdominal wall are positioned to retract the ribs and counteract rib protraction.
The rigid upper mid-trunk framing the body
Meeting all these multiple demands, the human body has arrived to its current form. Geometric constraints and mechanical forces in the ecological system would have influenced morphogenesis. The evolution of thorax in the mid-trunk is pertinent in framing the whole body. Increasing clustering of front-end functions and upper limbs skills up the evolutionary tree is associated with much change for the upper limbs as well as the mid-trunk, with regionalization and further evolution of vertebral column differentiation for specialized functions [87].
With the thoracic complex more or less hexagonal, and the upper limbs along it, the body perspective would be thus framed in a hexagonal perspective (Figure 4b), rather than the usual Cartesian antero-posterior and lateral plane (Figure 4a). Apart from the thorax profile being in hexagonal shape, the vertebrae also buttress the musculofascial scaffold in these directions (Figure 5). In fact, all thoracic motions and loads cause stress to the costvertebral attachments at both the vertebral body and the transverse process. The length of the transverse process also has an effect on the costvertebral complex. As the length of the transverse process varies (with its concomitant costtransverse articular surface), the leverage that is exerted on the costvertebral joints varies as well [88].
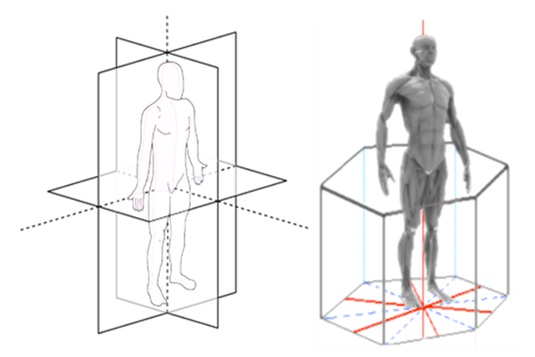 Figure 4: Body perspective. 4a (left) :Body perspective usually in antero-posterior and lateral plane. 4b (right): A better perspective is coding it in a hexagonal perspective. These accords with the hexagonal profile in thorax shape as well as the directions of vertebrae buttresses. This perspective is useful especially in interpreting lumbar-thoracic movements.
Figure 4: Body perspective. 4a (left) :Body perspective usually in antero-posterior and lateral plane. 4b (right): A better perspective is coding it in a hexagonal perspective. These accords with the hexagonal profile in thorax shape as well as the directions of vertebrae buttresses. This perspective is useful especially in interpreting lumbar-thoracic movements.
To further highlight the hexagonal buttresses with the vertebral main body located anteriorly and a bony arch located posteriorly, the two bilateral short stout pedicles join the arch to the vertebral body, and the two plate-like laminae span the pedicles. The arch supports two transverse, one spinous, and four articular processes. The directions of these pedicles are at the hexagonal diagonals, when the ribs are all accounted for (Figure 5). Formerly, picturing isolated vertebra with ribs excluded all along would miss the hexagonal diagonals, whereas re-enlightened understanding for the necessary presence of ribs to provide rigidity for the thoracic spine [28] should revive counting them in this buttress. Each architectural aspect of a vertebra has its major function. The vertebral body serves as the weight-bearing aspect, the bilateral superior and inferior processes determine the direction of motion and restrict abnormal movement, the vertebral arch encases the spinal cord and its coverings, and the spinous and transverse processes serve as bony levers for muscle and ligament attachment. The vertebra of mammals has evolved away from the one vertical plane of a fish (compressed by bilateral forces for swimming) to a structure with multi-dimensional planes (suited for actions in more planes). The human axial skeleton is configured by axial muscles that work three-dimensionally along the buttressed planes.
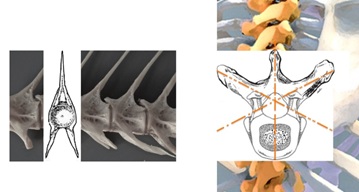 Figure 5: Biplanar vertebra in fish, adapted to swimming (left), evolved to human hexagonal vertebra (right) adapted to hexagonal buttressed actions (the empty diagonals in reality filled by ribs).
Figure 5: Biplanar vertebra in fish, adapted to swimming (left), evolved to human hexagonal vertebra (right) adapted to hexagonal buttressed actions (the empty diagonals in reality filled by ribs).
