
Natural Products Protect Lung Diseases by Targeting the Hippo Signaling Pathway
*Corresponding Author(s):
Jian ZhangShanghai Frontiers Science Center Of Optogenetic Techniques For Cell Metabolism, Shanghai Key Laboratory Of New Drug Design, School Of Pharmacy, East China University Of Science & Technology, Shanghai, China
Email:zhangjian_tina@163.com
Abstract
Respiratory illness and diseases are common problems worldwide. Natural products have become a source of drug discovery for respiratory diseases. The Hippo signaling pathway is a highly conserved evolutionary pathway. This article summarizes the protective role of natural products in lung diseases such as pulmonary arterial asthma, acute lung injury, pulmonary arterial hypertension and idiopathic pulmonary fibrosis and explains how they exert protective effects through the Hippo signaling pathway. In addition, natural products targeting the Hippo signaling pathway may be promising drug candidates for lung diseases. Consequently, further research is required to focus on the mechanism of natural products and drug targets in Hippo signaling pathway, especially in lung diseases.
Introduction
Plants or herbs containing natural compounds have been used for centuries in traditional medicine and have made significant contributions to the pharmacological treatment of various diseases [1]. Natural products have emerged as a valuable source of novel bioactive lead compounds in drug discovery, characterized by multi-target effects, stable therapeutic efficacy, low side effects and no drug dependence [2].
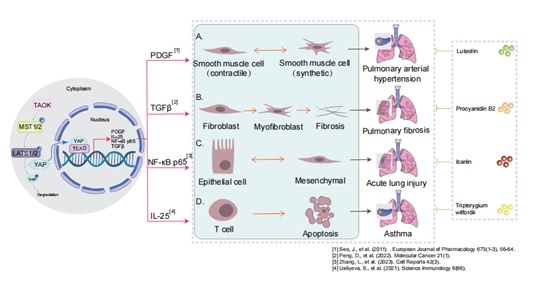
Over the years, natural products have been the subject of extensive investigation for their potential in the treatment of lung diseases, with a particular focus on their impact on different classical signaling pathways [3]. EA (Ellagic Acid) has been shown to significantly suppress fibroblast activation and ECM (Extracellular Matrix) production through inhibition of the Wnt/Akt and Erk signaling pathways [4]. Tripterine and procyanidin B2 improve the occurrence and the development of lung diseases through the Hippo signaling pathway [5-7].
The Hippo signaling pathway has been reported to play an important role in lung development and alveolar differentiation [8]. Beyond impaired alveolar differentiation, lung diseases involve multiple pathological processes. Chronic inflammation drives tissue damage through cytokine release and immune cell infiltration [9]. Fibrosis, characterized by excessive extracellular matrix deposition (e.g., collagen), disrupts lung architecture in conditions like idiopathic pulmonary fibrosis, YAZ/TAP induces myofibroblast differentiation and excessive extracellular matrix deposition, leading to tissue scarring. Vascular remodeling—including endothelial dysfunction and pulmonary hypertension—compromises gas exchange [10]. Airway remodeling features goblet cell hyperplasia, smooth muscle hypertrophy, and submucosal gland enlargement in obstructive diseases such as asthma and COPD, the Hippo signaling pathway function is associated with defective alveolar epithelial regeneration and airspace enlargement [11]. Additionally, oxidative stress, protease-antiprotease imbalances, and epithelial-mesenchymal transition contribute to progressive tissue injury and functional decline [12]. We will summarize the role and mechanism of natural products in Pulmonary Hypertension (PAH), asthma and Acute Lung Injury (ALI) through the Hippo signaling pathway.
Hippo Signaling Pathway
The Hippo signaling pathway, first identified in Drosophila melanogaster, is one of the earliest developmentally conserved pathways [13,14]. It gets its name from the key member of the pathway, Hippo protein kinase, which has been studied for 20 years [15].
The pathway consists of a series of conserved kinases (MST1/2 and LATS1/2 , as examples) [16] that regulate cell proliferation, apoptosis, stem cell self-renewal [17,18], which are involved in a variety of biological functions, such as tissue development, maintenance of tissue homeostasis, and regenerative repair [19]. The imbalance in the Hippo pathway leads a wide range of diseases, such as heart disease, liver disease, lung disease, and immune dysfunction [20,21].
