Neck and Brain Venous Lesions in Meniere’s Disease
*Corresponding Author(s):
Aldo BrunoDepartment Of Vascular, GEPOS Clinic, Telese Terme, Italy
Tel:+39 3357494926,
Email:dottaldobruno@gmail.com
Abstract
Objectives: The authors evaluate the incidence of Chronic Cerebro-Spinal Venous Insufficiency (CCSVI) in patients with Meniere’s Disease (MD).
Methods: Between April 2013 and April 2020, 622 patients with diagnosis of Meniere’s Disease were included, all were submitted to duplex ultrasound and Magnetic Resonance Angiography (MRI) with TOF 2D and 3D reconstruction and compared to a control population.
Results: Chronic Cerebo-Spinal Venous Insufficiency (CCSVI) was demonstrated in 81.4% of patients with Meniere’s disease vs. 12.7% of the volunteers. Visible defects were present in 90% of the cases. There was a high correlation, round 90%, between ultrasound exam and MRI.
Conclusion: Our results suggest that there is a high incidence of neck and brain veins in patients suffering of Menière's disease.
Keywords
Cronic Cerebro-Spinal Venous Insuffucuency (CCSVI); Endolymphatic Hydrops (EH); Magnetic Resonance Angiography (MRI); Menierè Disease (MD)
INTRODUCTION
Meniere’s disease is a chronic disease that affects the inner ear and is related to the presence of Endolymphatic Hydrops (EH). It is characterized by four symptoms: mono or bilateral hearing impairment, tinnitus, aural fullness, and recurrent dizziness [1].
The pathology was first described by Prospero Ménière in 1861, he was the first who identified the inner ear as the organ of our equilibrium [1].
EH appears to be the pathological substrate necessary, but it is not responsible for the entire cochlear and vestibular symptomatology.
The diagnosis is actually done on the criteria stated by the Barany Society that 2015 located new guidelines [2,3]. The number of cathegories of Meniere’s disease is now reduced to “definite” and “probable”.
The “definite” is characterized by relapsing dizziness associated with low- to medium-frequency sensorineural hearing loss and fluctuating aural symptoms (hearing, tinnitus and/or fullness) in the affected ear confirmed by an audiometric examination. Duration of vertigo episodes is limited to a period between 20 min and 12 h. “Probable” Menière'sdisease is a broader concept defined by episodic vestibular symptoms associated with fluctuating aural symptoms occurring in a period from 20 min to 24 h that are reported by the patients but are not confirmed by audiometric test [2].
MD occurs in approximately 0.5 per 100.000 people, but there is no absolute data security and in some countries there is a much higher incidence such as England (140 per 100.000 people).
Diagnostic guidelines from the American Academy of Otology in 1985 and 1995 defined MD as an idiopathic syndrome of endolymphatic hydrops. Endolymphatic Hydrops (EH), after the experience of Merchant, is a pathologic substrate necessary but not sufficient to determine a clinically evident disease and its responsability in determining the cochlear and vestibular pathology is not yet demonstrated. At the moment Endolimpatic Hydrops is a symptom as vertigo, hearing loss, fulness, tinnitus [4-9].
The demonstration of the endolymphatic hydrops was at the beginning only feasible on a temporal bone sample but recently, the objective diagnosis by MRI has become possible using intravenous or intratympanic gadolinium injection [10-14].
The disease does not have a unanimously determined predominance, does not have a prevalence of sex, is more common in the Caucasian race [15], almost always begins unilaterally but tends to bilateralization in a significant percentage of cases that progressively increases over the years [16-20].