With this stabilized thorax system, fine tuning of the cluster of forward and skill tasks are achieved with support. For targeted movements, trade-offs are expected when the speed and accuracy of the task is complicated and when the centre of balance is not maintained. Body control strategies then involve the diaphragm, stabilized by the ribs and abdominal-axial muscles, and the lower trunk for good performance and momentum.
Body configuration adjustments down the lower body for function
To start with, the thoracic spine, due to the articulations with the rib cage and the kyphotic sagittal alignment, has unique biomechanical properties compared to other parts of the spine. Thoracic disc spaces are fairly parallel rather than wedge shaped. The primary thoracic kyphosis is produced by hard bone being shaped. The thoracic spine is a unique load-bearing structure in the human body. On a daily basis, it resists repeated physiological forces of axial loading, flexion and extension, lateral bending, and twisting while protecting the spinal cord and providing a foundation to the thorax. Under the influence of geometric constraints and mechanical forces and constraints, movements tend to be referenced on a coordinative system between the axial and appendicular orientations. The pelvic girdle stabilizes between the thoracic cage, the spine and lower limbs, for a diversity of locomotor dynamical mechanics with core stability and strength for power to overcome the surrounding environment and terrains.
Functional stability is a dynamic process. The axial skeleton configured with axial muscles is working together with three-dimensional fascial tensile continuum with connective tissues that support muscles with mechanical advantage in force even using the skin to increase leverage [89]. For the appendage-based axial system, the osteoligamentous parts with their characteristics would be passively moulding, while the neuromuscular parts with their reflexive and exertive drives and armaments actively working up endeavours in a flexible balance for internal and external needs as a whole.
As the axial skeleton is regionalized in function and morphology, the dominant extremities has enhanced the axial-based locomotion to become appendage-based. The lower limbs, in support of the more rigid upper body case, would be influenced or induced to conform to act in directions effective for the framed planes. A comparatively greater number of vertebrae in lumbar region in human that decoupled the thoracic and pelvic regions also allow for more freedom to a wider adaptive coverage of locomotor biomechanical capabilities.
Body configuration adjustingup from new directional planes required from lower limbs
Locomotion is characterized by the use of asymmetrical gaits associated with extensive flexions and extensions of the body axis. The upper body case is rigid but to some extent mouldable, as chest compliance is achieved through an anatomical ribcage, sternum design, and flexible joints in the dorsal spine. In general, lateral bending and long-axis torsion become less pronounced during symmetrical gaits in mammals compared to tetrapods [24]. Sagittal bending, which has become one of the apomorphic characteristics of mammalian locomotion [82,83,84], in a way compensate against the possibility of lateral bends and twists. Simply, the locomotor forces produced by the limbs and the inertia of the body have resulted in an increased need for dynamic muscular stabilization in the sagittal plane.
Nonetheless, twists and turns do occur. In daily living, lumbar-thoracic movements can be exaggerated and get out of alignment. Over the long term, one side dominance by right arm movement, chewing on one side, sitting crossleg or other unilateral habits are common. In fact, the sacrum as the large triangular bone at the base of the spine, with joints connecting the spine to the two wings of the pelvis and articulate with the hip bones for a crucial stabilizing mass, is not fused (from the sacral vertebrae S1–S5) until the ages of 18-30 years to allow for the twists and turns to establish. There are four physiological sagittal spinal curvatures to increase endurance to axial compression forces and allow adequate postural balance, and these curves have to stay within effective ranges to achieve a static and dynamic balance, a correct functioning of the muscles and an adequate distribution of the loads [90]. Intense motions in the sagittal plane can occur in the rib-free lumbar region due to zygapophysesbein vertical [91-93]; the secondary cervical and lumbar lordoses are produced not by bone but by fibrocartilage.
The asymmetrical deviations of the lower body would malleate the upper body as required, even up the relatively mobile thoracolumbar junction to the stiffed upper thoracic spine. With the activation of synergistic muscles contributing the net moments in a target direction, the lumbar movements are contributed by lower limb actions. The sagittal balance is the head-tail alignment positioning of C7 with respect to the sacrum. The high flexible extraneous need of present-day human beings has demanded a different load for tension. In adaptive movements, the trunk may shift, the pelvis may deviate or be tilted off. After the sacrum is fused, there may be additional strain when later positions and movements are sustainably not congruent with the former set pattern. These incur twists and rotations along the lower limbs up the body cage. The overly tilted pelvis also may put the lower limbs on a different alignment of the body action axis and gravity pull. For example, the fascia lata lifted by the iliac crest with a different pelvis tilt and the lower limb muscles would act in a different axis. Variability in motion coupling between in the thorax and appendicular lower body contribute to such observations. The whole up and down structure reposes to attain and establish the final form construct.