Hippo signaling pathway is a kinase cascade in which MST1/2 kinase /Salvador (SAV1) complex phosphorylate and activate LATS1/2 kinase [22-24]. The transcriptional coactivators YAP and TAZ—key downstream effectors—are phosphorylated by LATS1/2 [25,26]. Upon dephosphorylation, YAP/TAZ complex is translocated to the nucleus, where it interacts with TEAD1-4 and other transcription factors to induce the expression of genes that promote cell proliferation and inhibit apoptosis [27]. Moreover, the Hippo signaling pathway plays a role in cell-contact inhibition [28].
The Hippo pathway is orchestrated through a tightly controlled kinase cascade. MST1/2 and LATS1/2 kinases are activated by upstream regulators including scaffold proteins (Merlin, KIBRA, RASSF) and the LIM-domain protein Ajuba, which facilitate signal integration [29,30] . Phosphatases dynamically modulate MST1/2 and YAP/TAZ phosphorylation status [31,32], and ubiquitination controls LATS1/2 and YAP/TAZ protein stability [32]. Cytoskeletal tension further fine-tunes LATS1/2 activity [33]. Concurrently, cytoplasmic retention complexes containing 14-3-3, α-catenin, AMOT, and ZO-2 sequester YAP/TAZ at cell junctions, preventing nuclear accumulation. [34].
Upon pathway inactivation, dephosphorylated YAP/TAZ translocate to the nucleus [35] where they form transcriptional complexes with TEAD1-4. These complexes drive expression of proliferative (CTGF, Connective Tissue Growth Factor), survival (AXL, Receptor Tyrosine Kinase), and differentiation-related genes [36]. Notably, the TEAD-induced Ajuba protein creates a negative feedback loop by directly inhibiting MST1/2 and LATS1/2 kinases [37], establishing a self-regulating circuit that maintains pathway homeostasis. This dual-layered regulation ensures precise spatial-temporal control of YAP/TAZ activity in response to mechanical and biochemical cues.
Briefly, the Hippo signaling pathway negatively regulates the transcriptional activity of its downstream effector YAP, which plays an important role in maintaining homeostasis of cell proliferation and apoptosis by limiting overgrowth in most tissues [38].
Natural Products and Lung Diseases
- The effects of natural products in Asthma
Asthma, caused by a combination of genetic and environmental factors, is a chronic inflammatory disease of the respiratory tract that affects approximately 300 million people worldwide and is responsible for at least 250,000 deaths annually [39,40]. The global median incidence rate of asthma is 402 people per 100000 people, and the incidence rate of children under 10 years old is growing [41].
Asthma is a heterogeneous respiratory disease characterized by reversible bronchial obstruction [42]. The airway epithelium is the first structural barrier against inhaled environmental damage and plays a key role in the development of allergic airway inflammation [43]. Current asthma medications (e.g., bronchodilators, corticosteroids, theophyllines) alleviate symptoms but exhibit side effects due to narrow therapeutic windows, while inhaled corticosteroids are more effective, yet none provide a cure, leading to reduced quality of life [44,45].
Significant upregulation of the YAP protein in bronchial airway tissues was observed in an OVA-induced chronic asthma mouse model. Furthermore, the study proved that tripterine (Tripterygium wilfordii) treatment attenuated LPS-induced (Lipopolysaccharide-induced) airway epithelial barrier dysfunction by inhibiting the protein level of YAP/TAZ in the Hippo pathway, which in turn delayed the development of chronic asthma [6].
- The effects of natural products in ALI
ALI is a significant cause of severe respiratory failure, which is caused by infection, severe shock, or pulmonary trauma [46]. ALI is a life-threatening disease characterized by immune cell infiltration and diffuse alveolar injury, ultimately leading to pulmonary edema, hypoxemia, and organ failure, with high morbidity and mortality [47]. Meanwhile, the excessive lung inflammation and apoptosis of alveolar epithelial cells (AECs) play key roles in the pathogenesis of ALI [48].