There are still some uncertainties: A) Etiology: genetics, post-traumatic, post-inflammatory, allergic, immune and autoimmune, viral, hormonal, vascular, toxic, neoplastic (prolactinomy), etc., The origin is probably multifactorial with different incidence not always evident in each patient. It is well known the association between Menière's disease and migraine, and it has also been hypothesized that the two conditions have a common etiopathogenetic mechamism [21,22]. B) Pathogenesis: Multiple causes would work synergistically in determining a condition of cellulartoxicity on non-receptive cells of the inner ear, particularly those of the vascular stroma and of the endolimphatic sac, affected by the homeostatic mechanisms of the endolimpha. About the vascular hypothesis, already Godlowski, in 1972, described that the elevation of the hydrostatic pressure at the arterial end of the microcirculation in the striavascular is may increase the force that drives fluid from the capillaries into the endolymphatic space. In such an event, the hydrostatic pressure within the endolymph rises only if the fluid is not eliminated at an equal rate back into the blood at the venous end of the striavascularis, which finally drains into the Internal Jugular Vein (IJV) [23]. C) Therapy: Medical therapy reflects the etiopathogenetic uncertainties. No conservative medical therapy exists which shows evidence of efficacy in the control or treatment of the disease [23]. Intratimpanic therapies, conservative with steroid, or subablative chemistry with gentamicin, show some evidence of efficacy [23]. Surgical therapy: endolimphatic sac surgery [24], labyrinthectomy and selective vestibular nerve neurotomy are reserved for the few cases not otherwise controlled [25,26]. Certainly there is no consensus on the etiology and best treatment for Menière's disease so it is reasonable to look for new treatment strategies. In 2006, Zamboni presented for the first time the concept that a chronic disease of cerebral venous outflow could partially or totally induced neurodegenerative disease and coined the term Cerebral Spinal Venous Insufficiency (CCSVI), associating this condition in particular with Multiple Sclerosis [27]. In 2009, CCSVI was recognized as a nosological entity by the Consensus Document of the International Union of Phlebology on Venous Malformations [28]. Vascular anomalies cause brain function modification of the endothelial cell function by slowing the cerebrospinal venous outflow, which in turn worsens cellular perfusion with alterations to the blood-brain barrier [29]. The slowing down of the venous outflow causes an increase in venous pressure associated with a wave reflected by the arterial system, with a further increase in intravascular pressure to the capillary venous system that worsen the damage of the endothelial cells leading to small endothelial layer breaks. This causes diffusion of intravascular fluid, proteins and blood cells with local activation of inflammatory cascade, iron deposition, loss of oligodendrocytes and attraction of phagocytes, increase in interstitial fluid, and hence increased extracellular lymphatic fluid [30,31].
The evaluation of the increase in cerebral venous pressure was evaluated by mathematical models: Toro [32] developed a system based on MRI evaluations in which the stenosis of the major venous branches of the cerebral outflow causes endocranial hypertension with impact on the inner ear. There would be a stasis situation that would result in endothelial cellular toxicity with consequent cellular cell damage due to iron accumulation phenomena but also to osmotic alteration of extracellular fluid components [30-31]. The cerebral anomalies may also affect the inner ear structures, which also drain into the jugular venous system. According to the model proposed by Merchant for Menière's disease [33], the site of major interest should be the striavascularis with its fibrocytes actively involved in the endolymphatic homeostasis. We might hypothesize that at least one of the unknown causes of cellular toxicity postulated by Merchant could be the venous stasis resulting in cellular damage to the vascular stria, which would cause alterations in the metabolism and endolimphatic homeostasis that would lead to development of hydrops
Correlation Between Ccsvi and Menière Disease: Hypothesis Venous drainage of the inner ear occurs through the internal auditory vein, the cochlear aqueduct vein and the vestibular aqueduct vein, which are drained in the inferior petrosal sinus, the transverse sinus and finally in the inner jugular vein. In particular, in humans the vein of the vestibular aqueduct drains the venous blood of the utricle, the semicircular ducts, the saccule and the endolinphatic duct. The slowdown of the flow at this level would increase the pressure of the inner ear veins. Friis performed an interesting experimental study on the inner ear veins: he closed the aqueduct vestibular vein close to the endolinphaticsack in the guinea pigs and observed the formation of a portal flow of the inner ear and symptoms matching those described for Menière's disease [34]. This would happen as the direction of the venous flow in the inner ear is inverted, resulting in a portal flow, where venous capillary blood falls into the arterial capillary circulation. In addition, the endolinophatic sac, as described in many papers, contains glycosaminoglycans, proteins and a natriuretic hormone, whose modifications would result in changes in the composition of inner ear fluids with negative functional effects on dark cells [34-36].