Insight for a mouldable thorax is rendered by noting the high variability in the orientation of the facets of the zygapophyseal joints in 240 specimens from T1–L5 (over 4080 vertebrae were measured) [94]. An asymmetric orientation with differences between the left and right sides has been even remarked as a ‘normal characteristic in the thorax’. Axial rotation is dependent on the whole cage with ribs. One side rotation of the superior vertebra pushes the superior aspect of the head of the contralateral rib of the other side forward at the costvertebral join inducing its posterior rotation and anterior rotation of the contralateral rib, resulting in tightened ligaments. With effect of loading on ligament behaviour [95,96], the physical properties of spinal ligaments over time become shifted and change.
Both body orientations with actuational dynamics and the base of support significantly influence postural control [97,98]. A mutual relationship must exist between the body segment positions, the global body orientation and the gravitational field [99]. Alignment resets, or else be twisted.
Spinal Stability Counteracting Twists And Turns
The body is not simply form and structure in facilitating stability and activity. It needs to provide many functions and operations apart from building up processes and fittings to harness the power. These can be understood in terms of the mantle, operational organ systems and self-vitality subsystems that adaptively cater for body smooth operational mechanisms, not just in muscular terms, to the environment [1].
The lower-body core postural and pelvic muscles are also muscles for courting and mating. As these muscle groups and pelvic organs form one organ-cluster complex, the closely interactive, closely inter-coordinated parts serving reproduction, excretion, mating and coping behaviours and actuation with stress tolerance in living together form a self-vitality subsystem and has been collectively viewed as the Zang Kidney [4,100]. Throughout Chordata vertebrates, this hind part is long and strong (constituting more than half the whole-body mass) to drive the body towards its goals. In this retro-infra-peritoneal organ mass and structure, neuro-endocrine mechanisms subserve intricate interrelationship in between so that the organs and muscular parts together would advance functions for life. Through cultivated action, motivational activation of the back and pelvic musculature is basic to agility as well as coping and aggressive dynamics [100]. Programmed behavioural dynamics with facilitating and inhibitory drive mechanisms support poise and stabilized actuation of fine movements amounting to whole-body crafts and skills.
The Zang Kidney is one of the mainstays for the strength and maintenance of dynamics of the back and its proficiency support so that muscles are programmed to require little extraneous activity to maintain the erect posture. With its poor nourishment, the whole skeletal system cannot suffice [101]. When badly entrenched, the fascia and musculature integrity would be drained out. At its worst, the body and shape would be utterly broken down.
It may well be stressed that the body is oriented between snug states and fit states [1]. Both dynamics and resources can be viewed in Chinese medicine Yin-yang terms, alternating between releasing power or consolidating oneself (?/?). Conforming to the hexagonal upper body cage and redirecting action lines through pelvic girdle down to the lower limbs, Chinese medicine depicted 6 meridial lines (3 yang meridians on postero-lateral and 3 yin meridians on the frontal-medial aspects) over each lower limb. These meridial lines go up and down through the right and left side of the back and abdomen to the thorax (Figure 6).
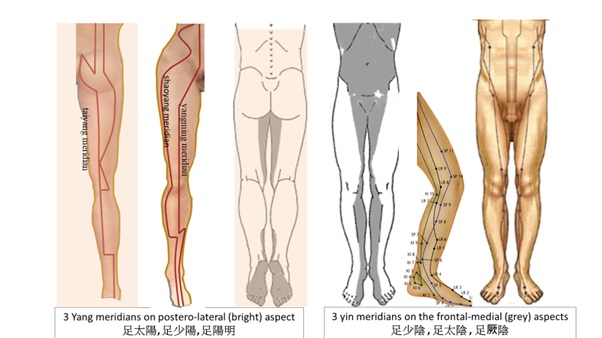 Figure 6: Three yang meridians on postero-lateral and three yin meridians on the frontal-medial aspects over each lower limb ascending up each side of the abdomen to the thorax.
Figure 6: Three yang meridians on postero-lateral and three yin meridians on the frontal-medial aspects over each lower limb ascending up each side of the abdomen to the thorax.