The key clinical bottleneck in ALI pharmacotherapy is that current anti-infectives (e.g., cephalosporins) only manage symptoms but cannot prevent disease progression, ultimately requiring surgical intervention [49]. An understanding of the mechanism of alveolar epithelial cell regeneration ability provides a theoretical basis for the treatment of ALI. It has been reported that the Hippo signaling pathway is involved in the repair process of ALI by improving the proliferation and differentiation of lung epithelial cells [50]. YAP has been reported to enhance the self-renewal of alveolar epithelial type II cells (AECIIs) and promote their differentiation into type I alveolar epithelial cells (AECIs) in lung injury. [8,51]. Furthermore, YAP-mediated proliferation and differentiation of AECIIs regulate AECIs in response to mechanical tension [8]. During alveolar regeneration, mechanical forces enhance AECII responsiveness, triggering nuclear YAP accumulation. YAP promotes AECII proliferation and differentiation into AECIs while driving pulmonary endothelial repair through angiogenic factors [52]. MST1/2 deletion and YAP target proteins further regulate epithelial cell dynamics. These findings implicate the Hippo pathway in ALI repair by modulating both epithelial regeneration and vascular remodeling [8].
Procyanidin B2 (PB2) has been demonstrated to elevate phosphorylation by inhibiting the expression of LATS1/2 and YAP in the Hippo signaling pathway at both the mRNA and protein levels [7]. And PB2 reduces the levels of inflammatory cells in the serum and lung tissue of sepsis induced ALI mice, alleviating LPS induced ALI in the peritoneum [7]. Therefore, PB2 has anti-inflammatory and lung-protective effects on sepsis-induced ALI and is a potential therapeutic drug.
- The effects of natural products in PAH
PAH is a chronic, progressive, and irreversible disease with a high mortality rate, characterized by a sustained increase in pulmonary vascular pressure and pulmonary vascular resistance, culminating in right heart failure and sudden death [53,54]. According to most European data, the estimated annual incidence rate of PAH is 5.8 adults per million, and the prevalence rate is 47.6 to 54.7 adults per million [55]. In recent years, notable advancements have been made in the diagnosis and treatment of PAH. However, it remains difficult to reverse the outcome and reduce the high mortality rate of PAH. The pathogenesis of PAH includes abnormal contraction of the pulmonary arteries, endothelial dysfunction, vascular remodeling and in situ thrombosis [56]. Vascular remodeling is mainly caused by abnormal proliferation and migration of pulmonary artery smooth muscle cells (PASMCs) [57].
The mainstay pharmacotherapies for pulmonary arterial hypertension (PAH) - including prostacyclin analogues, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors - primarily provide symptomatic relief and hemodynamic improvement without altering disease progression [58]. Therefore, there is a need for new target studies and new potential drug candidates. Hippo signaling pathway plays an important role in vascular remodeling [59,60]. Inactivation of LATS1 promotes YAP nuclear translocation, driving PASMC proliferation and vascular remodeling [61]. Consistently, smooth muscle-specific MST1/2 knockout in hypoxic PAH mice activates YAP via impaired phosphorylation, subsequently upregulating Akt-mTORC1 signaling [62].
Studies have shown that luteolin exerts protection by inhibiting YAP, which in turn inhibits the activation of the downstream PI3K/AKT pathway in part [63]. luteolin extract is a potential candidate drug in PAH.
- The effects of natural products in IPF
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, irreversible, and often fatal lung disease with unknown etiology and limited treatment options [64]. It is characterized by changes in the composition and homogeneity of peripheral lung cells, leading to an excessive accumulation of ECM and destruction of alveolar structure, resulting in respiratory failure and death [65]. According to the epidemiological survey of IPF, the global incidence rate of IPF ranges from 0.09 per 10000 people to 1.30 per 10000 people, and increases year by year [66].
The commonly used drugs for IPF are nintedanib and pirfenidone, which only delay its progression with certain side effects [67]. Nintedanib can cause diarrhea and other gastrointestinal symptoms, while pirfenidone is closely related to skin adverse reactions, especially photosensitivity, and has a certain probability of causing gastrointestinal intolerance [68]. Nintedanib-induced diarrhea typically occurs during early treatment, with most patients experiencing symptoms within 1–2 weeks of initiation [69,70]. Therefore, it is necessary to pay attention to new promising candidate drugs.