A thrombotic obstruction of the vestibular aqueduct vein has been observed in some patients with Menière's disease at autoptic examination. The presence of microthrombi would modify the flow dynamics so explaining the fluctuating symptoms typical of Menière's disease [37,38].
Over the years, the persistence of slow outflow with consequent injury to the labirinth endothelium veins would result in fibrosis of the endolimphatic sac and duct, decreased vascularization of the latter, increased local hydrostatic pressure, consequent inversion of the venous flow as predicted by the experimental model by Friis [34]. Anatomopathological studies performed on the inner ear in MD showed that striavascularis is atrophic and the number of vessels is highly reduced [37-38].
Merchant's experiment supports this hypothesis demonstrating the constant presence of endolimphatic hydrops in Menière's patients. However hydrops is not necessarily associated to the syndrome: this means that endolimphatic hydropsshould be considered as a histologic marker for Meniere's Disease rather than being directly responsible for its symptoms. The hydrops is in turn due to a primitive modification of the labyrinth homeostasis caused by cellular toxicity of type I and type II fibrocytes involved in the homeostasis of labyrinth fluids [33]. The reduced venous out flow, as demonstrated in CCSVI and in MS with concomitant CCSVI as underlined by Adams [33], occur probably even in Menière’s disease. It would affect the venous wall, increasing its permeability with formation of peripheral lymphocytic infiltrates and iron deposits which would cause cytotoxic damage to fibrocytes particularly in the highly vascularized districts (striavascularis). This problem in association with other causes, could lead to the development of the typical alterations and symptoms of the Menière’s disease. But, also changes in the Neuro-Vascular-Unit usually result in the impairment of Blood Brain Barrier (BBB) integrity and penetration of blood derived proteins, such as fibrinogen and plasimogen which has recently been suggested to clot and form insoluble pro-infiammatory fibrin deposit in the brain.
Another important aspect is the putative role of brain Lymphatic System in MD pathogenesis. It was described from Alpini and Loveau [39-40]. The authors hypothesized after showed, by brain scan, that CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature.
Finally a new data was supported by the data reported by O’Malley et al.,who found microglia-likecellsin the cochlea and in the vestibular apparatus of patients with autoimmune diseases such as Meniere disease. This aggression by microglia may determine a lesion with an area large enough to generate vertigo [42-43].
Based on these theoretical considerations, three Italian Schools have sought and found an relationship between CCSVI and Menière’s disease [44-47].
Two methods were used to diagnose CCSVI in patients with Meniere’s “definite” disease:
- • Duplex-ultrasound of the jugular system associated to transcranial doppler to evaluate the deep cerebral veins and veins reflux
- • MRI angiography of the neck and intracranial circle with venous phase [48]. This study allow a complete minimally invasive evaluation of the neck venous system, of the brachiocephalic veins and venous sinus. The main advantages are: a complete and precise evaluation of venous anomalies necessary to plan the eventual procedure, the evaluation of benign intracranial hypertension and repeatability. However there is not yet a consensus on the protocol study for CCSVI.
MATERIALS AND METHODS
This study was submitted and approvedby the Campania 2 Ethic Committee (Protocol n. 277/2013). All the treated patients included in the study signed the informed consent on the diagnostic exams and invasive procedure according to the Helsinki Declaration.
