
MR Imaging of Abdominal Pain in Pregnant Women: A Review of Common Obstetric and Non-Obstetric Pathologies
*Corresponding Author(s):
Lucia ManganaroDepartment Of Radiological, Oncological And Pathological Sciences, Umberto I Hospital, “Sapienza” University Of Rome, Vle Regina Elena, 324 00161 Rome, Italy
Tel:+39 3338151295,
Email:lucia.manganaro@uniroma1.it
Abstract
Acute abdominal pain in pregnancy is a common presenting symptom in the emergency setting, often requiring a quick imaging diagnosis to exclude etiologies associated with high maternal and fetal morbidity/mortality and guide proper patient management. Although US is the first-line imaging modality, many of the common causes of acute abdominal pain in pregnancy are not readily diagnosed sonographically. The current ACR guidelines asserts the general safety of MR imaging in pregnant women, but its use should be limited to cases of inconclusive Ultrasound (US) findings or unclear differential diagnosis. With the aim to discuss the most common causes of abdominal pain in pregnant women and the role of MR imaging in an emergency setting, a structured search using PubMed database was performed including all relevant original articles and reviews published during 2010-2022. In general, studies show that MRI of pregnant women with abdominal pain is increasingly used in emergency setting as a useful diagnostic tool because its safety profile and greater diagnostic accuracy than US. As a result of the rapid technical development of ultrafast sequences, acquisition times have been significantly reduced making the use of MR more feasible. Finally, MRI represents a valuable problem-solving tool and can also improve patient care and reduce the number of unnecessary surgical procedures.
Keywords
Abdominal pain; Magnetic resonance imaging; Obstetric emergency; Pregnancy
Introduction
Abdominal pain in pregnancy is a common presenting symptom in the emergency setting. Several causes of abdominal pain can occur in pregnant women including gynecologic, gastrointestinal, hepatobiliary, and genitourinary pathologies. During pregnancy, an early diagnosis of the underlying cause of abdominal pain is more difficult because of several confounding factors related to normal changes occurring during gestation such as nonspecific leukocytosis, displacement of abdominal and pelvic structures from their normal locations by the enlarged gravid uterus and a difficult abdominal examination. In addition, clinical symptoms such as nausea and vomiting, are vague and nonspecific. Conditions causing abdominal pain can range from self-limiting diseases to severe complications associated with maternal and fetal morbidity/mortality requiring prompt surgical intervention. Although Ultrasound (US) remains the first-line imaging modality, Magnetic Resonance Imaging (MRI) is gaining favor in the emergent setting due to its increased diagnostic accuracy, relatively quick acquisition time and excellent safety profile. This review discusses the role of MR imaging in evaluating the common obstetric and non-obstetric causes of acute abdominal pain in pregnant women.
Search Criteria and Study Selection
Based on the above assumptions, a structured search using PubMed database was performed from December 2021 and included all relevant original articles and reviews, published in and after 2010. The search used the following key word combinations: [(MRI) OR (Magnetic Resonance Imaging)] AND [(OBSTETRIC) OR (PREGNANCY) OR (PREGNANT)] AND [(EMERGENCY) OR (URGENCY)] (n=850), also combined, depending on the specific domain of interest, with [(APPENDICITIS)] (n:135); [(CROHN)] AND [(Inflammatory bowel disease) OR (IBD)] (n:17); [(OVARIAN) OR (OVARY) OR (ADNEXAL) OR (ADNEXA)] AND [(TORSION)] (n:104); [(PYELONEPHRITIS)] (n:16); [(NEPHROLITHIASIS)] (n:13); [(CHOLECYSTITIS)] (n:19); [(PANCREATITIS)] (n:118); [(Uterine rupture)] (n:26); [(ECTOPIC)] (n:332). All papers published on human subjects were included. Citations and references of the retrieved studies were used as additional sources. These were then evaluated for their content and relevance to this review article; editorial comments, conference abstracts and short communications were excluded. Studies concerning MRI technical development were included. Ultimately, 137 articles were deemed relevant and used as the literature basis of this review. With reference to these papers data, the purpose of this review is to summarize the most common pathological causes of abdominal pain in pregnant women, discussing the role and support of MR imaging in an emergency setting.
MR Imaging -Safety and Protocol
Magnetic Resonance Imaging has proven to be an excellent multiplanar imaging technique in the pregnant patient with acute abdominal and pelvic pain, because of the absence of ionizing radiation exposure and excellent imaging quality for soft tissue contrast. To date, there are no specific contraindications to the use of MRI in pregnant patients. The American College of Radiology (ACR) guidelines asserts that MR examination of the pregnant women is generally safe to both fetus and mother, in any stage of pregnancy. Nevertheless, the use of MRI should be limited to cases of inconclusive Ultrasound (US) findings or unclear differential diagnosis, always evaluating the potential risk against the benefit of MRI in the pregnant patient [1]. Potential negative effects of MRI result from heating of the fetus and amniotic fluid due to the use of radiofrequency pulses. However, Specific Absorption Rate (SAR, a measure of the Radio Frequency - RF- energy deposition, defined as power absorbed per unit mass of the tissue, expressed in W/kg) is typically monitored and kept below the threshold necessary increase fetal temperature to potentially teratogenic levels. Studies have not found harmful effects on pregnant women or the fetus: a recent study by Ray JG et al., compared the effects of MRI exposure in pregnancy with no exposure and noted no increase in harm to the fetus or in early childhood [2]. In addition, recent research has shown that also 3T-scan can be performed safely using a very low SAR [3]. The MRI protocol for acute abdominal pain during pregnancy involves the use of sequences that minimize motion artifacts due to respiration and fetal movements. Examination time should be optimized and minimized retaining diagnostic accuracy. Most abbreviated MRI protocols use a combination Single-Shot Fast-spin echo (SSFSE/HASTE [half-Fourier acquisition single-shot turbo-spin echo]) and T2-weighted (T2W) fat-saturated sequences in all three planes, along with axial gradient-echo T1-weighted in-phase and out-of-phase sequences, without IV contrast, patient preparation, or antiperistaltic agents shortens the examination duration [4]. The need for additional sequences can be decided based on the real-time imaging results. For example, Diffusion-Weighted Sequences (DWI) may be needed to diagnose inflammatory conditions such as appendicitis or crohn’s disease [5]. Gadolinium-based contrast agents in pregnancy is considered a category C drug, with teratogenic effects having been demonstrated in animals without any definite effects on human fetuses [6]. According to current ACR guidelines, Intravenous (IV) gadolinium should not be routinely used in pregnancy, as gadolinium-based contrast agents have been shown to cross the placental barrier with theoretic adverse effect on fetal development [1,7]. The decision to administer gadolinium IV in pregnancy should be considered only in very select situations and only after carefully balancing the risk and potential benefit.
Non-Obstetric Pathologies
Appendicitis
Acute appendicitis is the most common non-obstetric surgical disease during pregnancy. The incidence is estimated at 1 in 500 to 1 in 635 pregnancies per year [8-10]. The clinical diagnosis of appendicitis is more difficult during pregnancy for several reasons: the appendix is often displaced from the right lower quadrant due to the enlarged gravid uterus, and mild leukocytosis can be physiologic during pregnancy. Early identification and operative management are critical in pregnant women because of a higher rate of perforation and associated complications, which can also result in premature labor, fetal morbidity, and mortality [9,11,12]. In addition, incorrect preoperative diagnosis results in unnecessary operations [13]. According to American College of Radiology (ACR) criteria, Ultrasound (US) remains the first-line imaging modality in cases of suspected appendicitis during pregnancy [14]. However, it is operator dependent and often has limited diagnostic accuracy because of the difficulty in visualizing the appendix due to the upward displacement of pelvic structures by the gravid uterus. In this context, the use of MRI is the preferred imaging modality for the diagnosis of indeterminate cases of appendicitis, allowing better visualization of the appendix than US [15-18]. Several studies have confirmed the excellent diagnostic performance of MRI proposing it as first-line imaging technique, especially when US findings are equivocal [19-25]. A meta-analysis study reported that the sensitivity and specificity of MRI for the diagnosis of acute appendicitis were 96% (95% CI 95%-97%) and 96% (95% CI 95%- 97%), respectively. In a subgroup of pregnant patients, the sensitivity and specificity of MRI were 94% (95% CI 87%-98%) and 97% (95% CI 96%-98%), respectively [26]. A large study cohort on 204 pregnant patients with suspected appendicitis confirms MRI to be of high diagnostic value in the workup of acute appendicitis with 100% Negative Predictive Value (NPV) and sensitivity and 99.5% specificity [27]. Last not least, MRI significantly reduces the rate of unnecessary operations and surgery overall in pregnant women [20,28]. Studies showed that the routine use of MR imaging into the clinical workup for suspicion of appendicitis in pregnant patients was associated with a decrease in the Negative Laparotomy Rates (NLR) of 47% [29,30].
On MRI, acute appendicitis appears as an increased size appendix filled with fluid, 7 mm or more in diameter, and associated peri-appendicular inflammation. Other typical findings include wall thickening (>2 mm), hyperintense T2 appearance of the appendix lumen due to fluid or edema, and absence of blooming. Fat-suppressed T2-weighted and Diffusion-Weighted Imaging (DWI) are useful sequences to diagnose inflammation of the appendix and peri-appendicular fat. An appendicolith appears hypointense on all sequences (Figure 1). Associated abscess formation would appear as a well-defined T2 hyperintense fluid collection [7,9-11,31]. Colonic stool potentially be used as a natural positive contrast agent as it is characterized by relatively high signal on T1-Weighted Imaging (T1WI). The “T1 bright appendix sign”, defined as an area of high linear signal intensity on T1WI filling the appendix, has been proposed as a specific finding useful for the detection of appendices and the diagnosis of a normal appendix in pregnant women with suspected appendicitis [32,33]. With the aim of optimizing the MRI protocol, it was observed that the addition of coronal and sagittal SSH-TSE T2WI to the MR protocol did not improve the accuracy for the diagnosis of acute appendicitis in pregnant women, although its visualization could be improved by adding coronal images. From these results, if the radiologist can diagnose appendicitis on axial T2WI, coronal and sagittal images acquisition may be omitted, resulting in reduced scan time [34].