Recent studies have shown that YAP/TAZ in the Hippo signaling pathway is a key coordinator of fibroblasts activation with increased synthesis of ECM, and expression of pro-fibrotic factors [71]. Overexpression of YAP has been shown to promote the proliferation and migration of fibroblasts, induce collagen production, and inhibit epithelial cell differentiation, thereby exacerbating the disease progression of idiopathic pulmonary fibrosis [72]. In addition, the inhibition of YAP signaling blocks TGF-β-induced fibroblast to myofibroblast transformation and ECM deposition, whereas activation of YAP is sufficient to promote fibroblast differentiation and ECM deposition [73].
Recent research reports that Icariin (ICA) attenuates BLM-induced pulmonary fibrosis in rat model by inhibiting inflammatory response, profibrotic activity, and expression of YAP and collagen [64]. The treatment of ShaShen MaiDong (Adenophora stricta Miq) resulted in inhibition of YAP/TAZ and phosphorylation of YAP. ShaShen MaiDong suppresses IPF and and alleviates BLM induced pulmonary fibrosis in mouse by simultaneously regulating the TGF - β/Smad3, AKT/MAPK, and YAP/TAZ pathways, indicating that they are potential natural compounds for treating IPF [74]. In summary, ICA and SMT can treat bleomycin induced pulmonary fibrosis by inhibiting the Hippo signaling pathway, which may be promising candidate drugs.
Conclusion And Prospect
Beyond the roles in PAH, asthma, ALI, and IPF summarized above, the Hippo pathway also contributes to the pathogenesis of chronic obstructive pulmonary disease (COPD) and pneumonia.
COPD affects nearly 400 million people and is already the third leading cause of death worldwide , which is characterized by persistent respiratory symptoms and progressive airflow obstruction documented by spirometry [75]. The occurrence and development of COPD is associated with an abnormal inflammatory response of the lungs to toxic particles or air pollution [76,77]. Studies have shown that E-calmodulin activates Hippo signaling pathway in lung epithelial cells [78]. Exposure to air pollution has been demonstrated to result in a reduction in E-calmodulin expression and YAP phosphorylation in A549 cell proliferation and A549 cell senescence, which may subsequently trigger emphysema in patients with COPD [78].
Pneumonia is an infection of the lungs caused by bacteria, viruses, or other microorganisms. The mortality rate of pneumonia accounts for 30% of all respiratory system deaths [79]. The alveolar epithelium plays a key role in protecting the lungs from inhaled infectious agents. Surface-active protein C-expressing (SPC-expressing) AECIIs undergo proliferation and differentiation after infection, which is reported to be associated with increased expression of YAP and TAZ in nuclear [8]. Deficiency of YAP in AECIIs leads to a sustained accumulation of inflammatory cells in the lungs in bacterial pneumonia [80]. In YAP/TAZ-deficient mice, the signaling pathways regulating the resolution of lung inflammation are significantly dysregulated, the lungs exhibit prolonged inflammatory response [8]. Given the critical role of YAP/TAZ in lung disease pathogenesis, targeting this pathway may represent a promising therapeutic strategy.
Oleanolic acid, a small molecule natural product, has been reported to inhibit ECM degeneration in osteoarthritis by regulating the Hippo/YAP and Wnt/β-catenin pathways [81]. Liquiritigenin (LQ) has been shown to be antioxidant, anti-inflammatory, antitumor, and antidiabetic activities. LQ treatment induced YAP phosphorylation and inhibits YAP/TAZ activation, which ultimately blocks HSC activation and the development of liver fibrosis [82]. Cordycepin administration upregulates the expression of MST1 and LATS1, thereby inhibiting the expression of YAP1, and thus exerts anti-cancer effects [83]. Therefore, evaluating oleanolic acid and Liquiritigenin (LQ)—natural compounds targeting the Hippo/YAP pathway—has become a warranted strategy for future research into lung disease treatment.
Natural products have been studied for a long period of time and the relevant drugs are widely used in clinical practice.This review underscores the targeting of the Hippo signaling pathway as an emerging therapeutic strategy for lung diseases and provides a comprehensive overview of promising natural product-derived drug candidates to modulate this pivotal pathway.
