
Neurotransmitters: A Computational and bio mathematical approach at the time of COVID-19
*Corresponding Author(s):
Bin ZhaoSchool Of Science, Hubei University Of Technology, Wuhan, Hubei, China
Tel:+ 86 130 2851 7572,
Email:zhaobin835@nwsuaf.edu.cn
Abstract
COVID-19 has already swept millions of lives and created the deep black cloud made up of negative emotions. Acanthus ilicifolius was used as Traditional Indian medicine (TIM) and Traditional Chinese medicine (TCM). The plants showed many clinical properties. Still, the neurological related functions and disorders are not well explored in this plant. Complex interplay of positive and negative emotions orchestrated by intricately associated neuronal circuits, neurotransmitters coupled with endocrinal in- fluence holds responsible for human behavior, considered as the root of human civilization, is currently facing existential crisis during COVID-19 pandemic. In the present study, an attempt was made to identify the interaction between A. ilicifolius natural compounds and Echinacoside as reference compounds were to study the neurotransmitters functions through biomathematical and computational method. Initially, in silico molecular docking was performed to identify the potent natural compounds against neurological disease. The results show among 8 natural compounds, 26.27-Di(nor)-cholest-5,7,23-trien-22-ol, 3-methoxymethoxy, Cholest-5-en-3-ol (3, Beta.)-, carbonochloridate, Cholesterol and Echinacoside exhibited maximum interaction with all the target proteins. Especially, Echinacoside exhibited the maximum interaction with (Serotonin) 5-hydroxytryptamine receptor 2A (-17.077), Sodium-dependent serotonin transporter (-15.810) and (Histamine) Histamine H2 receptor (-17.556). These two neurotransmitters act as a major concern related to the mental disorders and neurological functions. The natural compounds may potent inhibitor for neurological disorders.
Keywords
Acanthus ilicifolius, Biomathematics; Echinacoside, In silico, Neurotransmitters.
Introduction
In Ayurvedic medicine (Sahachara), A. ilicifolius one among the 9 plants to treat rheumatic complaints. Leaves and roots are used as a treatment for asthma, paralysis, Anti-inflammatory, Antimicrobial, used as an antidote for snake venom and Hepatoprotective function. Neurotransmitters function which are the important physiological phenomena of human beings. A presynaptic nerve cell is induced by certain stimulus called neurotransmitters, a molecule which can stimulate or inhibit a postsynaptic cell which is released into the body. Glycine, serotonin, Gamma Amino Butyric Acid (GABA), endorphins, norepinephrine, acetylcholine, and dopamine are some of the neurotransmitters found in the body [1]. Our nervous system appears as a vast network of specialized cells, of which, neurons play an important role in information processing based on the location, morphology, chemistry, and connectivity of the cell types [2].
Neurons are available in an enormous amount and they communicate with each other through neurotransmitters. It acts as chemical messengers to synchronize the signals transmitted from neuron to neuron and also play a central role in proteins involved in neurotransmitter synthesis and inactivation, neurotransmitter receptors and brain function. These brain chemicals interact with target sites through receptors located in the brain also regulate a wide variety of processes throughout the body (organs, glands, and muscles) [3].
Neurotransmitters such as PEA and glutamate are responsible for the urinary measurement level and ends up in reflective of peripheral biosynthesis of urine by dopamine and serotonin. Nephrons in the kidney act as filter circulating neurotransmitters or the precursors from the blood into the urine [4]. Irrespective of the origin of production of neurotransmitters, the dysregulation of it leads to a disease state. For example, glutamate regulates as a brain’s major excitatory neurotransmitter but high glutamate results in celiac disease [5] and hyperthyroidism [6] and low level of glutamate cause migraines [7].
In the same way, histamines are the immune-modulator and neurotransmitter. High histamine involves allergies and low histamine lead to mild depression, fatigue, and weight gain and tension headaches. The pleasure and reward centre in the brain is associated with the dopamine neurotransmitter. High of anxiety and stress are due to the high level of dopamine [8] and low level of it lead to Alzheimer’s disease [9]. Norepinephrine regulate fight or flight response with bipolar disorder [10] and anxiety (high level) [11, 12] and Alzheimer’s disease (low level) [13]. The major inhibitory neurotransmitter, GABA, found elevated in ovarian cancer patients [14] and preeminent in sleep difficulties and anxiety patients with low level of GABA. Whereas, serotonin play the feelings of happiness and well-being. The high level of serotonin leads to high blood pressure, anxiety, and irritability and low level of it cause depression [15].