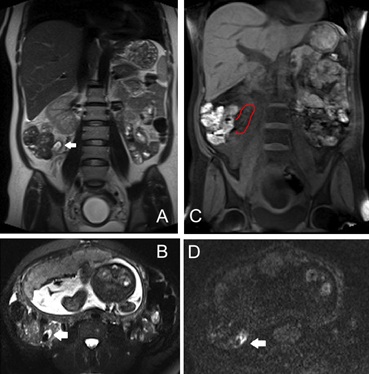 Figure 1: Coronal T2WI (A), axial fat suppressed T2WI (T2 FS; B) and coronal T1WI FS (C) show a thickened and dilated tubular appendix with intraluminal fluid and appendicolith (white arrows; red line) and mild peri-appendiceal T2-hyperintense signal consistent with inflammation. Inflammation is also visible on DWI image (D).
Figure 1: Coronal T2WI (A), axial fat suppressed T2WI (T2 FS; B) and coronal T1WI FS (C) show a thickened and dilated tubular appendix with intraluminal fluid and appendicolith (white arrows; red line) and mild peri-appendiceal T2-hyperintense signal consistent with inflammation. Inflammation is also visible on DWI image (D).
Inflammatory Bowel Disease (IBD) and Bowel obstruction
The bowel disorders causing acute abdominal pain most found in pregnancy are Inflammatory Bowel Disease (IBD) and bowel obstruction. The typical age of onset for IBD, with special regard to Crohn’s disease, is 15-30 years, therefore affecting young women during their childbearing period. Most pregnant women with IBD are diagnosed before pregnancy. There are no data to suggest that Crohn’s Disease (CD) is worse during pregnancy, and severe exacerbations of CD requiring acute surgery are rare. Literature also reports rare cases of Ulcerative Recto-Colitis (RU) complicated by toxic megacolon in pregnancy [35] (Figure 2). Surgical indications for a pregnant patient are similar with non-pregnant, and include hemorrhage, perforation, obstruction and abscess. The presence of active inflammatory also increase the risk of preterm delivery and decreased neonatal weight. Inflammatory bowel diseases must be differentiated from other acute abdominal diseases in pregnancy and the diagnosis may often be delayed, especially if the patient has never been previously diagnosed with CD [36,37]. In cases strongly suspected for new-onset CD or an exacerbation of established CD, an accurate anatomical information and evaluation of CD complications is mandatory. Moreover, imaging may be valuable in planning the mode of delivery, as cesarean section is usually recommended in patients with active perianal disease [38]. The terminal ileum is the most affected portion of the gastrointestinal tract: mural thickening, high signal intensity in the wall on T2-weighted images, and edema in the perivisceral fatty tissue is typical findings but may also be in appendicitis or ileitis (Figure 3). The presence of perianal disease including abscesses, fistulas, intestinal stenosis, is a more specific diagnostic finding as well as a clue to cesarean delivery [5,11,39].
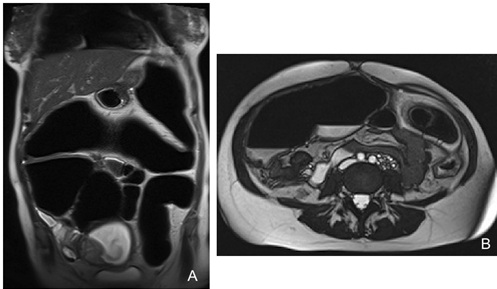 Figure 2: Pregnant patient at 16 Weeks of Gestation (GW) with acute abdominal pain and a history of ulcerative recto-colitis complicated by toxic megacolon .Coronal (A) and axial (B) T2-weighted images show marked gas distension of the colon with endoluminal air-fluid levels.
Figure 2: Pregnant patient at 16 Weeks of Gestation (GW) with acute abdominal pain and a history of ulcerative recto-colitis complicated by toxic megacolon .Coronal (A) and axial (B) T2-weighted images show marked gas distension of the colon with endoluminal air-fluid levels.
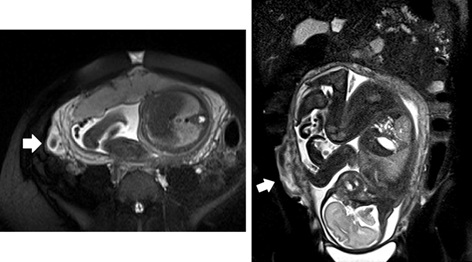 Figure 3: Crohn’s disease in pregnant women. Axial and coronal fat suppressed T2-weighetd images show mural thickening of the terminal ileum, T2-high signal intensity of the wall associated with hyperintensity of perivisceral fatty tissue due to inflammation (white arrows).
Figure 3: Crohn’s disease in pregnant women. Axial and coronal fat suppressed T2-weighetd images show mural thickening of the terminal ileum, T2-high signal intensity of the wall associated with hyperintensity of perivisceral fatty tissue due to inflammation (white arrows).
Bowel obstruction is another important cause of acute abdominal pain in pregnancy; when it caused by volvulus or internal hernia prompt surgical intervention is necessary to reduce maternal and fetal mortality and morbidity [40-42]. During pregnancy, the development of sigmoid volvulus can occur as result of a redundant or abnormally mobile sigmoid colon, displaced and led to twist around its point of fixation by the enlargement of the uterus. The high contrast resolution of MRI can detect typical findings suggesting the diagnosis of volvulus in pregnancy. The coffee bean sign is a common imaging finding typically encountered in sigmoid volvulus: it is represented by two closed and dilated apposed loops, recalling the appearance of a “coffee bean”. The split wall sign is another typical imaging finding due to the partial twisting of sigmoid loop: the intestinal walls are separated by adjacent mesenteric fat planes; a soft-tissue mass with a swirling internal architecture was identified, showing a whirl sign. Finally, MRI can be useful to characterize the etiology of obstruction and the need for surgery [43].
Adnexal Torsion
Adnexal torsion is a gynecologic surgical emergency caused by partial or complete rotation of the ovary and/or the Fallopian tube around its vascular axis. Pregnancy increases the risk of adnexal torsion because of laxity of ligamentous structures: up to 25% of cases of adnexal torsion occur in pregnant women with a higher incidence in early pregnancy (before 20 weeks of gestation), often related to the presence of a corpus luteum cyst or a mature cystic teratoma, which can give rise to torsion. Para-ovarian cysts account for only a small per- centage of adnexal torsions in pregnancy [44,45]. The incidence of ovarian torsion during pregnancy also increases in cases of ovarian hyperstimulation related to associated reproductive technology [46]. The lower incidence in late pregnancy may be explained by reduced mobility of the ovary because of the enlarged uterus [11,44,47]. Diagnosis of adnexal torsion is based on clinical symptoms and supported by imaging findings. Early diagnosis is crucial for pregnant women because treatment decisions based on the diagnosis are associated with a risk of damage to the reproductive organs by necrosis, early missed abortions, and life-threatening fetal events due to preterm labor if surgical treatment is not performed at the optimal time [48]. Currently, Ultrasonography (US) is the primary accepted imaging technique for adnexal torsion, but it is extremely limited for visualization of the ovaries during the second and third trimesters of pregnancy with the risk of delaying diagnosis and surgical management. MRI can provide diagnostic images with excellent soft-tissue resolution, and may be useful in cases when US results are inconclusive or nondiagnostic, especially in advance gestation [44,46,49]. On T2 sequences, MRI features of adnexal torsion include ovarian enlargement with edema, prominent peripheral follicles, thickened fallopian tube, twisted vascular pedicle, periovarian fat stranding and free fluid (Figure 4). On T1-weighted images, the signal intensity varies according to the age of intraparenchymal blood products. Late torsion demonstrates increased signal intensity on T2-weighted images owing to necrosis [45]. DWI better describes early ischemia, and the ADC signal intensity could predict the severity of hemorrhagic infarction in the twisted ovary. In Kato et al.’s series, the ADC signal intensity was significantly lower in patients with ovarian torsion with hemorrhagic infarction than in those without infarction [45,50]. Massive Ovarian Edema (MOE) is a rare condition characterized by marked unilateral (rarely bilateral) ovarian enlargement due to gross diffuse stromal edema. Its etiology is still poorly understood, but probably involves obstruction of venous and lymphatic drainage because of compression of ovarian vessels between the gravid uterus and pelvic sidewall or intermittent/partial torsion. At MR imaging, ovarian edema appears as ovarian enlargement, homogeneous low signal intensity on T1WI and high signal intensity on T2WI in the ovarian stroma, and peripheral displacement of ovarian follicles. On imaging, MOE may be indistinguishable from ovarian torsion, but a history of weeks to months of intermittent pelvic pain suggests ovarian edema [51,52].
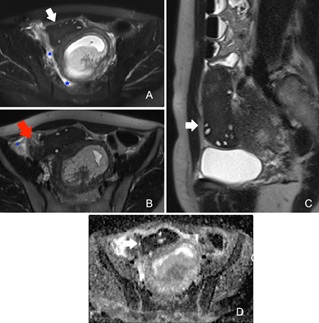 Figure 4: Adnexal torsion in pregnant patient. Axial T2-weighted images with and without fat suppression (A, B) and sagittal T2-WI (C) show enlargement of right ovary with peripheral follicles (white arrows), twisted vascular pedicle (red arrow), periovarian fat stranding and free fluid (blue stars). T2 and ADC signal intensity (D) of the ovarian parenchyma was significantly lower due to necrotic-hemorrhagic evolution.