Acknowledgement
This work was supported in part by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0528200 to J.Z); and the National Natural Science Foundation of China (82370068 and 81771513 to J.Z.).
References
- Lee S-H (2024) Therapeutic Effects of Natural Products on Human Diseases. Life 9: 1166.
- Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, et al. (2015) Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances 8: 1582-1614.
- Ji-hong Y, Yu M, Ling-hong Y, Jing-jing G, Ling-li X, et al. (2023) Baicalein attenuates bleomycin-induced lung fibroblast senescence and lung fibrosis through restoration of Sirt3 expression. Pharmaceutical Biology 1: 288-297.
- Li X, Huang K, Liu X, Ruan H, Ma L, et al. (2021) Ellagic Acid Attenuates BLM-Induced Pulmonary Fibrosis via Inhibiting Wnt Signaling Pathway. Frontiers in Pharmacology 12: 639574.
- Yao J, Fang X, Zhang C, Yang Y, Wang D, et al. (2020) Astragaloside IV attenuates hypoxia-induced pulmonary vascular remodeling via the Notch signaling pathway. Molecular Medicine Reports 1: 89.
- Gao J, Wang W (2018) Tripterine alleviates lipopolysaccharide-induced airway epithelial barrier dysfunction through suppressing the Hippo pathway. RSC Advances 69: 39696-39702.
- Kim GO, Park DH, Bae J-S (2023) Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway. International Journal of Molecular Sciences 9: 7930.
- LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, et al. (2019) Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. Journal of Clinical Investigation 5: 2107-2122.
- Ortiz-Zapater E, Bagley DC, Hernandez VL, Roberts LB, Maguire TJA, et al. (2022) Epithelial coxsackievirus adenovirus receptor promotes house dust mite-induced lung inflammation. Nature Communications 1: 6407.
- Jia Z, Wang S, Yan H, Cao Y, Zhang X, et al. (2023) Pulmonary Vascular Remodeling in Pulmonary Hypertension. Journal of Personalized Medicine 2: 366.
- Jones RL, Noble PB, Elliot JG, Mitchell HW, McFawn PK, et al. (2016) Airflow obstruction is associated with increased smooth muscle extracellular matrix. European Respiratory Journal 6: 1855-1857.
- Arafa SS, Elnoury HA, Badr El-Din S, Sakr MA, Hendawi FF, et al. (2025) Acetamiprid-induced pulmonary toxicity via oxidative stress, epithelial-mesenchymal transition, apoptosis, and extracellular matrix accumulation in human lung epithelial cells and fibroblasts: Protective role of heat-killed Lactobacilli. Food and Chemical Toxicology 198: 115322.
- Meng Z, Moroishi T, Guan KL (2016) Mechanisms of Hippo pathway regulation. Genes Dev 1: 1-17.
- Mo JS, Park HW, Guan KL (2014) The Hippo signaling pathway in stem cell biology and cancer. EMBO reports 6: 642-656.
- Harvey KF, Pfleger CM, Hariharan IK (2003) The Drosophila Mst Ortholog, hippo, Restricts Growth and Cell Proliferation and Promotes Apoptosis. Cell 4: 457-467.
- Ma S, Meng Z, Chen R, Guan K-L (2019) The Hippo Pathway: Biology and Pathophysiology. Annual Review of Biochemistry 1: 577-604.
- Zhong Z, Jiao Z, Yu F-X (2024) The Hippo signaling pathway in development and regeneration. Cell Reports 3: 113926.
- Liu Y, Zhang B, Zhou Y, Xing Y, Wang Y, et al. (2023) Targeting Hippo pathway: A novel strategy for Helicobacter pylori-induced gastric cancer treatment. Biomedicine & Pharmacotherapy 161: 114549.
- Cao Z, An L, Han Y, Jiao S, Zhou Z (2023) The Hippo signaling pathway in gastric cancer. Acta Biochimica et Biophysica Sinica 6: 893-903.
- Driskill JH, Pan D (2021) The Hippo Pathway in Liver Homeostasis and Pathophysiology. Annual Review of Pathology: Mechanisms of Disease 1: 299-322.