Neurodegenerative disorders in the nervous system are characterized by the accumulation of abnormal protein aggregation and oxidative stress was due to environmental and genetic influences. Alzheimer’s disease and Parkinson’s disease were found to be the common neurodegenerative disorder [16]. Yet another neurodegenerative disease caused by prions end up with multiple sclerosis and spongiform encephalopathies. So, based on the detailed literature survey and our previous work in the mathematical model [17]. In the present study was designed for a new drug candidate or the use of traditional medicinal plant extracts in neurotransmitters disease was done with in silico molecular docking and mathematical model. An exploration was done with Acanthus ilicifolius with 7 natural compounds extracted from leaves Echinacoside from the Cistanche deserticola was used to study the binding properties of 11 neurotransmitter such as, Muscarinic acetylcholine receptor M1, Muscarinic acetylcholine receptor M2, Muscarinic acetylcholine receptor M3, Neuronal acetylcholine receptor subunit alpha-7, D2 dopamine receptor, Gamma-aminobutyric acid type B receptor subunit 1, Glutamate receptor ionotropic, kainate 1, Beta2 adrenoceptor, 5-hydroxytryptamine receptor 2A, Sodium-dependent serotonin transporter, Histamine H2 receptor. Followed by the common standard drug was used for the comparison.
Materials And Methods
Collection and Authentication of Plant
A ilicifolius leaves were collected from Cuddalore District, Tamil Nadu, India and authenticated by the Botanical Survey of India, Tamil Nadu Agriculture University, Coimbatore, Tamil Nadu, India. (BSI/SC/5/23/09-10/Tech. 306). A voucher specimen of the plant has been deposited at the Herbarium of Botanical Survey of India.
Preparation of the leaf extracts
The fresh leaves of A. ilicifolius were washed and shade dried at room temperature (28 ± 2°C). The dried leaves were powdered by the electrical blender. 25 gms of A. ilicifolius leaf powder was used for methanol extraction in the Soxhlet apparatus [18]. The solvent was boiled gently at 64 C in a heating mantle until the extraction was done. Then the solvent was evaporated using a rotary vacuum evaporator to yield a viscous dark green residue of methanol leaf extracts.
Identification of phytochemicals
The GC-MS of A. ilicifolius methanolic leaf extract was identified compounds are 26.27-Di (nor)-cholest-5, 7, 23-trien-22-ol, 3-methoxymethoxy (RT=12.31). 9H –purin-6-amine, N, 9-bis (trimethylsilyl)-8-((trimethylsilyl) oxy) (RT=14.09). Cyano colchicines (RT=6.06). 3Beta-methoxy-5-cholesten-19-oic acid (RT=18.46) [19]. Cholest-5-en-3-ol (3, Beta.)-, carbonochloridate (RT=25.978), Cholesterol (RT=27.518), Cholest-5-en-3-ol (3, Beta.)-, propionate (RT=28.51) and Echinacoside [20]. Infrared spectroscopy identifies the functional group present in the above-listed compounds, the presence of alcohols and phenols in the O-H region at 3389 cm-1.
In silico studies
Preparation of Ligands and standard drug
The seven major phytochemical compounds considered are: 26.27-Di (nor)-cholest-5, 7, 23-trien-22-ol, 3-methoxymethoxy, 9H –purin-6-amine, N, 9-bis (trimethylsilyl)-8-((trimethylsilyl) oxy), Cyanocolchicines and 3Beta-methoxy-5-cholesten-19-oic acid. Cholest-5-en-3-ol (3, Beta.)-, carbonochloridate, Cholesterol, Cholest-5-en-3-ol (3, Beta.)-, propionate and Echinacoside structures were retrieved from Protein data bank (PDB) and ISIS Draw 2.3 software (freeware) (http://chemfan.pg.gda.pl/Oprogramowanie/Program/Draw23.exe) was used to design the ligands. Analogs were changed into MOL files and 3D optimization was done by ChemSketch 3D viewer of ACDLABS 8.0. The standard drug was used for comparison. Acetylcholine, Dopamine, GABA, Glutamic acid, Norepinephrine, Serotonin, and Histamine were collected from PubChem.
ADME property of active components
Lipinski rule of five is used to check the Adsorption, Distribution, Metabolism, and Excretion (ADME) orally active drug in humans. This was done by ADME tool. OSIRIS Property Xplorer was used to validate the drug molecule (Active phytochemicals) which has an inhibitory effect on the modeled target protein. Properties such as mutagenicity, irritant, tumorigenic and drug likeliness of the phytochemicals were studied [21, 22].