Figure 4: Adnexal torsion in pregnant patient. Axial T2-weighted images with and without fat suppression (A, B) and sagittal T2-WI (C) show enlargement of right ovary with peripheral follicles (white arrows), twisted vascular pedicle (red arrow), periovarian fat stranding and free fluid (blue stars). T2 and ADC signal intensity (D) of the ovarian parenchyma was significantly lower due to necrotic-hemorrhagic evolution.
Nephrolithiasis and Pyelonephritis
Renal colic is one of the most common non-obstetric cause for abdominal-pain-related hospitalization during pregnancy [12,53]. Physiologic hydronephrosis is reported in up to 90% of pregnancies and is more often right sided. This physiologic dilation of the collecting system is due in part to progesterone-dependent smooth muscle relaxation and in part to compression of the ureter by the gravid uterus [11,13]. This physiologic hydronephrosis must be differentiated from an obstructing ureteral calculus: dilatation distal to the sacral promontory is much more suspicious for an obstructive process above, beyond the physiologic hydro-nephrosis of pregnancy. Flank or abdominal pain and microscopic hematuria are the most common clinical features; other non-specific symptoms include nausea and vomiting. Accurate diagnosis of obstructive hydronephrosis is important due to the increased risk of pre- mature labor and infection. Furthermore, the presence of ureteral obstruction may be further complicated by infection such as pyelonephritis [54].
Magnetic Resonance Urography (MRU) without contrast agents should be considered as a second-line modality during pregnancy when the use of US is inconclusive and there are ongoing symptoms despite conservative management [54,55]. MRU using heavily T2-weighted ‘water’ images with thick slabs is useful to detect the urinary system and the ureters, differentiating physiological urinary tract dilatation from stone-related obstruction. The biggest disadvantage of MRI is its limited ability to depict small calculi: nevertheless thin-slice, high-resolution, highly T2-weighted Fast Spin Echo (FSE) sequences can improve the ability of MRU for detection of small stones. Obstructing stone on MRU appear as a T2 hypointense filling defect with proximal ureteral dilation. Peri-renal and peri-ureteral edema are other MRI features that are indicative of pathologic hydronephrosis These changes are better appreciated on fat- suppressed images (Figure 5). The presence of the double kink sign (ureteral kinking at the pelvic brim with a column of urine seen down to the level of the ureterovesical junction) suggests an obstructing distal ureteral calculus [9,55,56]. When corroborated with ureteroscopy findings, the positive predictive value of MRI for diagnosis of stones among pregnant women is 80% [57]. MRI can also depict complications such as pyelonephritis. Acute pyelonephritis is among the leading causes for antepartum hospitalization, associated with increased risk of septicemia, low birth weight and preterm delivery [58]. On MR imaging, pyelonephritis appears as an edematous enlargement of the kidney, with parenchymal areas of heterogenous signal intensity (often linear and wedge-shaped) on T2WI and limited proton diffusion on DWI [59] (Figure 6). One limiting factor in pregnant patients is the inability to use gadolinium-based contrast agents, which would otherwise demonstrate the characteristic striated or wedge-shaped pattern of renal parenchymal enhancement. Renal and perirenal abscesses may also develop in complicated pyelonephritis, which are visualized as T2 hyperintense collections with restricted diffusion [60] (Figure 7). Studies have also shown that the DWI sequence provides information on renal function and is a viable alternative to dynamic contrast MRI or CT, relative to their general contraindication in pregnant or lactating women [61,62].
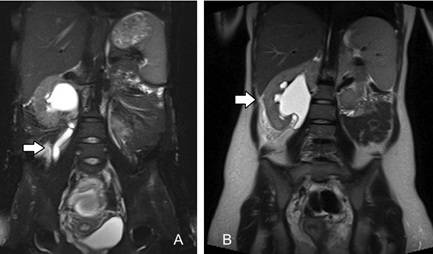 Figure 5: Acute uncomplicated pyelonephritis with hydronephrosis in pregnancy. Coronal Fat-Suppressed (FS) T2-weighted image(A) and T2-weighted image without FS (B) show severe hydroureteronephrosis associated with diffuse signal T2-hyperintensity of peri-renal and peri-ureteral soft tissue due to edema. Case contributed by Dr Maurizio Del Monte.
Figure 5: Acute uncomplicated pyelonephritis with hydronephrosis in pregnancy. Coronal Fat-Suppressed (FS) T2-weighted image(A) and T2-weighted image without FS (B) show severe hydroureteronephrosis associated with diffuse signal T2-hyperintensity of peri-renal and peri-ureteral soft tissue due to edema. Case contributed by Dr Maurizio Del Monte.
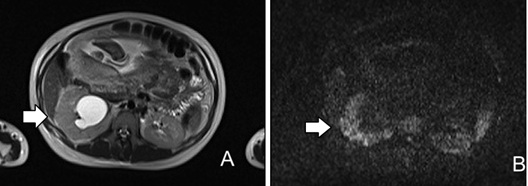 Figure 6: Pregnant woman with right side pain. Axial T2-weighted image (A) and axial Diffusion Weighted Image (DWI) (B) show mild parenchymal heterogeneity of the right kidney related to edema with striated appearance (A) and multiple wedge-shaped areas of restricted diffusion (B) consistent with acute pyelonephritis.
Figure 6: Pregnant woman with right side pain. Axial T2-weighted image (A) and axial Diffusion Weighted Image (DWI) (B) show mild parenchymal heterogeneity of the right kidney related to edema with striated appearance (A) and multiple wedge-shaped areas of restricted diffusion (B) consistent with acute pyelonephritis.
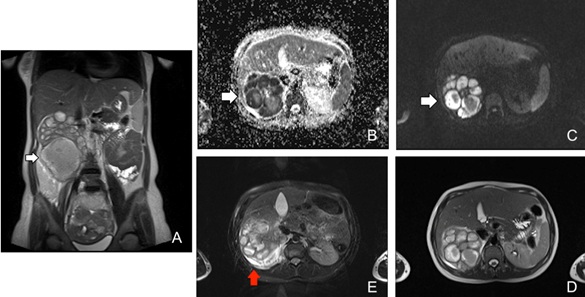 Figure 7: Pregnant woman affected by acute pyelonephritis complicated by renal abscesses (white arrows). Coronal T2-WI and axial T2-WI (A, D) show multiple renal fluid collections (white arrows) with restricted diffusion and low ADC value (B,C). Perirenal fat stranding and effusion are better showed on fat suppressed T2-WI (red arrow, E).
Figure 7: Pregnant woman affected by acute pyelonephritis complicated by renal abscesses (white arrows). Coronal T2-WI and axial T2-WI (A, D) show multiple renal fluid collections (white arrows) with restricted diffusion and low ADC value (B,C). Perirenal fat stranding and effusion are better showed on fat suppressed T2-WI (red arrow, E).
Acute Cholecystitis
Acute cholecystitis, usually a complication of cholelithiasis, is the second most common reason for non-obstetric surgery in pregnancy, due to an increased incidence of gall- stones in pregnant women9.US is the first line imaging technique. In equivocal cases, MR Cholangiopancreatography (MRCP) has a high sensitivity and specificity for detecting biliary disorders and associated complications [63]. Typical cholecystitis MRI findings include all bladder distension (>5 cm transverse diameter), edematous wall thickening (>3 mm) and pericholecystic free fluid like an increased signal intensity surrounding the gallbladder on T2WI. Associated obstructive stones can be visualized as a signal void in the cystic duct or gallbladder neck [64]. Biliary obstruction can result in acute cholangitis and biliary sepsis. Imaging findings in acute cholangitis include dilated intrahepatic/extra- hepatic biliary ducts, periductal edema (T2 hyperintense), intrahepatic abscesses and ascites (Figure 8).
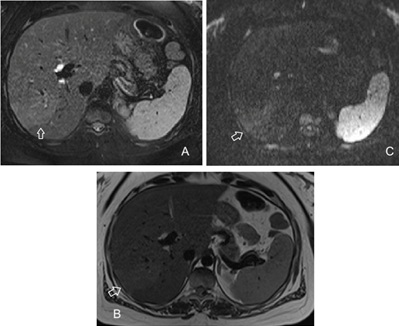 Figure 8: Pregnant woman with abdominal pain and vomiting and a history of choledocholithiasis. Axial T2-weighted image with and without fat suppression (A, B) show a mild periductal and intrahepatic edema (hyperintense) which corresponds to an area of slight diffusion restriction (C).
Figure 8: Pregnant woman with abdominal pain and vomiting and a history of choledocholithiasis. Axial T2-weighted image with and without fat suppression (A, B) show a mild periductal and intrahepatic edema (hyperintense) which corresponds to an area of slight diffusion restriction (C).