- Nguyen-Lefebvre AT, Selzner N, Wrana JL, Bhat M (2021) The hippo pathway: A master regulator of liver metabolism, regeneration, and disease. The FASEB Journal 5: 21570.
- Casati G, Giunti L, Iorio AL, Marturano A, Galli L, et al. (2021) Hippo Pathway in Regulating Drug Resistance of Glioblastoma. International Journal of Molecular Sciences 24: 13431.
- Han H, Nakaoka HJ, Hofmann L, Zhou JJ, Yu C, et al. (2022) The Hippo pathway kinases LATS1 and LATS2 attenuate cellular responses to heavy metals through phosphorylating MTF1. Nature Cell Biology 1: 74-87.
- Qin M, Geng E, Wang J, Yu M, Dong T, et al. (2024) LATS2 condensates organize signalosomes for Hippo pathway signal transduction. Nature Chemical Biology 6: 710-720.
- Liu H, Sun M, Wu N, Liu B, Liu Q, et al. (2023) TGF-β/Smads signaling pathway, Hippo-YAP/TAZ signaling pathway, and VEGF: Their mechanisms and roles in vascular remodeling related diseases. Immunity, Inflammation and Disease 11: 1060.
- Kwon H, Kim J, Jho EH (2021) Role of the Hippo pathway and mechanisms for controlling cellular localization of YAP/TAZ. The FEBS Journal 19: 5798-5818.
- Zhao B, Ye X, Yu J, Li L, Li W, et al. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes & Development 14: 1962-1971.
- Koo JH, Guan K-L (2018) Interplay between YAP/TAZ and Metabolism. Cell Metabolism 2: 196-206.
- Kim JE, Finlay GJ, Baguley BC (2013) The Role of the Hippo Pathway in Melanocytes and Melanoma. Frontiers in Oncology 3: 123.
- Yang J, Song DH, Kim CH, Kim MH, Jo HC, et al. (2022) Expression of the Hippo Pathway Core Components in Endometrial Cancer and Its Association with Clinicopathologic Features. Diagnostics 12: 2973.
- Yu F-X, Zhao B, Panupinthu N, Jewell JL, Lian I, et al. (2012) Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell 4: 780-791.
- Li F-L, Fu V, Liu G, Tang T, Konradi AW, et al. (2022) Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nature Chemical Biology 10: 1076-1086.
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. (2011) Role of YAP/TAZ in mechanotransduction. Nature 7350: 179-183.
- Giampietro C, Disanza A, Bravi L, Barrios-Rodiles M, Corada M, et al. (2015) The actin-binding protein EPS8 binds VE-cadherin and modulates YAP localization and signaling. Journal of Cell Biology 6: 1177-1192.
- Hao X, Zhang Y, Shi X, Liu H, Zheng Z, et al. (2022) CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3ζ. Journal of Experimental & Clinical Cancer Research 1: 281.
- Moon S, Lee S, Caesar JA, Pruchenko S, Leask A, et al. (2020) A CTGF-YAP Regulatory Pathway Is Essential for Angiogenesis and Barriergenesis in the Retina. iScience 6: 101184.
- Rozengurt E, Sinnett-Smith J, Eibl G (2018) Yes-associated protein (YAP) in pancreatic cancer: at the epicenter of a targetable signaling network associated with patient survival. Signal Transduction and Targeted Therapy 3: 11.
- Kim C-L, Lim S-B, Choi S-H, Kim DH, Sim YE, et al. (2024) The LKB1–TSSK1B axis controls YAP phosphorylation to regulate the Hippo–YAP pathway. Cell Death & Disease 15: 76.
- Martinez FD (2007) Genes, environments, development and asthma: a reappraisal. European Respiratory Journal 1: 179-184.
- Dharmage SC, Perret JL, Custovic A (2019) Epidemiology of Asthma in Children and Adults. Front Pediatr 7: 246.
- Xu Q, Zhou Q, Chen J, Li T, Ma J, et al. (2023) The incidence of asthma attributable to temperature variability: An ecological study based on 1990-2019 GBD data. Sci Total Environ 904: 166726.
- Bradding P (2007) Mast cell regulation of airway smooth muscle function in asthma. European Respiratory Journal 5: 827-830.