Collection of Target proteins for HCC
The lists of target proteins for neurotransmitters were collected through a literature survey and structures were retrieved from the Protein Data Bank (PDB) shown in Table 1.
|
S.No |
Gene name |
PDB ID |
Protein name |
Reference |
|
1 |
Acetylcholine |
5CXV |
Muscarinic acetylcholine receptor M1 |
[23,24] |
|
3UON |
Muscarinic acetylcholine receptor M2 |
|||
|
4U14 |
Muscarinic acetylcholine receptor M3 |
|||
|
5AFH |
Neuronal acetylcholine receptor subunit alpha-7 |
|||
|
2 |
Dopamine |
6CM4 |
D2 dopamine receptor |
[25] |
|
3 |
GABA |
4MR7 |
Gamma-aminobutyric acid type B receptor subunit 1 |
[26] |
|
4 |
Glutamic acid |
2ZNT |
Glutamate receptor ionotropic, kainate 1 |
[27] |
|
5 |
Norepinephrine |
2R4S |
Beta2 adrenoceptor |
[28] |
|
6 |
Serotonin |
5TUD |
5-hydroxytryptamine receptor 2A |
[29] |
|
5I71 |
Sodium-dependent serotonin transporter |
|||
|
7 |
Histamine |
3SN6 |
Histamine H2 receptor |
[30] |
Table 1:. List of neurotransmitters receptors.
Molecular Docking of A. ilicifolius phytochemicals against HCC target proteins
The structure was minimized using OPLS-2005 force field with Polack-Ribiere Conjugate Gradient (PRCG) algorithm. The Schrodinger Glide program version 2017 has been used for docking. The best 10 poses and corresponding scores have been evaluated using Glide in single precision mode (GlideSP) for each ligand. For each screened ligand, the pose with the lowest Glide SP score has been taken as the input for the Glide calculation in extra precision mode (Glide XP). The docking was carried out with the following non-default settings in Glide SP and Glide XP both [31].
Results And Discussion
Structure and function of the neurotransmitters by Bio-Mathematical model
Typical neuron mathematical equation
A typical neuron is generally classified into three parts namely cell body, dendrites, and axon [32]. The cell body contains the nucleus and associated intracellular structures whereas, dendrites are the extension of the cell body. Axon carries information from the cell body to other cells (receiving cell). Dendrites and axons, both extensions of the cell body, are also referred to as processes.
For the typical neuron, a simple model is as follows:
N =K(C+D+A) (2.1)
where N is the neuron; C is the Cell body; D is the Dendrites; A is the Axon; K is the diffusion coefficient. Factors that relate to the K, are the neuron weight, degree of ionization, neuron space configuration, and the condition that whether it combined with the three distinct parts.
Cell body
Cell body assimilates the synaptic input and transmits the determined message to another cell by the axon. It was found to be responsible for the diversity of biochemical process such as transforming glucose into high-energy compounds to other parts of the neuron, highly active proteins serve as chemical messengers between cells are manufactured and packaged and specialized organelles perform the cell’s function. Sheng Chen [33] proposed a mathematical theory for hormonal functions in the cell body were as follows:
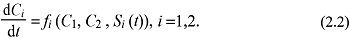 where fi is one hormonal function of the cell body, and fi is made up of three main parts:
where fi is one hormonal function of the cell body, and fi is made up of three main parts:
f1 (C1,C2 , S1 (t)), f2 (C1,C2 , S2 (t)).
Dendrites
Dendrites expand its sensitive receptive surface to the surrounding nervous tissue, reflects the function of the cell and the functional properties can be predicted from the pattern of dendritic branching. The thin branching and treelike forms increase the chance for synaptic connections in the brain [34]. Dendrites in the many neurons present with a special form of synaptic connection called dendritic spines. They are small (1-2 µm), a thorn-like protuberance from the dendrite and are the major anatomical feature of neurons in the human nervous system.
According to the definition of dendritic spines, suppose that the dendritic spines are e = x + y . If for any x (0 ), y (0) satisfy the condition
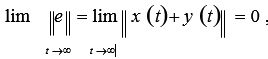 then we say that system (2.1) and system (2.2) achieve modifiable structures.
then we say that system (2.1) and system (2.2) achieve modifiable structures.