Pancreatitis
Acute pancreatitis is a rare condition in pregnancy and it’s most often secondary to gallstones or sludge, followed by hyperlipidemias [65-67]. Previously, acute pancreatitis during pregnancy was a serious condition and the maternal mortality rate was high, but the mortality rate has recently decreased because diagnosis is reached earlier, and maternal and neonatal intensive care have improved. More than 50% of cases occur in the third trimester [68-72]. The clinical presentation is like that in non-pregnant women, although pregnancy status may limit the diagnostic and surgical options for management. The management is primarily conservative, but surgery is indicated in severe disease [73,74]. Epigastric pain, nausea, vomiting, anorexia, and elevated lipase are common symptoms. Focal or diffuse enlargement and increased T2-signal of the pancreas, peripancreatic fat inflammation and fluid collections, are typical MRI findings of acute pancreatitis (Figure 9). Sequelae of acute pancreatitis such as abscess formation or pancreatic necrosis are important causes of morbidity and mortality to both mother and fetus [9,11,75].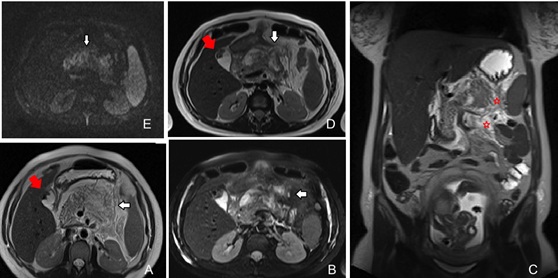 Figure 9: A 27 gestational weeks pregnant patient with epigastric pain and hypertriglyceridemia. MR imaging findings show an interstitial edematous pancreatitis. Axial and coronal T2-weighted images with and without fat suppression (A, B, C, D) demonstrate a diffuse pancreatic edema (T2-hyperintense signal) with peripancreatic fluid and restricted diffusion (E) due to acute inflammation (white arrow and red stars); cholelithiasis (red arrow).
Figure 9: A 27 gestational weeks pregnant patient with epigastric pain and hypertriglyceridemia. MR imaging findings show an interstitial edematous pancreatitis. Axial and coronal T2-weighted images with and without fat suppression (A, B, C, D) demonstrate a diffuse pancreatic edema (T2-hyperintense signal) with peripancreatic fluid and restricted diffusion (E) due to acute inflammation (white arrow and red stars); cholelithiasis (red arrow).
Obstetric Pathologies
Uterine rupture
Uterine Rupture (UR) is a rare complication of pregnancy associated with significant maternal and fetal mortality. Typically, UR occurs in the third trimester of pregnancy and is rarely seen in the first and second trimesters [76]. The risk of uterine rupture in pregnancy is increased in patient with a history of previous uterine surgery, with rupture occurring at the site of scar: cesarean section, myomectomy, adeno-myomectomy, hysteroscopic resection, surgical treatments for ectopic pregnancies, and curettage performed for premature interruption of pregnancy or retained placenta. Other risk factors have also been reported, such as multiple gestation, polyhydramnios, abnormal placentation and induction of labor [77-79].
As above mentioned, one of the most important risk factors is a previous history of myomectomy. According to the large clinical trials, case reports, and review articles, the incidence of uterine rupture during pregnancy after Laparoscopic Myomectomy (LM) is estimated to be less than 1% when the surgical procedures are performed appropriately [80-83]. Tian et al., reported a post-myomectomy uterine scar dehiscence rate of 4.9%, with no cases of UR; in contrast, Koo et al., reported 3 cases of uterine rupture in pregnancy among 523 patients undergoing laparoscopic myomectomy (0,6%) [84,85]. Spontaneous rupture of unscarred gravid uterus is an extremely rare occurrence. In this case, it has been reported that the fundus and the uterine cornual area are the most common rupture sites. Some studies also suggest a potential correlation with a preexisting Mullerian duct anomalies associated with a focal weakness of the uterine cornual myometrium [86-88]. Clinically, uterine rupture can present as abdominal pain, vaginal bleeding and alteration in uterine contractions. Worst-case scenario, pregnant women may present with hypotension, shock, hematuria, and abrupt fetal distress with fetal heart rate abnormalities [89]. Prompt and accurate diagnosis is essential to limit morbidity and mortality. Although it remains the first-line imaging modality, ultrasonography may have great difficulty in identifying myometrial flap. The US diagnosis of uterine rupture mainly relies on secondary signs such as presence of free fluid, intraperitoneal or extraperitoneal hematoma, intrauterine blood, and empty uterus. At the same time, uterine rupture is a real surgical emergency and MRI should be considered only when the patient is hemodynamically stable. MRI provides a wider field of view to rule out other causes of abdominal pain in the pregnant woman or identify any other cause of increased incidence of rupture, such as congenital anomalies or fetal macrosomia. On MRI, uterine rupture appears as a focal defect of the myometrium that may be associated with intramural hematoma and hemoperitoneum. Other features include fetal parts in an extrauterine location, intra-amniotic hemorrhage, and focal bulging of membranes through the site of dehiscence (Figure 10).
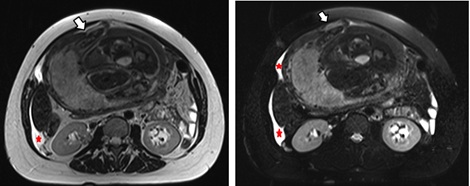 Figure 10: A 36 gestational weeks pregnant woman with sudden onset acute abdominal pain and inconclusive US findings. No significant history of abdominal surgery. Axial T2-weighted images show a focal defect of the anterior aspect of myometrial wall with fetus hand outside the uterus (white arrows). Significant abdominal effusion is also present (red stars).Case contributed by Dr Maurizio Del Monte.
Figure 10: A 36 gestational weeks pregnant woman with sudden onset acute abdominal pain and inconclusive US findings. No significant history of abdominal surgery. Axial T2-weighted images show a focal defect of the anterior aspect of myometrial wall with fetus hand outside the uterus (white arrows). Significant abdominal effusion is also present (red stars).Case contributed by Dr Maurizio Del Monte.
Ectopic pregnancy
Ectopic Pregnancy (EP), defined as the implantation and growth of a fertilized ovum outside the endometrial cavity, is the most common emergency that occurs in the first trimester of pregnancy, and usually results in pregnancy failure. In typical cases, ectopic pregnancy is easily diagnosed based on Clinical Features, Transvaginal Ultrasonography (TVUS) findings, combined with rapid assay for Human Chorionic Gonadotropin (hCG) levels: increased serum beta-hCG level and an empty uterus on TVUS are the most compelling early diagnostic clues. However, patient factors (such as: obesity, presence of bowel gas and pain) may limit the utility of ultrasonography. In this regard, MRI is generally reserved for equivocal cases and when further information of the ectopic pregnancy is required to guide clinical management. MRI is increasingly used in cases with unusually localized ectopic pregnancy, and as an additional imaging modality in complicated cases [76,90,91]. MRI also allows detection of fresh hemorrhage, specific localization of the abnormal implantation site with high spatial resolution, and identification of associated congenital uterine abnormalities [92,93]. Specific MRI findings that are diagnostic of EP include an empty uterine cavity, a cystic mass-like structure separate from the ovary and surrounded by low signal intensity acute hematoma on T2WI and hemoperitoneum.
The most common location for ectopic pregnancy is the fallopian tube, accounting for 97 % of the cases (50%-80% in the ampulla, 10%-25% in the isthmus, and 5%-17% in the fimbria) [94,95]. Most patients have no symptom during early pregnancy. However, symptoms such as lower abdominal pain develop as the concept us grows and vaginal or intra-abdominal bleeding occurs because of miscarriage or rupture. MRI shows an oval or round extrauterine Gestational Sac (GS) lateral to the uterus, with a cystic appearance and a typical thick trilaminarwall on T2WI. This “three rings” appearance is rarely observed in other adnexal masses and can be used as a key imaging feature. The thin outer hypointense ring is formed by the adjacent tubal wall, while the thin inner hypointense ring is formed by extraembryonic coelom and amnion without blood vessels. The thick middle T2-hyperintense ring is composed of chorionic villi tissue, which contains abundant fetal capillaries and maternal blood in the interstitium [94,95]. The gestational sac may contain solid components, fresh blood, or fluid-fluid level. Fresh or acute tubal hematoma occurs for the rupture of tubal intramural arterioles caused by trophoblastic invasion, and appears as T1 hyperintense, with a low signal on T2-weighted images [96-98]. Diffusion-weighted imaging has also been utilized in MR imaging of ectopic pregnancy. A “ring of restriction sign” can be seen in the wall of the gestational sac, due to the high cellularity and rate of proliferation of trophoblasts, vascular-fibroblastic proliferation, and macromolecules within the cytoplasm and extra-cellular matrix [99]. Fat-suppressed T1-weighted images can improve detection of hemorrhage [97]. Hemoperitoneum appears as mildly hyperintense on T1 images and hypointense on T2 images depending on the age of the blood products. Although hemoperitoneum in the pouch of Douglas in a pregnant woman has a 93% positive predictive value for EP, other causes of hemoperitoneum such as ruptured ovarian cysts, corpus luteum, placenta accreta and spontaneous abortion must be considered [96,100].
Contrast-enhanced MRI has also been reported to be useful for the diagnosis of EP because it demonstrates enhancement in the GS and the affected tubal wall. IV administration of contrast medium may help to detect tubal rupture as an interruption of tubal wall enhancement and the presence of acute hematoma, as shown by hemorrhage located outside the implantation site on T1 and T2-weighted images [98,101,102]. However, the contrast-enhanced MRI should be performed only when a normal intrauterine pregnancy is excluded by US and unenhanced MR sequences. Otherwise, gadolinium-based contrast agents should be avoided [94]. Magnetic resonance imaging is also useful in excluding potential mimics of EP. The classic differential diagnosis of the gestational sac is a corpus luteum cyst. On MRI, the distinction can be facilitated by focusing on wall signal intensity, location (intra-ovarian or extra-ovarian) and enhancement pattern. A typical corpus luteum appears as an intra-ovarian cyst-like structure with a thickened wall and homogeneous mural enhancement. The GS of an ectopic pregnancy it usually locates extra-ovarian, and the dot-like enhancement pattern and the “three rings” T2WI-appearance is specific for a GS. The finding of a dilated tubal wall is another key MRI finding suspicious for a tubal pregnancy [98].