- Calvén J, Ax E, Rådinger M (2020) The Airway Epithelium—A Central Player in Asthma Pathogenesis. International Journal of Molecular Sciences 23: 8907.
- Cardet JC, Papi A, Reddel HK (2023) "As-Needed" Inhaled Corticosteroids for Patients With Asthma. J Allergy Clin Immunol Pract 3: 726-734.
- Volmer T, Effenberger T, Trautner C, Buhl R (2018) Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. European Respiratory Journal 4: 1800703.
- Tao H, Xu Y, Zhang S (2023) The Role of Macrophages and Alveolar Epithelial Cells in the Development of ARDS. Inflammation 1: 47-55.
- Li Y, Cao Y, Xiao J, Shang J, Tan Q, et al. (2020) Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death & Differentiation 9: 2635-2650.
- Xiao K, He W, Guan W, Hou F, Yan P, et al. (2020) Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death & Disease 10: 863.
- Salazar-Puerta AI, Rincon-Benavides MA, Cuellar-Gaviria TZ, Aldana J, Vasquez Martinez G, et al. (2023) Engineered Extracellular Vesicles Derived from Dermal Fibroblasts Attenuate Inflammation in a Murine Model of Acute Lung Injury. Advanced Materials 35: 2210579.
- Hu C, Sun J, Du J, Wen D, Lu H, et al. (2019) The Hippo–YAP pathway regulates the proliferation of alveolar epithelial progenitors after acute lung injury. Cell Biology International 10: 1174-1183.
- Jia X, Wu B, Huang J, Fan L, Yang M, et al. (2021) YAP and Wnt3a independently promote AECIIs proliferation and differentiation by increasing nuclear β-catenin expression in experimental bronchopulmonary dysplasia. International Journal of Molecular Medicine 1: 195-206.
- Zhou B, Flodby P, Luo J, Castillo DR, Liu Y, et al. (2018) Claudin-18–mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. Journal of Clinical Investigation 3: 970-984.
- Mocumbi A, Humbert M, Saxena A, Jing Z-C, Sliwa K, et al. (2024) Pulmonary hypertension. Nature Reviews Disease Primers 1: 1.
- Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM (2020) Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation 8: 678-693.
- Reinders S, Didden E-M, Ong R (2024) Survival, morbidity, and quality of life in pulmonary arterial hypertension patients: a systematic review of outcomes reported by population-based observational studies. Respiratory Research 1: 373.
- Santos-Gomes J, Ribeuz HL, Brás-Silva C, Antigny F, Adão R (2022) Role of Ion Channel Remodeling in Endothelial Dysfunction Induced by Pulmonary Arterial Hypertension. Biomolecules 4: 484.
- Babicheva A, Zhao T, Yuan JXJ (2019) KCNK3 Channel: A New Player in the Field of Pulmonary Vascular Disease. Circulation Research 7: 696-698.
- Gessler T (2018) Inhalation of repurposed drugs to treat pulmonary hypertension. Advanced Drug Delivery Reviews: 34-44.
- He J, Bao Q, Yan M, Liang J, Zhu Y, et al. (2017) The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. British Journal of Pharmacology 8: 1354-1361.
- Masliantsev K, Karayan-Tapon L, Guichet P-O (2021) Hippo Signaling Pathway in Gliomas. Cells 1: 184.
- Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, et al. (2016) HIPPO–Integrin-linked Kinase Cross-Talk Controls Self-Sustaining Proliferation and Survival in Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine 7: 866-877.
- Kudryashova TV, Dabral S, Nayakanti S, Ray A, Goncharov DA, et al. (2022) Noncanonical HIPPO/MST Signaling via BUB3 and FOXO Drives Pulmonary Vascular Cell Growth and Survival. Circulation Research 5: 760-778.
- Zuo W, Liu N, Zeng Y, Xiao Z, Wu K, et al. (2021) Luteolin Ameliorates Experimental Pulmonary Arterial Hypertension via Suppressing Hippo-YAP/PI3K/AKT Signaling Pathway. Frontiers in Pharmacology 12: 663551.
- Podolanczuk AJ, Raghu G (2024) Idiopathic pulmonary fibrosis mortality: update on trends in the modern treatment era. European Respiratory Journal 2: 2401305.