On the basis of adaptive control methods, we can give the following equations and the stability of neural networks with the dendritic structure:
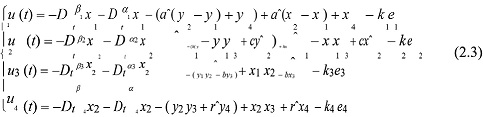 where e1 = x1 + y1 , e2 = x2 + y2 , e3 = x3 + y3 , e4 = x4 + y4 , ki > 0, (i =1, 2,3, 4).If t → ∞ , then e → 0 , and system (1) and system (2) achieve modifiable structures indicating the possibility that the pineal gland, a primary source of dendrites.
where e1 = x1 + y1 , e2 = x2 + y2 , e3 = x3 + y3 , e4 = x4 + y4 , ki > 0, (i =1, 2,3, 4).If t → ∞ , then e → 0 , and system (1) and system (2) achieve modifiable structures indicating the possibility that the pineal gland, a primary source of dendrites.
If we put (3) and system (1) to the system (2), then the following error equations can be obtained between the groups for some fractional differential equations and the central nervous system: 
Where ea = a - aˆ, eb = b -b, ec = c - cˆ, eh = h - h, er = r - rˆ are the parameter estimation errors.
Next, according to (4), we design the adaptive update law for each parameter estimation error:
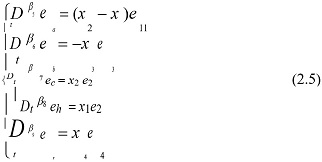 where 0 < βi < 1, (i = 5, 6, 7,8, 9), and (5) are obtained by eliminating the dendritic potentials from the underlying compartmental model or cable equations [35].
where 0 < βi < 1, (i = 5, 6, 7,8, 9), and (5) are obtained by eliminating the dendritic potentials from the underlying compartmental model or cable equations [35].
Axon
Axon is the excitable membrane that extends to the region of synaptic contact and generates or propagate the action potential. Generally, cells contain one axon but there may be off branches or collaterals to transmit the action potential to the brain. The distinctive length of the axon is the action potential and there occurs a Turing-like instability condition as a precursor for pattern formation in a spatially organized network.
According to ea = a - aˆ, eb = b -b, ec = c - cˆ, eh = h - h, er = r - rˆ and (5), we can get the parameters of the adaptive control law: 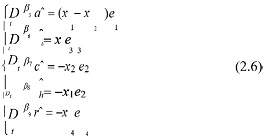
According to (4) and (5), we get the total error of the system: 
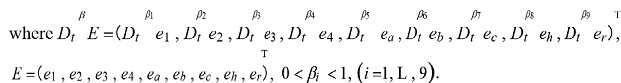 Then we consider Eq. (2.7), and expand the formula, we obtain:
Then we consider Eq. (2.7), and expand the formula, we obtain: 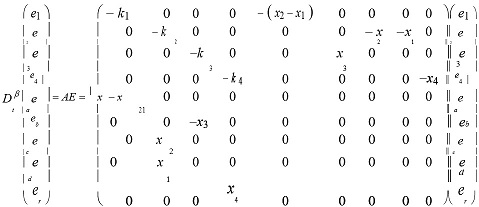
Setting P = E9 . Then we obtain the following result:
AP + PAT
A+AT =-Q
 where ki > 0, (i =1, 2,3, 4), Q = diag (2k1 , 2k 2 , 2k3 , 2k4 , 0, 0, 0, 0, 0) .
where ki > 0, (i =1, 2,3, 4), Q = diag (2k1 , 2k 2 , 2k3 , 2k4 , 0, 0, 0, 0, 0) .
It is easy to see that Q = diag (2k1 , 2k2 , 2k3 , 2k4 , 0, 0, 0, 0, 0) is a semi-positive definite matrix. Then, the state variable of (7) E =?e1 , e2 , e3 , e4 , ea , eb , ec , eh , er? is asymptotically stable, that is, e1 , e2 , e3 , e4 , ea , eb , ec , eh , er approach zero asymptotically with time. Therefore, we achieve the neuronal functions in the endoplasmic reticulum by a number of the adaptive robust set of fractional differential equations anti-synchronization indicating how the dispersion relation depends on the spatial distribution of the axon-dendritic weights with respect to both network and dendritic coordinates [36].
These primary afferent axons come in different diameters and can be divided into different groups based on their size. Here, in order of decreasing size, are the different nerve fiber groups: A-alpha (13-20 µm), A-beta (6-12 µm), A-delta (1-5µm) and C-nerve fibers(.2-1.5µm). A-alpha, A-beta, and A-delta nerve fibers are insulated with myelin. C-nerve fibers are unmyelinated. The thickness of the nerve fiber is correlated to the speed with which information travels in it - the thicker the nerve fiber, the faster information travels in it.