Scar pregnancy
Cesarean Scar Pregnancy (CSP) is a rare form of ectopic pregnancy in which the embryo implantation occurs within the scar of a previous cesarean section; its incidence is increasing because of the worldwide increase in the number of cesarean deliveries. Regarding etiopathogenesis, it has been hypothesized that poor vascularization of the lower anterior uterine segment impairs healing after cesarean procedures, making this area vulnerable to formation of small defects into which a trophoblast can implant. Multiple cesarean sections are associated with larger areas of scarring, which may in turn increase the risk of a scar implantation [103,104]. CSP is considered a potentially life-threatening condition and its delayed diagnosis may result in complications, such as uterine rupture and hemorrhage with significant maternal morbidity and potential hysterectomy. As an alternative to surgical treatment via laparotomy and hysterotomy, nonsurgical conservative therapy options include systemic and local methotrexate administration, which were reportedly quite successful [105].
The first-line diagnostic tool for CSP remains TVUS [106,107]. However, the use of magnetic resonance imaging after initial ultrasound may provide additional information useful for directing patient management and therapy (conservative versus surgical treatment). MRI provides a more detailed anatomic assessment to minimize surgical invasion as much as possible [108,109]. In the further evaluation of CSS, MRI generally has a higher degree of accuracy than US and can identify the CSS, decidual layer, and myometrium separately; in contrast, these three tissues are often not so distinct in the US images. The T2WI sagittal section is the best view for the CSS, the GS and the decidual layer of a CSP. The CSS defect mainly showed the typical signal hypointensity of fibrous tissue [110,111], and most GSs present as a normal early pregnancy [112]. Most CSPs present as a thin-walled diverticulum at the CSS defect, and the CSS defect becomes weaker with the growth of the GS. CSPs were categorized into three types, based on the features of the CSS and the growth pattern of the GS: a thin-walled diverticulum at the CSS defect with a GS fully embedded (type I) or partially embedded in the diverticulum and partially growing into the uterine cavity (type II); a niche is visible in the CSS defect and the GS is mainly embedded in the isthmus (type III) [111] (Figure 9). The contrast enhancement could be helpful in identifying the silhouette of the GS and the content of the GS when they are confounded by the surrounding hemorrhage, even if not strictly necessary for the evaluation of all CSPs [101] (Figures 1 and 12).
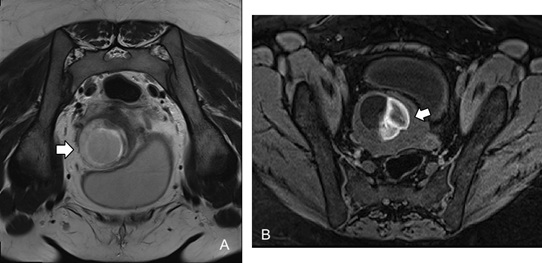 Figure 11: Cesarean scar pregnancy. Axial T2-weighted image (A) shows a gestational sac with a cystic appearance and a severe myometrial thinning (white arrow); fat-suppressed T1-weighted image (B) shows hemorrhage (white arrow).
Figure 11: Cesarean scar pregnancy. Axial T2-weighted image (A) shows a gestational sac with a cystic appearance and a severe myometrial thinning (white arrow); fat-suppressed T1-weighted image (B) shows hemorrhage (white arrow).
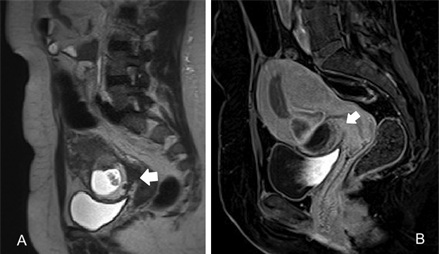 Figure 12: Cesarean scar pregnancy. Sagittal T2-weighted image (A) shows a gestational sac containing the small fetus (cystic mass-like) within a cesarean delivery scar (typical T2-hypointensity of fibrous tissue; white arrow). The contrast enhancement (B) demarcates the silhouette of the gestational sac.
Figure 12: Cesarean scar pregnancy. Sagittal T2-weighted image (A) shows a gestational sac containing the small fetus (cystic mass-like) within a cesarean delivery scar (typical T2-hypointensity of fibrous tissue; white arrow). The contrast enhancement (B) demarcates the silhouette of the gestational sac.
Placenta accreta spectrum
Placenta Accreta Spectrum (PAS) disorders occur when the placenta adheres abnormally to the uterine myometrium. PAS includes three degrees of myometrial trophoblastic invasion: accreta, where placental villi show robust attachment but no invasion of the myometrium; increta, where villi invade the myometrium but not through the entire myometrial depth; percreta, where villous invasion crosses the entire myometrial thickness extending to adjacent organs. Main risk factors include abnormal sites of placental implantation (such as placenta previa) and previous cesarean delivery [113,114]. Pregnancies with invasive placentas are more likely to develop potentially fatal massive hemorrhage because of damage to the myometrial circulation from infiltrative phenomena, often requiring emergency hysterectomy [115,116]. Radical hysterectomy is universally accepted as treatment; up to 40–60% of the peripartum hysterectomies are due to invasive placenta [117]. However, the emergence of new techniques such as the uterine artery angioembolization approach, and the use of chemotherapy agents (methotrexate), are alternative therapies also described in the literature [118]. It’s therefore evident that prenatal diagnosis of invasive placenta plays an essential role in the delivery planning. In recent years, although Ultrasound (US) still represents the first-line examination for the prenatal diagnosis, MRI has been increasingly used in the evaluation of placental invasiveness [119-121]. Thanks to the high resolution of soft tissue contrast and the wide field of view, MRI can provide more accurate topographic and morphologic information regarding the placenta and clarify the degree of invasion optimizing the planning of surgical management [122]. Several studies identified some distinctive MR signs of placental invasion, such as the presence of T2-dark intraplacental bands, myometrial thinning, focal disruption of placental-myometrial interface, heterogeneous placental signal intensity, abnormal placental bulging, abnormal placental vascularity, and percretism signs (direct invasion of adjacent organs) [104,123-130] (Figure 13). A correlation between these MR signs and maternal outcome should there be [131,132]. Chen et al., [129] showed that dark band, percretism signs, and placental protrusion signs were more frequently observed in patients with poor clinical outcome (massive/minor bleeding and hysterectomy), while Delli Pizzi et al., reported percretism signs and myometrial thinning as two most predictive MR features of poor outcome [133]. Other recent studies have focused attention on new quantitative MRI techniques and their potential to improve diagnostic rates and increase the understanding of PAS-pathophysiology by revealing more details of the aberrant placental hemodynamics [134,135]. In this regard, the study by Rachel L. et al showed that the IVIM parameter of Perfusion Fraction (Pf) was significantly higher in pregnant women with PAS than in the healthy control group, given the larger, more proliferative vasculature and increased regional blood flow in the abnormally adherent placenta [136].
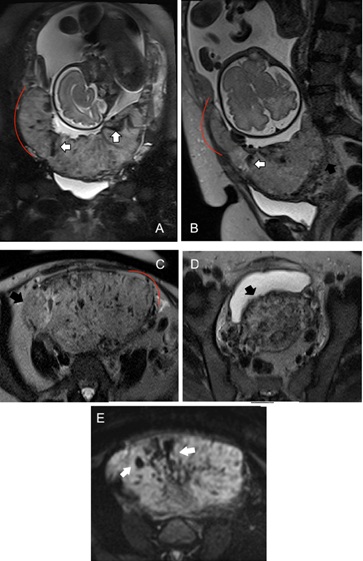 Figure 13: Pregnant patient at 31 weeks of gestation with placenta previa and suspected US of placenta accreta disease. On MRI, Coronal, axial and sagittal T2-weighted images (A, B, C) show T2-dark intraplacental bands (white arrows), focal areas of placental bulging and severe myometrial thinning with disruption of placental-myometrial interface (red lines). Axial T2-weighted images (C, D) show percretism signs with invasion of the right parameters and posterior bladder wall (black arrows). The heterogeneous signal intensity and abnormal placental vascularity are also showed on DW image (E).
Figure 13: Pregnant patient at 31 weeks of gestation with placenta previa and suspected US of placenta accreta disease. On MRI, Coronal, axial and sagittal T2-weighted images (A, B, C) show T2-dark intraplacental bands (white arrows), focal areas of placental bulging and severe myometrial thinning with disruption of placental-myometrial interface (red lines). Axial T2-weighted images (C, D) show percretism signs with invasion of the right parameters and posterior bladder wall (black arrows). The heterogeneous signal intensity and abnormal placental vascularity are also showed on DW image (E).
Conclusion
In conclusion, MRI is a useful imaging modality for the evaluation and differential diagnosis of several pathologic conditions that may affect pregnant patients, especially when US findings are inconclusive and equivocal. MRI is increasingly used in the emergency setting because its safety profile and greater diagnostic capability than US, due to its high tissue contrast resolution and wide field of view. We also believe that radiologists should be familiar with most common causes of abdominal pain in pregnancy and their MR imaging features.
References
- Expert Panel on MR Safety, Kanal E, Barkovich AJ, Bell C, Borgstede JP, et al. (2013) ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 37: 501-530.
- Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL (2016) Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 316: 952-961.
- Krishnamurthy U, Neelavalli J, Mody S, Yeo L, Jella PK, et al. (2015) MR imaging of the fetal brain at 1.5T and 3.0T field strengths: Comparing specific absorption rate (SAR) and image quality. J Perinat Med 43: 209-220.
- Islam GMN, Yadav T, Khera PS, Sureka B, Garg PK, et al. (2021) Abbreviated MRI in patients with suspected acute appendicitis in emergency: A prospective study. Abdom Radiol (NY) 46: 5114-5124.
- Horowitz JM, Hotalen IM, Miller ES, Barber EL, Shahabi S, et al. (2020) How Can Pelvic MRI with Diffusion-Weighted Imaging Help My Pregnant Patient? Am J Perinatol 37: 577-588.