Between April 2013 and April 2020, 622 patients were included in the study (380 females - 242 males). Mean age was 53.1 +/- 15.3 years. All of them had a “definite” Menière's disease, monolateral in 521 cases, bilateral in 101, according to A.A.O. 2015 criteria. The onset of symptoms varied between 1 and 28 years (Table 1).
|
Patients: n.622 Age 47.4+/- 11.43 P. Value .45 Disease Duration, yr. 0.5-28 P. Value 0.48 Pure-Tone Audiometry: 57.9 THI: 50.2, DHI 54,6 DHI: Dizziness Handicap Inventory THI: Tinnitus Handicap Inventory.
|
Table 1: Clinical Characteristics of Patients with concomitant Meniere’s Disease and C.C.S.V.I.
All the patients were submitted to duplex ultrasound of the deep cerebral and neck veins according to the criteria of the International Society for Neurovascular Disease 2011, modified by the 2014 guidelines (Table 2) [48]. The exam was performed also on a control population that included 102 healthy volunteers who did not have a neurologic or audio vestibular disease (54 females and 48 males).
|
1)Reflux in the IJVs and/or VVs in orthostatic and supine postures |
Pathological when reversal flow lasted >88 sec. |
7/12 (58,3%) |
277/622 (44,5%) |
|
2) Bidirectional flow (or reflux) in the intracranial veins and sinuses |
Using the same intracranial approach and QDP system |
10/12 (83,3%) |
498/622 (80%) |
|
3) B-mode abnormalities/stenoses of the IJVs including: A. Morphological stenosis
B. Hemodynamic stenosis |
Presence of severe reduction of the CSAof IJVs in the supine position (
Significant stenoses with simultaneous presence of intraluminal defects such as webs, septa, or malformed valves and hemodynamic changes (block, reflux, increased velocity flow) |
11/12 (91,7%)
5/12 (41,7%) |
560/622 (90%)
433/622 (69,7%) |
|
4) Flow not Doppler-Decteable in IJVs aand or VVs despite numerous forced inspirations |
Tested in both sitting and supine position |
0% |
277/622 (44,5%) |
|
5) Negative cross- sectional area in the IJVs |
Value is obtained by measuring the difference in IJV cross sectional area between the supine and upright positions |
0% |
21/622 (3,3%) |
Table 2: Zamboni Diagnostic Parameters for C.C.S.V.I. and Incidence in Patient with Meniere’s Disease and Healthy Controls.
|
CCSVI-Positive CCSVI-Positive Healthy Controls: MD Patients: 12/102 (12,7%) 498/622 (80%)
|
Patients with “definite” Meniere’s disease and duplex ultrasound CCSVI diagnosis were submitted to cerebral and neck MRI angiography (GE Brivo MR 355 machine1.5 T.). The MRI study protocol consist of TOF (2D) images to study arterial and venous flow dynamic and 3D contrast-enhancement MR to evaluate vascular anomalies: atresia, aplasia, truncular malformations, valve problems.
The CSA (cross sectional area) was used to evaluate the degree of stenosis of the target vessels: value < 25 mm2 at or below C3 was considered as a stenosis, on the contrary above C3 the vessel was considered stenotic for CSA < 12.5 mm2.
The inner ear was evaluated with multiplanar reconstructions before and after contrast medium.
STATISTICAL ANALYSIS
Patient’s demographics were evaluated with t-test; the significance of the association between "definite" Meniere's disease and CCSVI was determined by comparison with the control population with Fisher's exact test; the comparison of the initial audiologic staging was evaluated with the χ2 test. The comparison between the initial DHI and THI in the two group of patients and the pre and postoperative DHI and THI were evaluated with the Unpaired t test and so the comparison pre and post PTA Pure Tone Average (Figure 1).