With snug, an energy-efficient state for progress, the body’s routine basic life activities run well. Lack of both energy and nourishment can suffer the axial and appendicular system. Sarcopenia and osteopenia are often related to long term poor conditioning of these meridial systems, particularly over the (yangming meridian) which characteristically runs along with the highly vascular parts. Therapy need attend to the blocks entrenched.
To fit the surrounding needs on the other hand, the body is positioned adaptively in the parts that are geared to the needed operations confronting the surrounding environment. Interactive possibilities for change in the separate parts evolved in adaptation to the living terrains. Thus, there is a high diversity in pelvic shape [102], with associated differences in propensities. The weight-bearing structures have to be in good alignment and the ligaments are healthy. Their interactive relation would be shaped to become habitual patterns through modifications of neuromuscular reflexive modes. Consolidated with osteoligamentous fascial configurations, these modes and patterns would become more or less fixed within a certain range when the terrains and surroundings are minimally variable for an individual. With more variable and exaggerated surroundings, the interactive relational patterns would be strained and need be adjusted.
In Chinese medicine, there is the meridian fascial tensile system that runs fairly close to the meridian lines described above for the springiness of the axial and appendicular system. Overly strained or exhausted, it may affect the leg, the ankles (taiyang meridian) or the shin (yangming meridian) [103] along the lower limb meridians. Notably, the shaoyang-meridial fascial tensile system, running along the lateral trunk and associated with the postero-lateral pelvic-hip musculature (Figure 7), influence the back and walking. When the sacrum at the pelvis is affected, the lower back is stiffened and walking difficult in this ill-directed shaoyang-meridian block [104].
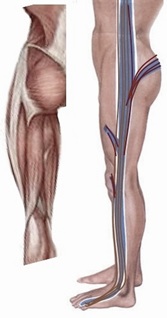 Figure 7: The lateral meridian shaoyang??? (right) with its meridial fascial tensile system is shown (in brown), running along the lateral trunk and associated with the postero-lateral pelvic-hip musculature shown in muscle anatomy (left).
Figure 7: The lateral meridian shaoyang??? (right) with its meridial fascial tensile system is shown (in brown), running along the lateral trunk and associated with the postero-lateral pelvic-hip musculature shown in muscle anatomy (left).
The axial back centrally from heels up to the head would determine the release of power [105]. It is stabilized by some 39 muscles [106,107].Throughout the back, uneven tension can be associated with related problems. The thighs are related mainly to L1-3 levels, the diaphragm to L1 (for yangming meridian). As L3 is related to taiyang meridian and L5 to shaoyang meridian, body asymmetry, slant and twists could be the result of tensile differences over them. The belt vessel (dai meridian) is winding around the trunk from L2 to L4 [108] as a stabilization tensile force. More will be discussed in future articles.
Conclusive Remarks
Understanding of the form and structure of the axial and appendicular system is important for clinical workup and therapy. The evolutionary root and adaptive changes as well as the basic nourished development are meaningful. As upper and lower body regions are particularly specialized morphologically and physiologically for their functions, each axial segment has its own build-in advantage for living. The rigid thoracic cage braces the upper trunk for ventilation needs. Between the retractile components of the upper trunk and lower limbs, the pelvic girdle anchors the diverse locomotor mechanics for stability and control. The mobility of the lower body affects the whole appendicular-based axial system, the mid-trunk allowing mouldability to a certain extent. The skeleto-musculo-fascial structure as a whole establishes itself on functional stability and agility as the parts interactively relate. Then the pelvis, the thoracic cage, the spine and lower limbs would together harness for the diversity of body and locomotor dynamical mechanics with core stability and strength for power to overcome surroundings and terrains.
The rigidity of the thorax, besides offering protection inside, is advantageous to support skilful hand movements outside. Constrained by the costvertebral and costtransverse articulations as well as spinal and costal relationships, its hexagonal profile becomes a dominant frame for the whole body. Over the large variety of locomotor movements and skills including walking, running, jumping, hopping, crawling, climbing, sliding and skipping, it is notable that muscles often fulfill different functions even during the course of one behavior. When there are extraneous demands of the lower body, forces could impact up the rigid mid-trunk region. Adaptive mouldabilty of the body form would be observed.