- Katzberg RW, McGahan JP (2011) Science to Practice: Will Gadolinium-enhanced MR Imaging Be Useful in Assessment of At-Risk Pregnancies? Radiology 258: 325-326.
- Wieseler KM, Bhargava P, Kanal KM, Vaidya S, Stewart BK, et al. (2010) Imaging in Pregnant Patients: Examination Appropriateness. Radiographics 30: 1215-1229.
- Kave M, Parooie F, Salarzaei M (2019) Pregnancy and appendicitis: A systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg 14: 37.
- Wang PI, Chong ST, Kielar AZ, Kelly AM, Knoepp UD, et al. (2012) Imaging of Pregnant and Lactating Patients: Part 2, Evidence-Based Review and Recommendations. AJR Am J Roentgenol 198: 785-792.
- Beddy P, Keogan MT, Sala E, Griffin N (2010) Magnetic Resonance Imaging for the Evaluation of Acute Abdominal Pain in Pregnancy. Semin Ultrasound CT MR 31: 433-441.
- Spalluto LB, Woodfield CA, DeBenedectis CM, Lazarus E (2012) MR Imaging Evaluation of Abdominal Pain during Pregnancy: Appendicitis and Other Nonobstetric Causes. Radiographics 32: 317-334.
- Katz DS, Klein MA, Ganson G, Hines JJ (2012) Imaging of Abdominal Pain in Pregnancy. Radiol Clin North Am 50: 149-171.
- Prodromidou A, Machairas N, Kostakis ID, Molmenti E, Spartalis E, et al. (2018) Outcomes after open and laparoscopic appendectomy during pregnancy: A meta-analysis. Eur J Obstet Gynecol Reprod Biol 225: 40-50.
- Smith MP, Katz DS, Lalani T, Carucci LR, Cash BD, et al. (2015) ACR Appropriateness Criteria® Right Lower Quadrant Pain--Suspected Appendicitis. Ultrasound Q 31: 85-91.
- Ramalingam V, LeBedis C, Kelly JR, Uyeda J, Soto JA, et al. (2015) Evaluation of a sequential multi-modality imaging algorithm for the diagnosis of acute appendicitis in the pregnant female. Emerg Radiol 22: 125-132.
- Burke LM, Bashir MR, Miller FH, Siegelman ES, Brown M, et al. (2015) Magnetic resonance imaging of acute appendicitis in pregnancy: A 5-year multiinstitutional study. Am J Obstet Gynecol 213: 693.
- Ditkofsky NG, Singh A (2015) Challenges in Magnetic Resonance Imaging for Suspected Acute Appendicitis in Pregnant Patients. Curr Probl Diagn Radiol 44: 297-302.
- Abbasi N, Patenaude V, Abenhaim HA (2014) Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG 121: 1509-1514.
- Patel D, Fingard J, Winters S, Low G (2017) Clinical use of MRI for the evaluation of acute appendicitis during pregnancy. Abdom Radiol (NY) 42: 1857-1863.
- Burns M, Hague CJ, Vos P, Tiwari P, Wiseman SM (2017) Utility of Magnetic Resonance Imaging for the Diagnosis of Appendicitis during Pregnancy: A Canadian Experience. Can Assoc Radiol J 68: 392-400.
- Konrad J, Grand D, Lourenco A (2015) MRI: first-line imaging modality for pregnant patients with suspected appendicitis. Abdom Imaging 40: 3359-3364.
- Jung JY, Na JU, Han SK, Choi PC, Lee JH, et al. (2018) Differential diagnoses of magnetic resonance imaging for suspected acute appendicitis in pregnant patients. World J Emerg Med 9: 26-32.
- Cho SU, Oh SK (2021) Diagnostic accuracy of magnetic resonance imaging for acute appendicitis during pregnancy: A systematic review. Ulus Travma Acil Cerrahi Derg 27: 271-277.
- Al-Katib S, Sokhandon F, Farah M (2016) MRI for appendicitis in pregnancy: Is seeing believing? clinical outcomes in cases of appendix nonvisualization. Abdom Radiol (NY) 41: 2455-2459.
- Wi SA, Kim DJ, Cho ES, Kim KA (2018) Diagnostic performance of MRI for pregnant patients with clinically suspected appendicitis. Abdom Radiol (NY) 43: 3456-3461.
- Duke E, Kalb B, Arif-Tiwari H, Daye ZJ, Gilbertson-Dahdal D, et al. (2016) A Systematic Review and Meta-Analysis of Diagnostic Performance of MRI for Evaluation of Acute Appendicitis. AJR Am J Roentgenol 206: 508-517.
- Kereshi B, Lee KS, Siewert B, Mortele KJ (2018) Clinical utility of magnetic resonance imaging in the evaluation of pregnant females with suspected acute appendicitis. Abdom Radiol (NY) 43: 1446-1455.
- Lukenaite B, Luksaite-Lukste R, Mikalauskas S, Samuilis A, Strupas K, et al. (2021) Magnetic resonance imaging reduces the rate of unnecessary operations in pregnant patients with suspected acute appendicitis: A retrospective study. Ann Surg Treat Res 100: 40-46.
- Rapp EJ, Naim F, Kadivar K, Davarpanah A, Cornfeld D (2013) Integrating MR Imaging into the Clinical Workup of Pregnant Patients Suspected of Having Appendicitis Is Associated with a Lower Negative Laparotomy Rate: Single-Institution Study. Radiology 267: 137-144.
- Hansen W, Moshiri M, Paladin A, Lamba R, Katz DS, et al. (2017) Evolving Practice Patterns in Imaging Pregnant Patients With Acute Abdominal and Pelvic Conditions. Curr Probl Diagn Radiol 46: 10-16.
- Dewhurst C, Beddy P, Pedrosa I (2013) MRI evaluation of acute appendicitis in pregnancy. J Magn Reson Imaging 37: 566-575.
- Shin I, An C, Lim JS, Kim MJ, Chung YE (2017) T1 bright appendix sign to exclude acute appendicitis in pregnant women. Eur Radiol 27: 3310-3316.
- Jang KM, Kim SH, Choi D, Lee SJ, Rhim H, et al. (2011) The value of 3D T1-weighted gradient-echo MR imaging for evaluation of the appendix during pregnancy: Preliminary results. Acta Radiol 52: 825-828.
- Shin I, Chung YE, An C, Lee HS, Kim H, et al. (2018) Optimisation of the MR protocol in pregnant women with suspected acute appendicitis. Eur Radiol 28: 514-521.
- Brunelli R, Perrone S, Perrone G, Galoppi P, De Stefano MG, et al. (2019) New-onset ulcerative colitis in pregnancy associated to toxic megacolon and sudden fetal decompensation: Case report and literature review. J Obstet Gynaecol Res 45: 1215-1221.
- Burgers J, Ruiz O, Rivers J (2015) Perforated Crohn’s disease presenting during pregnancy. BMJ Case Rep 2015: bcr2015210109.
- Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, et al. (2012) Complications From Inflammatory Bowel Disease During Pregnancy and Delivery. Clin Gastroenterol Hepatol 10: 1246-1252.
- Stern MD, Kopylov U, Ben-Horin S, Apter S, Amitai MM (2014) Magnetic resonance enterography in pregnant women with Crohn’s disease: Case series and literature review. BMC Gastroenterol 14: 146.
- Palomba S, Sereni G, Falbo A, Beltrami M, Lombardini S, et al. (2014) Inflammatory bowel diseases and human reproduction: A comprehensive evidence-based review. World J Gastroenterol 20: 7123-7136.
- Webster PJ, Bailey MA, Wilson J, Burke DA (2015) Small bowel obstruction in pregnancy is a complex surgical problem with a high risk of fetal loss. Ann R Coll Surg Engl 97: 339-344.
- Vassiliou I, Tympa A, Derpapas M, Kottis G, Vlahos N (2012) Small Bowel Ischemia due to Jejunum Volvulus in Pregnancy: A Case Report. Case Rep Obstet Gynecol 2012: 485863.
- Cong Q, Li X, Ye X, Sun L, Jiang W, et al. (2014) Small bowel volvulus in mid and late pregnancy: Can early diagnosis be established to avoid catastrophic outcomes? 7: 4538-4543.
- Palmucci S, Lanza ML, Gulino F, Scilletta B, Ettorre GC (2014) Diagnosis of a sigmoid volvulus in pregnancy: Ultrasonography and magnetic resonance imaging findings. J Radiol Case Rep 8: 54-62.
- Ssi-Yan-Kai G, Rivain AL, Trichot C, Morcelet MC, Prevot S, et al. (2018) What every radiologist should know about adnexal torsion. Emerg Radiol 25: 51-59.
- Lee JH, Roh HJ, Ahn JW, Kim JS, Choi JY, et al. (2020) The Diagnostic Accuracy of Magnetic Resonance Imaging for Maternal Acute Adnexal Torsion during Pregnancy: Single-Institution Clinical Performance Review. J Clin Med 9: 2209.
- Asch E, Wei J, Mortele KJ, Humm K, Thornton K, et al. (2017) Magnetic resonance imaging performance for diagnosis of ovarian torsion in pregnant women with stimulated ovaries. Fertil Res Pract 3: 13.
- Lourenco AP, Swenson D, Tubbs RJ, Lazarus E (2014) Ovarian and tubal torsion: Imaging findings on US, CT, and MRI. Emerg Radiol 21: 179-187.
- Hasson J, Tsafrir Z, Azem F, Bar-On S, Almog B, et al. (2010) Comparison of adnexal torsion between pregnant and nonpregnant women. Am J Obstet Gynecol 202: 536.