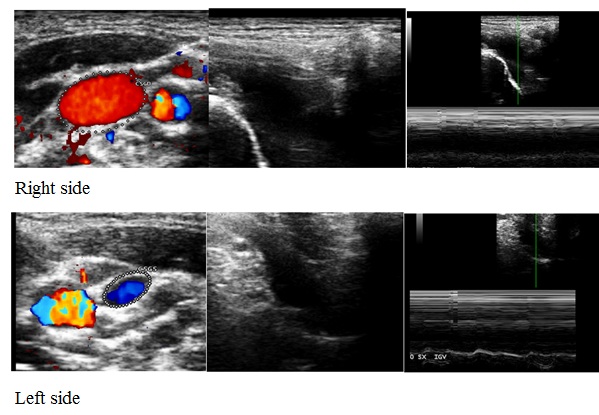 Figure 1: Clinical Case Patient with Hypoplasia IJV (Right Side and Left Side).
Figure 1: Clinical Case Patient with Hypoplasia IJV (Right Side and Left Side).
RESULTS
The patients with Meniere's disease and the healthy control volunteers were omogenous for age (53.1 +/- 15.34 years vs 49.7+/- 16.87; p=0.82).
CCSVI diagnosis according to Zamboni criteria was present in 506 of the 622 patients with Meniere's disease (81.4%) vs. 12/102 of the volunteers (12.7%) (p<0.0001), with no significant differences regarding sex and age between patients with and without CCSVI. The most common ultrasound anomalies were visible intraluminal defects (Criterion 3) in 90% of cases - presence of two-way flow in intracranial veins and sinuses (criterion 2) in 80%, the absence of flow both in VGIs and VVs and / or absence of flow in one projection and two-way in the other (Criterion 4) in 45% of cases, while Criterion 5 (DCSA greater or unchanged at 90 ° and 0 °) was found only in a small number of patients (3%). About the criteria 3 we found an incidence of about 18-20% a compression byhomoid muscle in J1-J2 and a compression of IJV in J3 by styloid process as it is described in the Eagle Syndrome [49] (Figures 2-4).
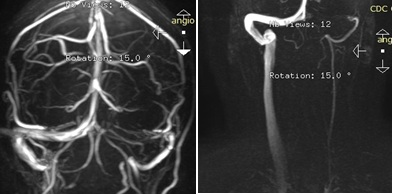 Figure 2: Patient with bilateral Meniere’s Disease with debut on the left in 2005 and on the right in the 2014. Sympthomatology: Vertigo, Balance Disorders, Fulness, Fluctuating hearing loss, Headache, Brain Fog and visual disturbances during vertigo.
Figure 2: Patient with bilateral Meniere’s Disease with debut on the left in 2005 and on the right in the 2014. Sympthomatology: Vertigo, Balance Disorders, Fulness, Fluctuating hearing loss, Headache, Brain Fog and visual disturbances during vertigo.
 Figure 3: Duplex Scan: Right. IJV reflux 0° and 90° con septum in J1. Left IJV: Valve malformation, hypoplasia with slow flow in both position.
Figure 3: Duplex Scan: Right. IJV reflux 0° and 90° con septum in J1. Left IJV: Valve malformation, hypoplasia with slow flow in both position.
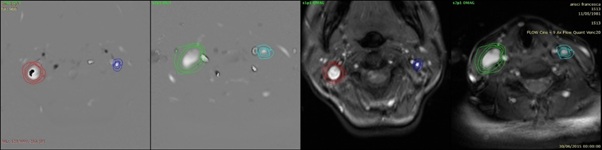 Figure 4: MRI: hypoplasia IJV left, occlusion, left upper transervse venous sinus, signs of benign intracranial hypertension, evaluatation of the flow in both IJVs with block of the flow of Left IJV.
Figure 4: MRI: hypoplasia IJV left, occlusion, left upper transervse venous sinus, signs of benign intracranial hypertension, evaluatation of the flow in both IJVs with block of the flow of Left IJV.
In case of monolateral Menière’s disease, the vein lesion was always found on the side of the diseased ear, while in cases of bilateral illness, the longest onset side was always found to have more severe venous lesions.