Movements tend to be referenced on this coordinative system between the axial and appendicular orientations. Lumbar-thoracic movements can be exaggerated and out of alignment. Adaptive mouldabilty facilitates functional stability and activity as a dynamic process. The six meridians in hexagonal coordinates mediate the fascia tensile systems up the lower limbs to the trunk. Tensile differences from the lower limbs can have significant impact onto the axial system, and understanding of meridial fascial tensile systems of Chinese medicine is pertinent. The interconnectivity array of differently differentiated musculo-fascial scaffold is a continuum of fascia tensile system in the interactive musculoskeletal framework.
In this interactive musculoskeletal framework, the constituting back and pelvic musculature of the Zang Kidney support for cultivated actions with firmness and agility. The Zang Kidney is a self-vitality subsystem that tunes the body to environment adaptively, remodeling itself out with operational organ systems as base. From its neurohumoral associated mechanisms, nourishment for its makeup would be particularly useful to build up the axial-appendicular system. Strengthening the self-vitality subsystems and tuning up the meridian fascia tensile systems are ways to booster up the whole system for stability and activity. With all these mechanisms understood, further descriptions coming will help to demonstrate these in related whole-body therapeutic methods.
References
- Yu ECL (2021) From Core and Mantle to Primary Integrality - A Brief Introduction of the Fit and Snug States. J Altern Complement Integr Med 7: 177.
- Yu ECL (2021) Body NPI Dimensions, the Neural, Perfusional, and Interconnective Matrix. ACAM 9: 71-78.
- Yu ECL (2021) Neuro-vascular reserve in developing snug and fit buildup. J Integ Med 10: 49-59.
- Yu ECL (2020) From Body Mantle to Internal Core - a Parallel Framework to Organ Systems. J Altern Complement Integr Med 6: 129.
- Gregorio AD, Levine M (1998) Ascidian embryogenesis and the origins of the chordate body plan. Current Op Gen Dev 8: 457-463.
- Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121: 333-346.
- Holland PWH, Fernandez JG (1996) Hox genes and chordate evolution. Devel Biol 173: 382-395.
- Galis F (1999) Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool 285: 19-26
- Narita Y, Kuratani S (2005) Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J Exp Zoll Part B 304: 91-106.
- Kuratani S (2009) Modularity, comparative embryology and evo-devo: developmental dissection of evolving body plans. Dev Biol 332: 61-69.
- Arnold P (2020) Evolution of the mammalian neck from developmental, morpho-functional, and paleontological perspectives. J Mammal Evol 28: 173-183.
- Arnold P, Altava BE, Fischer MS (2017) Musculoskeletal networks reveal topological disparity in mammalian neck evolution. BMC Evol Biol 17: 251.
- Böhmer C (2017) Correlation between Hox code and vertebral morphology in the mouse: towards a universal model for Synapsida. Zool Lett 3: 1-8.
- Oxland TR(2016) Fundamental biomechanics of the spine--What we have learned in the past 25 years and future J Biomech 49: 817-832.
- Alizadeh M, Knapik GG, Mageswaran P, Mendel E, Bourekas E, et al. (2020) Biomechanical musculoskeletal models of the cervical spine: A systematic literature review. Clinical Biomechanics 71: 115-124.
- Fice JB, Siegmund GP, Blouin JS (2018) Neck muscle biomechanics and neural control. J Neurophysiol 120: 361-371.
- Yu ECL (2020) Core-vs-match model for Autism and Neuro-Developmental Disorders. J Paediatr Neonatol 2: 112.
- Ackland DC, Merritt JS, Pandy MG (2011) Moment arms of the human neck muscles in flexion, bending and rotation. J Biomech 44: 475-486.
- Peterson BW, Choi H, Hain T, Keshner E, Peng GC (2001) Dynamic and kinematic strategies for head movement control. Ann NY Acad Sci 942: 381-393.
- Vasavada AN, Peterson BW, Delp SL (2002) Three-dimensional spatial tuning of neck muscle activation in humans. Exp Brain Res 147: 437-448.
- Yu ECL. Personal observations.
- Fogarty MJ, Sieck GC (2019) Evolution and Functional Differentiation of the Diaphragm Muscle of Mammals. Compr Physiol 9: 715-766.
- Jones KE, Benitez L, Angielczyk KD, Pierce SE (2018) Adaptation and constraint in the evolution of the mammalian backbone. BMC Evol Biol 18: 172.
- Schilling N (2011) Evolution of the axial system in craniates: Morphology and function of the perivertebral musculature. Front Zool 8: 4.