- Tanaka Y, Tsuboyama T, Yamamoto K, Terai Y, Ohmichi M, et al. (2018) A case of torsion of a normal ovary in the third trimester of pregnancy: MRI findings with emphasis on asymmetry in the diameter of the ovarian veins. Radiol Case Rep 14: 324-327.
- Kato H, Kanematsu M, Uchiyama M, Yano R, Furui T, et al. (2014) Diffusion-weighted Imaging of Ovarian Torsion: Usefulness of Apparent Diffusion Coefficient (ADC) Values for the Detection of Hemorrhagic Infarction. Magn Reson Med Sci 13: 39-44.
- Coakley FV, Anwar M, Poder L, Wang ZJ, Yeh BM, et al. (2010) Magnetic resonance imaging of massive ovarian edema in pregnancy. J Comput Assist Tomogr 34: 865-867.
- Gobara A, Yoshizako T, Yoshida R, Okada N, Makihara K, et al. (2016) Magnetic resonance imaging features of massive ovarian edema in pregnancy: Utility for decisions in expectant management. Springerplus 5: 1444.
- Dai JC, Nicholson TM, Chang HC, Desai AC, Sweet RM, et al. (2021) Nephrolithiasis in Pregnancy: Treating for Two. Urology 151: 44-53.
- Semins MJ, Matlaga BR (2013) Management of urolithiasis in pregnancy. Int J Womens Health 5: 599-604.
- Mullins JK, Semins MJ, Hyams ES, Bohlman ME, Matlaga BR (2012) Half Fourier Single-shot Turbo Spin-echo Magnetic Resonance Urography for the Evaluation of Suspected Renal Colic in Pregnancy. Urology 79: 1252-1255.
- Masselli G, Derme M, Bernieri MG, Polettini E, Casciani E, et al. (2014) Stone disease in pregnancy: Imaging-guided therapy. Insights Imaging 5: 691-696.
- White WM, Johnson EB, Zite NB, Beddies J, Krambeck AE, et al. (2013) Predictive Value of Current Imaging Modalities for the Detection of Urolithiasis During Pregnancy: A Multicenter, Longitudinal Study. J Urol 189: 931-934.
- Wing DA, Fassett MJ, Getahun D (2014) Acute pyelonephritis in pregnancy: An 18-year retrospective analysis. Am J Obstet Gynecol 210: 219.
- Das CJ, Ahmad Z, Sharma S, Gupta AK (2014) Multimodality imaging of renal inflammatory lesions. World J Radiol 6: 865-873.
- Aoyagi J, Odaka J, Kuroiwa Y, Nakashima N, Ito T, et al. (2014) Utility of non-enhanced magnetic resonance imaging to detect acute pyelonephritis. Pediatr Int 56: 4-6.
- Nikolaidis P, Dogra VS, Goldfarb S, Gore JL, Harvin HJ, et al. (2018) ACR Appropriateness Criteria ® Acute Pyelonephritis. J Am Coll Radiol 15: 232-239.
- De Pascale A, Piccoli GB, Priola SM, Rognone D, Consiglio V, et al. (2013) Diffusion-weighted magnetic resonance imaging: new perspectives in the diagnostic pathway of non-complicated acute pyelonephritis. Eur Radiol 23: 3077-3086.
- Baheti AD, Nicola R, Bennett GL, Bordia R, Moshiri M, et al. (2016) Magnetic Resonance Imaging of Abdominal and Pelvic Pain in the Pregnant Patient. Magn Reson Imaging Clin N Am 24: 403-417.
- Yu HS, Gupta A, Soto JA, LeBedis C (2016) Emergency abdominal MRI: Current uses and trends. Br J Radiol 89: 20150804.
- Stimac D, Stimac T (2011) Acute pancreatitis during pregnancy. Eur J Gastroenterol Hepatol 23: 839-844.
- Mali P (2016) Pancreatitis in pregnancy: Etiology, diagnosis, treatment, and outcomes. Hepatobiliary Pancreat Dis Int 15: 434-438.
- Sun L, Li W, Geng Y, Shen B, Li J (2011) Acute pancreatitis in pregnancy. Acta Obstet Gynecol Scand 90: 671-676.
- Hara T, Kanasaki H, Oride A, Ishihara T, Kyo S (2015) A Case of Idiopathic Acute Pancreatitis in the First Trimester of Pregnancy. Case Rep Obstet Gynecol 2015: 1-4.
- Qihui C, Xiping Z, Xianfeng D (2012) Clinical Study on Acute Pancreatitis in Pregnancy in 26 Cases. Gastroenterol Res Pract 2012: 271925.
- Zhang DL, Huang Y, Yan L, Phu A, Ran X, et al. (2013) Thirty-eight cases of acute pancreatitis in pregnancy: A 6-year single center retrospective analysis. J Huazhong Univ Sci Technolog Med Sci 33: 361-367.
- Vilallonga R, Calero-Lillo A, Charco R, Balsells J (2014) Pancreatitis aguda durante la gestación, experiencia de 7 años en un centro de tercer Cir Esp 92: 468-471.
- Charlet P, Lambert V, Carles G (2015) Pancréatites aiguës et grossesse:cas cliniques et revue de la littérature. J Gynécologie Obstétrique Biol Reprod 44: 541-549.
- Pandey R, Jacob A, Brooks H (2012) Acute pancreatitis in pregnancy: Review of three cases and anaesthetic management. Int J Obstet Anesth 21: 360-363.
- van der Woude CJ, Metselaar HJ, Danese S (2014) Management of gastrointestinal and liver diseases during pregnancy. Gut 63: 1014-1023.
- Nepal P, Wells M, Ojili V, Khandelwal K, Lalwani N, et al. (2021) Problem-solving with MRI in acute abdominopelvic conditions, part 1: gastrointestinal, hepatobiliary, and pancreatic diseases. Emerg Radiol 28: 1161-1172.
- Shanbhogue AK, Menias CO, Lalwani N, Lall C, Khandelwal A, et al. (2013) Obstetric (Nonfetal) Complications. Radiol Clin North Am 51: 983-1004.
- Yazawa H, Takiguchi K, Ito F, Fujimori K (2018) Uterine rupture at 33rd week of gestation after laparoscopic myomectomy with signs of fetal distress. A case report and review of literature. Taiwan J Obstet Gynecol 57: 304-310.
- Nagao Y, Osato K, Kubo M, Kawamura T, Ikeda T, et al. (2015) Spontaneous uterine rupture in the 35th week of gestation after laparoscopic adenomyomectomy. Int Med Case Rep J 9: 1-4.
- Otsubo Y, Nishida M, Arai Y, Ichikawa R, Taneichi A, et al. (2016) Association of uterine wall thickness with pregnancy outcome following uterine-sparing surgery for diffuse uterine adenomyosis. Aust NZJ Obstet Gynaecol 56: 88-91.
- Pistofidis G, Makrakis E, Balinakos P, Dimitriou E, Bardis N, et al. (2012) Report of 7 Uterine Rupture Cases After Laparoscopic Myomectomy: Update of the Literature. J Minim Invasive Gynecol 19: 762-767.
- Sun HD, Su WH, Chang WH, Wen L, Huang BS, et al. (2012) Rupture of a pregnant unscarred uterus in an early secondary trimester: a case report and brief review. J Obstet Gynaecol Res 38: 442-445.
- Parker WH, Einarsson J, Istre O, Dubuisson JB (2010) Risk Factors for Uterine Rupture after Laparoscopic Myomectomy. J Minim Invasive Gynecol 17: 551-554.
- Okada Y, Hasegawa J, Mimura T, Arakaki T, Yoshikawa S, et al. (2016) Uterine rupture at 10 weeks of gestation after laparoscopic myomectomy. J Med Ultrason (2001) 43: 133-136.
- Tian YC, Long TF, Dai YM (2015) Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res 41: 350-357.
- Koo YJ, Lee JK, Lee YK, Kwak DW, Lee IH, et al. (2015) Pregnancy Outcomes and Risk Factors for Uterine Rupture After Laparoscopic Myomectomy: A Single-Center Experience and Literature Review. J Minim Invasive Gynecol 22: 1022-1028.
- Mizutamari E, Honda T, Ohba T, Katabuchi H (2014) Spontaneous Rupture of an Unscarred Gravid Uterus in a Primigravid Woman at 32 Weeks of Gestation. Case Rep Obstet Gynecol 2014: 209585.
- Leroux M, Coatleven F, Faure M, Horovitz J (2014) Rupture utérine bilatérale sur utérus gravide non cicatriciel en dehors du travail. Gynécologie Obstétrique Fertil 42: 454-457.
- Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, et al. (2011) The prevalence of congenital uterine anomalies in unselected and high-risk populations: A systematic review. Hum Reprod Update 17: 761-771.
- Abdalla N, Reinholz-Jaskolska M, Bachanek M, Cendrowski K, Stanczak R, et al. (2015) Hemoperitoneum in a patient with spontaneous rupture of the posterior wall of an unscarred uterus in the second trimester of pregnancy. BMC Res Notes 8: 603.
- Filhastre M, Dechaud H, Lesnik A, Taourel P (2005) Interstitial pregnancy: Role of MRI. Eur Radiol 15: 93-95.
- Soewondo W, Kusumaningrum S, Putro PS, Indriyani I, Maryetty IP, et al. (2021) The use of FIESTA sequence MRI in successful management of abdominal pregnancy. Clin Imaging 77: 117-121.
- Srisajjakul S, Prapaisilp P, Bangchokdee S (2017) Magnetic resonance imaging in tubal and non-tubal ectopic pregnancy. Eur J Radiol 93: 76-89.
- Ramanathan S, Raghu V, Ladumor SB, Nagadi AN, Palaniappan Y, et al. (2018) Magnetic resonance imaging of common, uncommon, and rare implantation sites in ectopic pregnancy. Abdom Radiol (NY) 43: 3425-3435.