In about 8% of the patients we found a hypoplasia of an IJV, occlusion of the transerverse superior venous sinus of the same side and stenosis the IJV of the other side. In these cases the menieric ear was the one where the internal jugular vein was stenotic.
The most involved jugular segment was J1 (60%), in 30% there was a J3 injury and in 10% a J2.
DISCUSSION
Menière's Disease, although described for about 150 years ago, does not currently have a well defined etiology, pathogenesis, and definitive therapy with a high percentage of patients who do not respond to common therapeutic strategy. This study has shown that a high percentage of patients is affected by a modification of venous internal ear drainage due to changes in the jugular and azygos vein, whose treatment seems to offer a further therapeutic option for patients who do not respond to standard therapies.
CCSVI could therefore result in anatomical and functional alterations at the level of the non-receptive structures of the inner ear, particularly at the level of the striavascularis, which could alter the endo-facial homeostasis in a worse sense. This in addition to other cofactors could determin the outbreak of Menière's disease. The high incidence of stenosis of the veins responsible for endocranial circulatory drainage suggests that there could be a link between CCSVI and Meniere's disease and that CCSVI can be considered a further etiopathogenetic mechanism of this disease.
This study has developed new diagnostic criteria both for ultrasonographic examination and MRI of venous vessels of the neck and intracranial circulation, opening up new therapeutic horizons.
Endovascular treatment of jugular and azygos veins stenosis has proved to be a low-risk procedure with long-lasting effects: At 24 months follow-up only 4 patients (8%) developed a restenosis; results on Menière's disease symptoms were favourable in a high percentage of patients, especially on dizziness, which is the most disabling factor, with improved quality of life in 75% of cases.
CONCLUSION
The results of the present study confirm the close relationship between vascular disorders and MD. The ethical and feasibility limits remaina reason for discussion and comparison between otologists, but the results justify the interest in the senew look, considering that don’t existe at the moment a definitive care MD. Other experimental studies are needed in this field, which can be one of the promising paths for the interpretation and treatment of this com- plexdisease.
REFERENCES
- Ménière P (1816) Pathologieauriculaire: Mémoires sur des lésions de l’oreille interne donnant lieu à des symptoms de congestion céré Gaz Med Paris 16: 597-601.
- Lopez-Escamez JA, Carey J, Chung W-H, Goebel JA, Magnusson M, et al. (2015) Diagnosticcriteria for Menière’s disease. J Vestib Res 25: 1-7.
- Committee on Hearing and Equilibrium (1995) Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc., Otolaryngol Head Neck Surg 113:181-185.
- Wu Q, Dai C, Zhao M, Sha Y. (2015) The correlation between symptoms of definite Meniere’s disease and endolymphatic hydrops visualized by magnetic resonance imaging 126: 974-979.
- Schuknecht HF, Gulya AJ (1983) Endolymphatic hydrops. An overview and classification. Ann Otol Rhinol Laryngol 92: 1-20.
- Sperling NM, Paparella MM, Yoon TH, Zelterman D (1993) Symptomatic versus asymptomatic endolymphatic hydrops: a histopathologic comparison. Laryngoscope 103: 277-285.
- Kiang NYS. An auditory physiologist’s view of Ménière’s syndrome.
- Nadol JB Jr (1989) Second International Symposium on Ménière’s disease. Amsterdam: Kugler and Ghedini, Pg no: 13-24.
- Louza J, Krause E, Gürkov R (2015) Hearing function after intratympanic application of gadolinium-based contrast agent: A long-term evaluation. Laryngoscope 125: 2366-2370.
- Casselman JW, Kuhweide R, Ampe W, Meeus L, Steyaert L (1993) Pathology of the membranous labyrinth: comparison of T1- and T2-weighted and gadolinium-enhanced spin-echo and 3DFT-CISS imaging. AJNR Am J Neuroradiol 14: 59-69.