- Geed S, McCurdy ML, Kan PLEV (2017) Neuronal Correlates of Functional Coupling between Reach-and Grasp-Related Components of Muscle Activity. Front Neu ral Circuits 11: 7.
- Panjabi MM, Brand RA, White AA (1976) Mechanical properties of the human thoracic spine as shown by three-dimensional load-displacement curves.J Bone Joint Surg Am 58: 642-652.
- Lee D (1994)Manual Therapy for the Thorax: A Biomechanical Approach. Delta: Diane G Lee Physiotherapist Corporation, USA.
- Lee D (2003) The thorax – an integrated approach.Delta: Diane G Lee Physiotherapist Corporation, USA.
- Jia S, Lin L, Yang H, Fan J, Zhang S, et al. (2020)The influence of the rib cage on the static and dynamic stability responses of the scoliotic spine. Sci Rep 10:
- Brasiliense LBC, Lazaro BCR, Reyes PM, Dogan S, Theodore N, et al. (2011) Biomechanical contribution of the rib cage to thoracic stability. Spine 36: 1686-1693.
- Edmondston SJ, Ferguson A, Ippersiel P, Ronningen L, Sodeland S, et al. (2012) Clinical and radiological investigation of thoracic spine extension motion during bilateral arm elevation.J Orthop Sports Phys Ther 42: 861-869.
- Tachihara (2019) Characteristic Movement of the Ribs, Thoracic Vertebrae while Elevating the Upper Limbs - Influences of Age and Gender on Movements. The Open Orthopaedics Journal 13: 171.
- Theodoridis D, Ruston S (2002) The effect of shoulder movements on thoracic spine 3D motion.Clin Biomech (Bristol, Avon) 17: 418-421.
- Crosbie J, Kilbreath SL, Hollmann L, York S (2008) Scapulohumeral rhythm and associated spinal motion. Clin Biomech (Bristol, Avon) 23: 184-192.
- Watkins R, Watkins R, Williams L, Ahlbrand S, Garcia R, et al. (2005) Stability provided by the sternum and rib cage in the thoracic spine. Spine 30: 1283-1286.
- Mannen EM, Anderson JT, Arnold PM, Friis EA (2015) Mechanical contribution of the rib cage in the human cadaveric thoracic spine. Spine 40: 760-766.
- Liebsch C, Wilke HJ (2018) Chapter 3 - Basic Biomechanics of the Thoracic Spine and Rib Cage. Biomechanics of the Spine 2018: 35-50.
- Anderson DE, Mannen EM, Tromp R, Wong BM, Sis HL, et al. (2018) The rib cage reduces intervertebral disc pressures in cadaveric thoracic spines by sharing loading under applied dynamic moments. Journal of Biomechanics 70: 262–266.
- Liebsch C, Graf N, Appelt K, Wilke H-J (2017) The rib cage stabilizes the human thoracic spine: an in vitro study using stepwise reduction of rib cage structures. PLoS One 12: 2017.
- Kindig M, Li Z, Kent R, Subit D (2015) Effect of intercostal muscle and costovertebral joint material properties on human ribcage stiffness and kinematics. Comput Methods Biomech Biomed Engin 18: 556-570.
- Rosse C, Rosse PG, Hollinshead WH (1997) Hollinshead’s Textbook of Anatomy. Harper & Row, New York, USA.
- Haruyama K, Kawakami M, Otsuka T (2017) Effect of core stability training on trunk function, standing balance, and mobility in stroke patients. Neurorehabil Neural Repair 31: 240-249.
- Valdés RC, Calafat CB, Farrés MG, Gómez FMC, Valiño MH, et al. (2015) The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: a randomized controlled trial. Clin Rehabil 30: 1024-1033.
- Ting LT, Jie LM, Qian LY, Na ML, De JC (2018) Effects of core stability exercise on rehabilitation in stroke patients with hemiplegia: a meta-analysis. TMR Nondrug Therapy 1: 41-52.
- Nozaki D, Nakazawa K, Akai M (2005) Muscle activity determined by cosine tuning with a nontrivial preferred direction during isometric force exertion by lower limb. J Neurophysiol 93: 2614-2624.
- Kurtzer I, Pruszynski JA, Herter TM, Scott SH (2006) Primate upper limb muscles exhibit activity patterns that differ from their anatomical action during a postural task. J Neurophysiol 95: 493-504.
- Starck D (1978) Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage. Springer Verlag, Berlin, Heidelberg, New York, USA.