The model is described by a hyperbolic system of equations
ε( ∂ t + vi ∂ x ) pi = Σki j p j , 0 < x < ∞, t > 0, 1 ≤ i ≤ n,
where ki j ≥ 0 if i ≠ j , Σki j = 0 and 0 < ε = 1 . Here pi ( x ,t) is the thickness of the nerve fiber
in one of n nerve fiber groups, and x is the size of the nerve fiber. Setting
pm ( x ,t ) = lmQm ( x -evt , t) div(a(b(u ),∇u ))
where lm is determined by the boundary conditions at x = 0 and v is a weighted average of the velocities vi ( vi can be positive or negative). It is easy to prove that
Qm (s, t ) → Q (s, t) as ε → 0
where Q (s, t) is the bounded solution [37]
Overall, Cell body, dendrites, and axon are the three main parts of a neuron. We first consider the existence of positive solutions to parabolic nonlinear genetic equations of the form
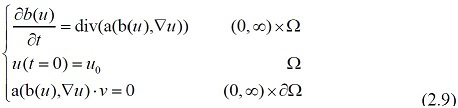 where a(b(u ),∇u ): = f (b(u ))∇c *[∇(u +V )] The dendrite receives the signal from other neurons; then the signal is computed at the synapse and transmitted to the cell body.
where a(b(u ),∇u ): = f (b(u ))∇c *[∇(u +V )] The dendrite receives the signal from other neurons; then the signal is computed at the synapse and transmitted to the cell body.
And c* represents the Legendre transform of a function c:¡ d → [0, ∞) , that is: 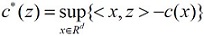
for z ∈ Rd . Here, the bounded domain of Rd is Ω including dendrites, cell body with a nucleus, axon; the outward unit normal to ∂Ω is ν . b : R → R is a monotone non-decreasing function;
V: Ω → R is a potential function; c : R4 → [0, ∞) is a convex function; f is a non-negative real-valued function, and u0 : Ω → R is a measurable function.
The rest is u :[0, ∞) ×Ω → R , u = u (t , x) .
If the signal into the cell body exceeds the holding threshold, the cell will fire and send the signal down to other neurons through axon [38].
Function and Transportation of Neurotransmitters
Neurotransmitters are the signaling molecules in neurons that play a vital role in transmitting neural signals through specific receptors, cytomembranes, and postsynaptic membranes. The end of axons of nerve cell secrete neurotransmitters (chemical agents), diffuse
And transmit a signal to adjoining cells like muscle cells, neurons, and glands across the synaptic gap by altering its electrical state.
Suppose that the neurotransmitters signals function F : [0, ∞ ) → R , where F ‘ = b−1 ?If
ρ := b(u) , ρ0 := b(u0 ), f ( x) = max( x, 0) . Then the nonlinear genetic equations (9) reduces to:
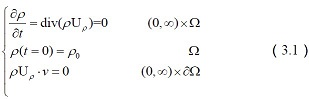
where U = -∇c * [∇( F ' (ρ) +V ) ] , ρ: Ω→ 0, ∞, ρ: 0, ∞Ω→ 0, ∞, ρ= ρ(t,x)
represents the transportation of neural signals by the time t ? t∈ [ 0, ∞) ?and position x. The summation of extracellular neurotransmitter concentration is:
E (ρ (t )) := ∫ Ω [F (ρ (t , x )) + ρ(t , x)V (x)]dx.
Through the above equations (10) and (11), we can find out that methionine enkephalin (ME), leucine enkephalin (LE), dopamine (DA) are able to diffuse freely into both 1-palmitoyl- 2-oleoyl-sn-glycero- 3- and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine membranes and are guided by the aromatic residues Tyr and Phe. Only a limited number of these neurotransmitters are allowed to penetrate into the membrane, which suggests an intrinsic mechanism by which the membrane is protected from being destroyed by excessive inserted neurotransmitters [39].
Transportation of GABA
GABA, a universal nonprotein amino acid, and functions variedly in different organisms (plants, fungi, and bacteria) and mammalian tissues. It acts as an inhibitory neurotransmitter and helps the neurons to recover from the worry, anxiety, and fretfulness [40].