- Ogawa T, Shichino S, Ueha S, Matsushima K (2021) Macrophages in lung fibrosis. International Immunology 12: 665-671.
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, et al. (2022) Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine 9: 18-47.
- Wilson KC, Raghu G (2015) The 2015 guidelines for idiopathic pulmonary fibrosis: an important chapter in the evolution of the management of patients with IPF. European Respiratory Journal 4: 883-886.
- Man RK, Gogikar A, Nanda A, Janga LSN, Sambe HG, et al. (2024) A Comparison of the Effectiveness of Nintedanib and Pirfenidone in Treating Idiopathic Pulmonary Fibrosis: A Systematic Review. Cureus 16: 54268.
- Proesmans VLJ, Drent M, Elfferich MDP, Wijnen P, Jessurun NT, et al. (2019) Self-reported Gastrointestinal Side Effects of Antifibrotic Drugs in Dutch Idiopathic Pulmonary Fibrosis patients. Lung 5: 551-558.
- Chianese M, Screm G, Salton F, Confalonieri P, Trotta L, et al. (2024) Pirfenidone and Nintedanib in Pulmonary Fibrosis: Lights and Shadows. Pharmaceuticals 6: 709.
- Papavassiliou KA, Sofianidi AA, Spiliopoulos FG, Gogou VA, Gargalionis AN, et al. (2024) YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis. Cells 18: 1519.
- Chen Y, Zhao X, Sun J, Su W, Zhang L, et al. (2019) YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death & Differentiation 9: 1832-1844.
- Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, et al. (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology 4: 344-357.
- Huang L, Yang X, Feng Y, Huang H-X, Hu J-Q, et al. (2025) ShaShen-MaiDong decoction attenuates bleomycin-induced pulmonary fibrosis by inhibiting TGF-β/smad3, AKT/MAPK, and YAP/TAZ pathways. Journal of Ethnopharmacology 337: 118755.
- Labaki WW, Rosenberg SR (2020) Chronic Obstructive Pulmonary Disease. Annals of Internal Medicine 3: 17-32.
- He Y, Qian DC, Diao JA, Cho MH, Silverman EK, et al. (2023) Prediction and stratification of longitudinal risk for chronic obstructive pulmonary disease across smoking behaviors. Nature Communications 1: 8297.
- Sin DD, Doiron D, Agusti A, Anzueto A, Barnes PJ, et al. (2023) Air pollution and COPD: GOLD 2023 committee report. European Respiratory Journal 5: 2202469.
- Chang J-H, Lee Y-L, Laiman V, Han C-L, Jheng Y-T, et al. (2022) Air pollution-regulated E-cadherin mediates contact inhibition of proliferation via the hippo signaling pathways in emphysema. Chemico-Biological Interactions 351: 109763.
- Hespanhol V, Bárbara C (2025) Pneumonia mortality, comorbidities matter? Pulmonology 3: 123-129.
- Tang W, Li M, Yangzhong X, Zhang X, Zu A, et al. (2022) Hippo signaling pathway and respiratory diseases. Cell Death Discovery 1: 213.
- Ma T, Ruan H, Lv L, Wei C, Yu Y, et al. (2023) Oleanolic acid, a small-molecule natural product, inhibits ECM degeneration in osteoarthritis by regulating the Hippo/YAP and Wnt/β-catenin pathways. Food & Function 22: 9999-10013.
- Lee EH, Park K-I, Kim K-Y, Lee J-H, Jang EJ, et al. (2019) Liquiritigenin inhibits hepatic fibrogenesis and TGF-β1/Smad with Hippo/YAP signal. Phytomedicine 62: 152780.
- Li X, Liu Q, Xie S, Wu X, Fu J (2024) Mechanism of action of cordycepin in the treatment of hepatocellular carcinoma via regulation of the Hippo signaling pathway. Food Science and Human Wellness 2: 1040-1054.
Citation: Guo Y, Jiao Z, Xu Y, Wang C, Jia T, et al. (2025) Natural Products Protect Lung Diseases by Targeting the Hippo Signaling Pathway. HSOA J Altern Complement Integr Med 11: 652.
Copyright: © 2025 Yifei Guo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