- Albers FW, Van Weissenbruch R, Casselman JW (1994) 3DFT-magnetic resonance imaging of the inner ear in Ménière's disease. Acta Otolaryngol 114: 595-600.
- Naganawa S, Nakashima T (2014) Visualization of endolymphatic hydrops with MR imaging in patients with Meniere's disease and related pathologies:currentstatuts of its methods and clinical significance Jpn J Radiol 32: 191-204.
- Naganawa S, Sugiura M, Kawamura M, Fukatsu H, Sone M (2008) Imaging of endolymphatic and perilymphatic fluid at 3T after intratympanic administration of gadolinium-diethylene-triamine pentaacetic acid. AJNR Am J Neuroradiol 29: 724-726.
- Hoa M, Friedman RA, Fisher LM, Derebery MJ (2015) Prognostic implications of and audiometric evidence for hearing fluctuation in Meniere’s disease. Laryngoscope
- Ohmen JD, White CH, Li X, Wang J, Fisher LM, et al. (2013) Genetic evidence for an ethnic diversity in the susceptibility to Ménière’s disease. Otol Neurotol 34: 1336-1341.
- Belinchon A, Perez-Garrigues H, Tenias JM (2012) Evolution of symptoms in Ménière's disease. Audiol Neurootol 17: 126-132.
- Seo T, Saka N, Sakagami M (2012) Furosemide-loading vestibular evoked myogenic potential testing can suggest developing bilateral involvement of unilateral Meniere's disease. Acta Otolaryngol 132: 632-636.
- Friberg U, Stahle J, Svedberg A (1984) The natural course of the Ménière´s disease. Acta Otolaryngol 406:72-77.
- Havia M, Kentala E (2004) Progression of symptoms of dizziness in Ménière´s disease. ArchOtolaryngol Head NeckSurg 130: 431-435.
- Tokumasu K, Fujino A, Yoshio S, Hoshino I (1995) Prognosis of Ménière´s disease by conservative treatment: retrospective study on the time course of the disease. Acta Otolaryngol (Suppl) 519: 216-218.
- Murofushi T, Ozeki H, Inoue A, Sakata A (2009) Does migraine-associated vertigo share a common pathophysiology with Menière's disease? Study with vestibular-evoked myogenic potential. Cephalalgia 29: 1259-1266
- Godlowski Z (1972) Hyperosmosis of endolymph as primary pathogenic mechanism of Meniere’s Disease and its clinical management. Acta OtolaryngolSuppl 299: 1-36.
- Syed MI, Ilan O, Nassar J, Rutka JA (2015) Intratympanic therapy in Meniere's syndrome or disease: up to date evidence for clinical practice. ClinOtolaryngol 40: 682-690.
- Pullens B, Verschuur HP, van Benthem PP (2013) Surgery for Ménière's disease. Cochrane Database of Systematic Reviews.
- Paparella MM (2008) Endaural labyrinthectomy. Ear, Nose Throat J 87: 204.
- Li CS, Lai JT (2008) Evaluation of retrosigmoid vestibular neurectomy for intractable vertigo in Ménière's disease: an interdisciplinary review. Acta Neurochirurgica 150: 655-661.
- Zamboni P (2006) The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R SocMed 99: 589-593.
- Lee BB, Bergan J, Gloviczki P, Laredo J, Loose DA, et al. (2009) Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP)-2009. International Angiology 28: 434-451.
- Talbert DG (2008) Raised venous pressure as a factor in multiple sclerosis. Med Hypothesese 70: 1112-1117.
- Zamboni P, Menegatti E, Weinstock-Guttman B, Dwyer MG, Schirda CV, et al. (2011) Hypoperfusion of brain parenchima is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: A cross sectional preliminary report. BMC Med 9:22
- Tsamopoulos NG, Kalodimou VE, and Vlachos S (2014) Chronic Cerebrospinal Venous Insufficiency in Multiple Sclerosis: The Hydrostatic-Immune Paradigm and the Flow Cytometry as a Diagnostic Tool. J Mult Scler 1:103.