The corresponding system of equations reduces to
 The major predictions from the above equations are as follows: (1) Uptake of GABA is totally sodium?dependent. (2) Although plots of 1/v versus 1/[Na]2 are nonlinear, the coupling ratio for transport (Na/GABA) is 2. (3) For transport to take place, the order of combination with a carrier must be Na, Na, GABA. (4) Maximal velocity will occur only at infinite Na and GABA concentrations. (5) There is a sigmoidal relationship between apparent maximal velocity (Va) and [Na]. (6) Kt, the [GABA] that gives a velocity equal to Va/2, rises and then falls as [Na] is increased from zero, passing through a maximum at 33.52 mM [Na]. (7) The relationship between initial velocity and [Na] is sigmoidal. (8) Jm, the rate of uptake with infinite [Na], is hyperbolically related to [GABA]; Jm approaches Vmax as [GABA] becomes very large. (9) KNa, the [Na] giving a velocity equal to Jm/2, declines rapidly from 10−7M to 10−5M GABA, but is essentially constant at 10−4M and above. (10) One GABA molecule is translocated per carrier molecule.
The major predictions from the above equations are as follows: (1) Uptake of GABA is totally sodium?dependent. (2) Although plots of 1/v versus 1/[Na]2 are nonlinear, the coupling ratio for transport (Na/GABA) is 2. (3) For transport to take place, the order of combination with a carrier must be Na, Na, GABA. (4) Maximal velocity will occur only at infinite Na and GABA concentrations. (5) There is a sigmoidal relationship between apparent maximal velocity (Va) and [Na]. (6) Kt, the [GABA] that gives a velocity equal to Va/2, rises and then falls as [Na] is increased from zero, passing through a maximum at 33.52 mM [Na]. (7) The relationship between initial velocity and [Na] is sigmoidal. (8) Jm, the rate of uptake with infinite [Na], is hyperbolically related to [GABA]; Jm approaches Vmax as [GABA] becomes very large. (9) KNa, the [Na] giving a velocity equal to Jm/2, declines rapidly from 10−7M to 10−5M GABA, but is essentially constant at 10−4M and above. (10) One GABA molecule is translocated per carrier molecule.
Transportation of Serotonin
Kogofsky [41] and other contributors cited that serotonin was one of the major neurotransmitters responsible for many biological processes like appetite, mood disorders, sleep, digestion, depression and generalized well-being.
The corresponding system of equations reduces to 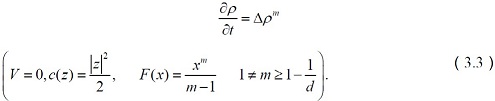
The above equations of serotonin transporter provide a novel genetic and behavioral primate model to study the molecular, neurodevelopmental, and psychopharmacological mechanisms that underlie genetic variation-associated complex behaviors, with specific implications for the understanding of normal and abnormal serotonin actions and the development of personalized Pharmacological treatments for psychiatric disorders.
Transportation of Acetylcholine
Acetylcholine was found mostly in neuromuscular junctions and are catalyzed by the acetylcholinesterase enzyme. It is responsible for learning, voluntary movement, sleep and memory and too much of it lead to depression and dementia in case of low level in the hippocampus region [42].
The corresponding system of equations reduces to 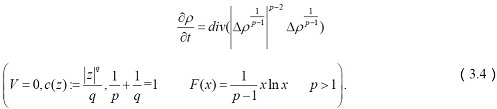
It is easy to see that the first intron of the ChAT gene encompasses the open reading frame encoding another protein, vesicular acetylcholine transporter (VAChT), which is responsible for the transportation of acetylcholine from the cytoplasm into the synaptic vesicles.
Transportation of Dopamine
Dopamine the inhibitory and excitatory neurotransmitter play the main role in the regulation of reward circuitry and pleasure centers and a dynamic brain chemical for memory and motor skills [43].
The corresponding system of equations reduces to
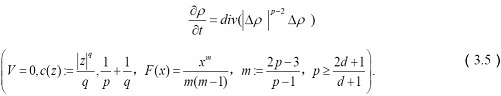 The major predictions from the above equations involving the effects of dopamine transporter(DAT) overexpression in MN-9D cells on the transportation of dopamine(DA) are as follows: some individuals may be simultaneously more responsive to the effects of environmental adversity and enrichment (i.e., differential susceptibility).
The major predictions from the above equations involving the effects of dopamine transporter(DAT) overexpression in MN-9D cells on the transportation of dopamine(DA) are as follows: some individuals may be simultaneously more responsive to the effects of environmental adversity and enrichment (i.e., differential susceptibility).
Transportation of Epinephrine
Epinephrine, otherwise called as adrenaline, a hormone responsible for its metabolism. It plays a key role in mental focus, attention, arousal, cognition, inhibits insulin excretion and elevates the number of fatty acids in the blood [44].