- Si MJ, Gui S, Fan Q, Han HX, Zhao QQ, et al. (2016) Role of MRI in the early diagnosis of tubal ectopic pregnancy. Eur Radiol 26: 1971-1980.
- Varma R, Parikh B, Beyer C, Ghosh S, Du D, et al. (2019) Magnetic resonance imaging of tubal ectopic pregnancy: Correlation with intraoperative findings. Clin Imaging 58: 194-200.
- Kao LY, Scheinfeld MH, Chernyak V, Rozenblit AM, Oh S, et al. (2014) Beyond Ultrasound: CT and MRI of Ectopic Pregnancy. Am J Roentgenol 202: 904-911.
- Kuroda M, Kuroda K, Kuwatsuru R, Kitade M, Kikuchi I, et al. (2011) Use of magnetic resonance analysis for clinical evaluation of the peripheral area of gestational sac in bleeding and non-bleeding ectopic pregnancy cases. Reprod Med Biol 11: 95-100.
- Takahashi A, Takahama J, Marugami N, Takewa M, Itoh T, et al. (2013) Ectopic pregnancy: MRI findings and clinical utility. Abdom Imaging 38: 844-850.
- Durur-Karakaya A, Seker M, Durur-Subasi ? (2018) Diffusion-weighted imaging in ectopic pregnancy: Ring of restriction sign. Br J Radiol 91: 20170528.
- Parker RA 3rd, Yano M, Tai AW, Friedman M, Narra VR, et al. (2012) MR Imaging Findings of Ectopic Pregnancy: A Pictorial Review. Radiographics 32: 1445-1460.
- Huang Q, Zhang M, Zhai R-Y (2014) The use of contrast-enhanced magnetic resonance imaging to diagnose cesarean scar pregnancies. Int J Gynecol Obstet 127: 144-146.
- Nishio N, Kido A, Kurata Y, Minami M, Tokunaga K, et al. (2020) Investigation of clinical utility of contrast-enhanced MRI in the diagnosis of ectopic pregnancy. Clin Radiol 75: 543-551.
- Chiang A-J, La V, Chou C-P, Wang P-H, Yu K-J (2011) Ectopic pregnancy in a cesarean section scar. Fertil Steril 95: 2388-2389.
- Derman AY, Nikac V, Haberman S, Zelenko N, Opsha O, et al. (2011) MRI of Placenta Accreta: A New Imaging Perspective. AJR Am J Roentgenol 197: 1514-1521.
- Yang X-Y, Yu H, Li KM, Chu YX, Zheng A (2010) Uterine artery embolisation combined with local methotrexate for treatment of caesarean scar pregnancy. BJOG 117: 990-996.
- Bij de Vaate AJ, Brölmann HA, van der Voet LF, van der Slikke JW, Veersema S, et al. (2011) Ultrasound evaluation of the Cesarean scar: Relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol 37: 93-99.
- Stirnemann JJ, Chalouhi GE, Forner S, Saidji Y, Salomon LJ, et al. (2011) First-trimester uterine scar assessment by transvaginal ultrasound. Am J Obstet Gynecol 205: 551.
- Wu R, Klein MA, Mahboob S, Gupta M, Katz DS (2013) Magnetic Resonance Imaging as an Adjunct to Ultrasound in Evaluating Cesarean Scar Ectopic Pregnancy. J Clin Imaging Sci 3: 16.
- Caserta NMG, Bacha AM, Grassiotto OR (2017) Cesarean scar ectopic pregnancy: Invasion of the bladder wall detected by magnetic resonance imaging. Radiol Bras 50: 197-198.
- Shin DS, Poder L, Courtier J, Naeger DM, Westphalen AC, et al. (2011) CT and MRI of Early Intrauterine Pregnancy. Am J Roentgenol 196: 325-330.
- Peng KW, Lei Z, Xiao TH, Jia FG, Zhong WX, et al. (2014) First trimester caesarean scar ectopic pregnancy evaluation using MRI. Clin Radiol 69: 123-129.
- Papaioannou GI, Syngelaki A, Poon LC, Ross JA, Nicolaides KH (2010) Normal ranges of embryonic length, embryonic heart rate, gestational sac diameter and yolk sac diameter at 6-10 weeks. Fetal Diagn Ther 28: 207-219.
- Silver RM, Branch DW (2018) Placenta Accreta Spectrum. N Engl J Med 378: 1529-1536.
- Higgins MF, Monteith C, Foley M, O'Herlihy C (2013) Real increasing incidence of hysterectomy for placenta accreta following previous caesarean section. Eur J Obstet Gynecol Reprod Biol 171: 54-56.
- Vintzileos AM, Ananth CV, Smulian JC (2015) Using ultrasound in the clinical management of placental implantation abnormalities. Am J Obstet Gynecol 213: 70-77.
- Jauniaux E, Collins S, Burton GJ (2018) Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol 218: 75-87.
- D'Arpe S, Franceschetti S, Corosu R, Palaia I, Di Donato V, et al. (2015) Emergency peripartum hysterectomy in a tertiary teaching hospital: A 14-year review. Arch Gynecol Obstet 291: 841-847.
- Tórrez-Morales F, Briones-Garduño JC (2017) Percretismo placentario con invasión de vejiga y recto. Cir Cir 85: 66-69.
- Fadl S, Moshiri M, Fligner CL, Katz DS, Dighe M (2017) Placental Imaging: Normal Appearance with Review of Pathologic Findings. Radiographics 37: 979-998.
- Rahaim NS, Whitby EH (2015) The MRI features of placental adhesion disorder and their diagnostic significance: Systematic review. Clin Radiol 70: 917-925.
- D'Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, et al. (2014) Prenatal identification of invasive placentation using magnetic resonance imaging: Systematic review and meta-analysis. Ultrasound Obstet Gynecol 44: 8-16.
- Zaidi SF, Moshiri M, Osman S, Robinson TJ, Siebert JR, et al. (2016) Comprehensive Imaging Review of Abnormalities of the Placenta. Ultrasound Q 32: 25-42.
- Ueno Y, Kitajima K, Kawakami F, Maeda T, Suenaga Y, et al. (2014) Novel MRI finding for diagnosis of invasive placenta praevia: Evaluation of findings for 65 patients using clinical and histopathological correlations. Eur Radiol 24: 881-888.
- Bour L, Placé V, Bendavid S, Fargeaudou Y, Portal JJ, et al. (2014) Suspected invasive placenta: Evaluation with magnetic resonance imaging. Eur Radiol 24: 3150-3160.
- Allen BC, Leyendecker JR (2013) Placental Evaluation with Magnetic Resonance. Radiol Clin North Am 51: 955-966.
- Alamo L, Anaye A, Rey J, Denys A, Bongartz G, et al. (2013) Detection of suspected placental invasion by MRI: Do the results depend on observer’ experience? Eur J Radiol 82: 51-57.
- Sato T, Mori N, Hasegawa O, Shigihara T, Fujimori K, et al. (2017) Placental recess accompanied by a T2 dark band: A new finding for diagnosing placental invasion. Abdom Radiol (NY) 42: 2146-2153.
- Valentini AL, Gui B, Ninivaggi V, Miccò M, Giuliani M, et al. (2017) The morbidly adherent placenta: When and what association of signs can improve MRI diagnosis? Our experience. Diagn Interv Radiol 23: 180-186.
- Chen T, Xu XQ, Shi HB, Yang ZQ, Zhou X, et al. (2017) Conventional MRI features for predicting the clinical outcome of patients with invasive placenta. Diagn Interv Radiol 23: 173-179.
- Ueno Y, Maeda T, Tanaka U, Tanimura K, Kitajima K, et al. (2016) Evaluation of interobserver variability and diagnostic performance of developed MRI-based radiological scoring system for invasive placenta previa. J Magn Reson Imaging 44: 573-583.
- Chen D, Xu J, Ye P, Li M, Duan X, et al. (2020) Risk scoring system with MRI for intraoperative massive hemorrhage in placenta previa and accreta. J Magn Reson Imaging 51: 947-958.
- Chu C, Liu M, Zhang Y, Yu L, Wang D, et al. (2021) Quantifying magnetic resonance imaging features to classify placenta accreta spectrum (PAS) in high-risk gravid patients. Clin Imaging 80: 50-57.
- Delli Pizzi A, Tavoletta A, Narciso R, Mastrodicasa D, Trebeschi S, et al. (2019) Prenatal planning of placenta previa: diagnostic accuracy of a novel MRI-based prediction model for placenta accreta spectrum (PAS) and clinical outcome. Abdom Radiol (NY) 44: 1873-1882.
- Chantraine F, Blacher S, Berndt S, Palacios-Jaraquemada J, Sarioglu N, et al. (2012) Abnormal vascular architecture at the placental-maternal interface in placenta increta. Am J Obstet Gynecol 207: 188.
- Lu T, Pu H, Cui W, Mei J, Huang MW, et al. (2019) Use of intravoxel incoherent motion MR imaging to assess placental perfusion in patients with placental adhesion disorder on their third trimester. Clin Imaging 56: 135-139.
- León RL, Brown BP, Persohn SA, Norris CD, Steinhardt NP, et al. (2021) Intravoxel incoherent motion MR imaging analysis for diagnosis of placenta accrete spectrum disorders: A pilot feasibility study. Magn Reson Imaging 80: 26-32.
Citation: Ercolani G, Ciulla S, Celli V, Ninkova R, Miceli V, et al. (2022) MR Imaging of Abdominal Pain in Pregnant Women: A Review of Common Obstetric and Non-Obstetric Pathologies. J Reprod Med Gynecol Obstet 7: 093.
Copyright: © 2022 Giada Ercolani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