- Toro EF (2016) Brain Venous haemodynamics, neurological disease and mathematical modelling. A review. Applied Mathematics and Computation 272: 542-579.
- Merchant SN, Adams JC, Nadol JB Jr (2005) Pathophysiology of Meniere's syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol 26: 74-81.
- Friis M., Klauss O (2007) Apotential Portal Flow inthe Inner Ear. Laryngoscope 117: 194-201.
- Nadol JB, Adams JC, Kim JR (1995) Degenerative changes in the organ of Corti and lateral cochlear wall in experimental endolymphatic hydrops and human Meniere’s disease. Acta Otolaryngol 519: 47-59.
- FribergU, Rask-Andersen H (2002) Vascular occlusion in the endolymphatic sac in Meniere’s disease. Ann Otol Rhinol Laryngol 11: 237-245.
- Kariya S, Cureoglu S, Fukushima H, Nomiya S, Nomiya R, et al. (2009) Vascular findings in the striavascularis of patients with unilateral or bilateral Ménière's disease: a histopathologic temporal bone study. Otol Neurotol 30: 1006-1012.
- Kariya S, Cureoglu S, Fukushima H, Kusunoki T, Schachern PA, et al. (2007) Histopathologic changes of contralateral human temporal bone in unilateral Ménière's disease.Otol Neurotol 28: 1063.
- Alpini DC, Di Berardini F, Mattei V, Manuela DM, Carlo B (2017) The Putative Role of Brain Lymphatic System in Ménière Disease Pathogenesis. J Lymphol Phlebol 1: 00001
- Loveau A, Herz J, Alme MN, Salvador AF (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nature Neurosciences 21: 1380-1391.
- Di Berardino F, Alpini DC, Bavera PM, Cecconi P, Farabola M, et al. (2015) Chronic cerebrospinal venous insufficiency in Ménière disease. Phlebology 30: 274-279.
- Echard A, Santos F, Di Stadio A, O'Malley J, McKenna J (2015) Microgliain Meniere Sindrome.In: Posterpresentation.7th InternationalSymposiumon MeniereDiseaseand Innerear disorders, Rome, Italy.
- O'Malley JT, Nadol JB Jr, McKenna MJ (2016) Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol Neurotol 37: 99-108.
- Filipo R, Ciciariello F, Attanasio G, Mancini P, Covelli E, et al. (2013) cerebrospinal venous insufficiency in patients with Ménière's disease. Eur.Arch.Othorinolaringology 272: 77-82.
- Bruno A, Mastrangelo D, et.al (2013) Chronic cerebrospinal venous insufficiency in Meniere Disease: diagnosis and treatment. Otorinolaringologia 63: 173.
- Bruno A, Califano L, Mastrangelo D, De Vizia M, Bernardo CD , et al. (2014) Chronic cerebrospinal venous insufficiency in Meniere’s Disease: Diagnosis and treatment. Veins and Lymphatic 3: 77-80.
- Bruno A, Napolitano M, Califano L, Attanasio G, Giugliano V, et al. (2016) The Prevalence of Chronic Cerebrospinal Venous Insufficiency in Meniere Disease: 24-Month Follow-up after Angioplasty. J Vasc Interv Radiol 28: 388-391.
- Zivadinov R, Bastianello S, Dake S, Ferral H, Haacke EM, et al. (2014) Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Int Rad 1: 1785-1794.
- Zamboni P, ScerratiA, Menegatti E, et. A (2019) The eagle jugular syndrome BMC Neurology (2019) 19: 333.
Citation: Bruno A, Attanasio G, Califano L, Giugliano V, De Vizia R, et al. (2020) Neck and Brain Venous Lesions in Meniere’s Disease. J Angiol Vasc Surg 5: 052.
Copyright: © 2020 Aldo Bruno, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.