The corresponding system of equations reduces to 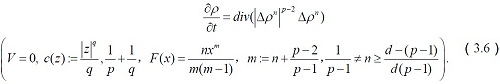
In the above equations, we established a dynamic mathematical model for detection of diabetes in blood with the help of parameters as epinephrine. In addition to this, we also incorporated a new parameter
in the existing model i.e. beta cells which has a great impact on the insulin.
Transportation of Glutamate
The exciting glutamate neurotransmitter required for memory and learning. Low level of glutamate results in poor brain activity and tiredness and high-level cause death to the neurons in the brain [45].
The corresponding system of equations reduces to
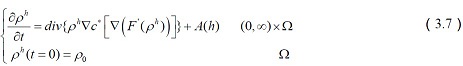 The above equations of glutamate transporters tell us that the control of glutamate concentrations is critical to the normal functioning of the central nervous system, and how glutamate transporters regulate glutamate concentrations to maintain dynamic signalling mechanisms between neurons.
The above equations of glutamate transporters tell us that the control of glutamate concentrations is critical to the normal functioning of the central nervous system, and how glutamate transporters regulate glutamate concentrations to maintain dynamic signalling mechanisms between neurons.
Transportation of Histamine
Histamine plays a major role in allergic reactions, affect emotions and behavior, control the sleep-wake cycle and promote the release of epinephrine and norepinephrine.
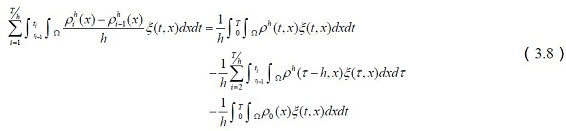 We use τ = t + h instead of the above expression to get
We use τ = t + h instead of the above expression to get
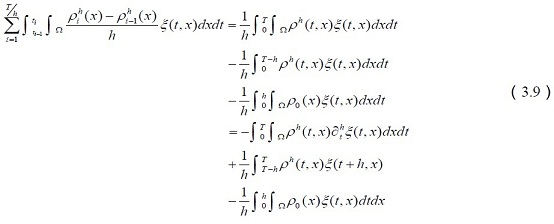
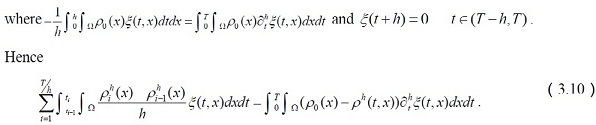 In silico molecular docking towards Neurodegenerative Disorders
In silico molecular docking towards Neurodegenerative Disorders
ADME/Tox filtering rules such as molecular weight, polar surface area, logP or number of rotatable bonds shown in Table 2. Target proteins were retrieved from PDB (Protein Data Bank). The Schrodinger Glide program version 2017 has been used for docking [46-48] shown in Table 3. The hydrogen interactions between ligands and target proteins are shown in Figure 1 (A, B, C).
|
S. No |
Ligand |
LogKaHSA |
Physical-chemical properties |
Log P |
||
|
Molecular Weight |
H2 donors |
H2 Acceptors |
||||
|
1 |
26.27-Di(nor)- cholest-5,7,23-trien-22-ol, 3- methoxymethoxy |
1.118 |
414.627 |
1,000 |
5,100 |
5.785 |
|
2 |
9H –purin-6-amine, N,9- bis(trimethylsilyl)-8-((trimethylsilyl)oxy) |
0.998 |
367.672 |
1,000 |
4,000 |
5.157 |
|
3 |
Cyanocolchicines |
-0.576 |
424.452 |
000 |
9,500 |
1.902 |
|
4 |
3Beta-methoxy-5- cholesten-19-oic acid |
1.414 |
430.670 |
1,000 |
3,700 |
7.011 |
|
5 |
Cholest-5-en-3-ol (3, Beta.)-, carbonochloridate, |
1.809 |
383.66 |
1 |
1.7 |
6.916 |
|
6 |
Cholesterol |
1.843 |
386.66 |
1 |
1.7 |
6.999 |
|
7 |
Cholest-5-en-3-ol (3, Beta.)-, propionate |
2.371 |
442.724 |
0 |
2 |
8.418 |
|
8 |
Echinacoside |
-2.248 |
786.736 |
12 |
28.8 |
-3.648 |
Table 2: ADME properties of active phytochemical components.