- Howell AB (1938) Morphogenesis of the architecture of hip and thigh. J Morph 62: 177-218.
- Jones CL (1979) The morphogenesis of the thigh of the mouse Mus musculus with special reference to tetrapod muscle homologies. J Morph 162: 275-310.
- Engberg I, Lundberg A (1969) An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta PhysiolScand 75: 614-630.
- Rasmussen SA, Chan AK, Goslow GEJ (1978) The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morph 155: 253-270.
- Kuhta PC, Trank TV, Smith JL (1998) Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687-1701.
- Smith JL, Kuhta PC, Trank TV (1998) Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1702-1716.
- Deban SM, Carrier DR (2002) Hypaxial muscle activity during running and breathing in dogs. J Exp Biol 205: 1953-1967.
- Carrier DR (1996) Function of the intercostal muscles in trotting dogs: Ventilation or locomotion? J Exp Biol 199: 1455-1465.
- Perry SF, Similowski T, Klein W, Codd JR (2010) The evolutionary origin of the mammalian diaphragm. Respir Physiol Neurobiol 171: 1-16.
- Buchholtz EA (2014) Crossing the frontier: a hypothesis for the origins of meristic constraint in mammalian axial patterning. Zoology 117: 64-69.
- Schilling N, Carrier DR (2010) Function of the epaxial muscles in walking, trotting, and galloping dogs: Implications for the evolution of epaxial muscle funtion in tetrapods. J Exp Biol 213: 1490-1502.
- Schilling N (2009) Metabolic profile of the perivertebral muscles of small therian mammals: Implications for the evolution of the mammalian trunk musculature. Zoology 112: 279-304.
- Rhalmi S, Yahia LH, Newman N, Isler M (1993) Immunohistochemical study of nerves in lumbar spine ligaments. Spine 18: 264-267.
- Yahia L, Newman N (1993) A scanning electron microscopic and immunohistochemical study of spinal ligaments innervation. Ann Anat 175: 111-114.
- Kiter E, Karaboyun T, Tufan AC, Acar K (2010) Immunohistochemical demonstration of nerve endings in iliolumbar ligament. Spine 35: 101-104.
- Filler AG (2007) Emergence and optimization of upright posture among hominiform hominoids and the evolutionary pathophysiology of back pain. Neurosurg Focus 23: 1-6.
- Yang K, King A (1984) 1984 Volvo award in biomechanics: mechanism of facet load transmission as a hypothesis for low-back pain. Spine 9: 557.
- Lorenz M, Patwardhan A, Vanderby R (1983) Load-bearing characteristics of lumbar facets in normal and surgically altered spinal segments. Spine 8: 122.
- Adams MA, Hutton WC (1980) The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J. Bone Jt Surg Br 62: 358-362.
- Dreischarf M, Rohlmann A, Bergmann G, Zander T (2011) Optimised loads for the simulation of axial rotation in the lumbar spine. J Biomech 44: 2323-2327.
- Rohlmann A, Zander T, Rao M, Bergmann G (2009) Applying a follower load delivers realistic results for simulating standing. J Biomech 42: 1520-1526.
- Wilke HJ, Rohlmann A, Neller S, Graichen F, Claes L, et al. (2003) ISSLS prize winner: A novel approach to determine trunk muscle forces during flexion and extension: A comparison of data from an In Vitro experiment and In Vivo measurements. Spine 28: 2585-2593.
- Slobounov SM, Newell KM (1996) Postural dynamics in upright and inverted stances. Journal of Applied Biomechanics 12: 185-196.
- Wikstrom EA, Tillman MD, Smith AN, Borsa PA (2005) A new force-plate technology measure of dynamic postural stability: The dynamic postural stability index. Journal of Athletic Training 40: 305-309.
- Cotoros D, Baritz M (2010) Biomechanical analyzes of human body stability and equilibrium. Paper presented at the Proceedings of the World Congress on Engineering, London, UK.
- Faries MD, Greenwood M (2007) Core training: stabilizing the confusion. Strength Cond J29: 10-25.
- Panjabi MM (1992) The stabilizing system of the spine: Part I function, dysfunction, adaptation, and enhancement. J Spinal Disord 5: 383-389.
- Panjabi MM (1992) The stabilizing system of the spine: Part II Neutral zone and instability hypothesis. J. Spinal Disord 5: 390-396.
- Buchanan TS, Rovai GP, Rymer WZ (1989) Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol 62: 1201-1212.
- Hoffman DS, Strick PL (1999) Step-tracking movements of the wrist. IV. Muscle activity associated with movements in different directions. J Neurophysiol 81: 319-333.
- Zuylen EJ, Gielen CC, Gon JJDV (1988) Coordination and inhomogeneous activation of human arm muscles during isometric torques. J Neurophysiol 60: 1523-1548.
- Farshadmanesh F, Byrne P, Keith GP, Wang H, Corneil BD, et al. (2012) Cross-validated models of the relationships between neck muscle electromyography and three-dimensional head kinematics during gaze behavior. J Neurophysiol 107: 573-590.
- Farshadmanesh F, Byrne P, Wang H, Corneil BD, Crawford JD (2012) Relationships between neck muscle electromyography and three-dimensional head kinematics during centrally induced torsional head perturbations. J Neurophysiol 108: 2867-2883.
- Ackland DC, Merritt JS, Pandy MG (2011) Moment arms of the human neck muscles in flexion, bending and rotation. J Biomech 44: 475-486.
- Howell AB (1944) Speed in animals. Univ Chicago Press, Chicago 1: 270.
- Hildebrand M (1959) Motions of the running cheetah and horse. J Mamm 40: 481-495.
- Gambaryan PP (194) How mammals run. John Wiley and Sons, New York 1: 367.
- Schilling N, Hackert R (2006) Sagittal spine movements of small therian mammals during asymmetrical gaits. J Exp Biol 209: 3925-3939.
- Fischer MS (1988) Die Lokomotion von Procavia capensis (Mammalia, Hyracoidea): Zur Evolution des Bewegungs systems bei Säugetieren. Abh Naturwiss Verein 33: 1-188.
- Jones K, Angielczyk K, Polly P, Head JJ, Fernandez V, et al. (2018) Fossils reveal the complex evolutionary history of the mammalian regionalized spine. Science 361: 1249- 1252.
- Goldthwait JE (1940) The rib joints. N Engl J Med 223: 568-573.
- Koob TJ, Long JH (2000) The Vertebrate Body Axis: Evolution and Mechanical Function. Integ Comp Biol 40: 1-18.
- Santonja-Medina F, Collazo-Diéguez M, Martínez-Romero MT, Rodriguez-Ferran O, Aparicio-Sarmiento A, et al. (2020) Classification System of the Sagittal Integral Morphotype in Children from the ISQUIOS Programme (Spain). J. Environ. Res. Public Health 17: 2467.
- Rockwell H, Evans FG, Pheasant HC (2005) The comparative morphology of the vertebrate spinal column. Its form as related to function. J Morph 63:87-117.
- Slijper EJ (1946) Comparative biological-anatomical investigations on the vertebral column and spinal musculature of mammals. Verh Kon AkadWetenschappen Amsterdam 1946, 45: 1-128.
- Washburn SL, Buettner-Janusch J (1952) The definition of thoracic and lumbar vertebrae. Am J Phys Anthrop 10: 251-252.
- Masharawi Y, Rothschild B, Dar G, Peleg S, Robinson D, Been E, Hershkovitz I. Facet orientation in the thoracolumbar spine: Three-dimensional anatomic and biomechanical analysis. Spine (Phila Pa 1976) 15: 29: 1755-163.
- Lucas SR, Bass CR, Salzar RS, Oyen ML, Planchak C et al, (2008) Viscoelastic properties of the cervical spinal ligaments under fast strain-rate deformations. Acta Biomater 4: 117-125.
- Mattucci SF, Moulton JA, Chandrashekar N, Cronin DS (2012) Strain rate dependent properties of younger human cervical spine ligaments. J. Mech. Behav. Biomed. Mater 10: 216-226.
- Nashner LM, McCollum G (1985) The organization of human postural movements: A formal basis and experimental analysis. The Behavioural and Brain Sciences 6: 135-172.
- Horak FB (2006) Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age and Ageing 35: 7-11.
- Blaszczyk JW, Michalski A (2006) Ageing and postural stability. Studies in Physical Culture and Tourism 13: 11-14.
- Yu ECL (2010) Redescription of Zang Kidney model-Anatomico-functional Tie. J. Chin. Med 22: 19.

Citation: Yu ECL, Wong K (2022) Mouldability of the Body Core in Adaptive Form. J Altern Complement Integr Med 8: 222.
Copyright: © 2022 Edwin Chau Leung Yu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