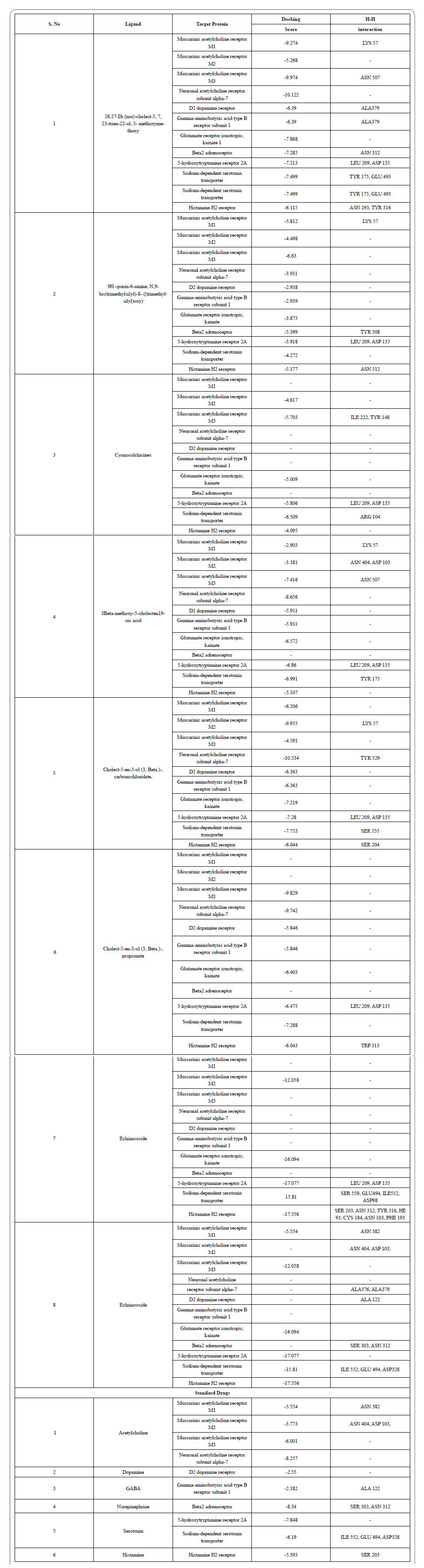 Table 3: Identification of new chemical entities through in-silico drug design method.
Table 3: Identification of new chemical entities through in-silico drug design method.
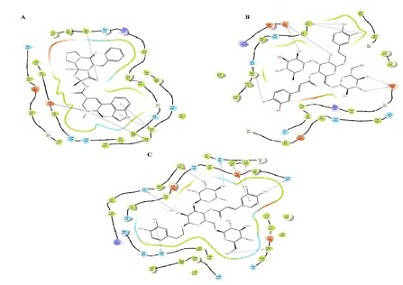 Figure 1: (A) Interaction between Echinacoside and 5-hydroxytryptamine receptor 2A (B) Interaction between Echinacoside and Sodium-dependent serotonin transporter (C) Interaction between Echinacoside and Histamine H2 receptor.
Figure 1: (A) Interaction between Echinacoside and 5-hydroxytryptamine receptor 2A (B) Interaction between Echinacoside and Sodium-dependent serotonin transporter (C) Interaction between Echinacoside and Histamine H2 receptor.
Totally, eight natural compounds with six standard drugs were docked against eleven target proteins, represent as neurotransmitters. The results show among the eight natural compounds, Echinacoside has shown the highest interaction with 5-hydroxytryptamine receptor 2A, Sodium-dependent serotonin transporter, and Histamine H2 receptor. The receptors are mainly involved in neurogenic disorders in human.
Conclusion
Neurotransmitters are molecules that inhibit or stimulate a postsynaptic cell, which is released into the body by the presynaptic nerve cell to produce a response to a certain stimulus. The development of neurotransmitters and its complex functions are influenced by numerous factors. In this study, some mathematical speculations have been proposed on the basis of structural and functional characteristics of the virtual neuron (especially the physiological phenomena of human beings) with a molecular docking and bio mathematical approach to formulating some speculations to the consolidation of the identification of neurotransmitters function. This could pave a way to formulate more mathematical speculations related to the neuron, and finally, these data and approaches will be useful for constructing virtual neuron with the help of biomathematics. The interaction between natural compounds and neurotransmitter studies shows the good interaction with all the compounds. Especially, 26.27-Di(nor)-cholest-5,7,23-trien-22-ol, 3-methoxymethoxy, Cholest-5-en-3-ol (3, Beta.)-, carbonochloridate, Cholesterol and Echinacoside exhibited maximum interaction with all the target proteins. Among the other compounds, echinacoside shows highest interaction with (Serotonin) 5-hydroxytryptamine receptor 2A (-17.077), Sodium-dependent serotonin transporter (-15.810) and (Histamine) Histamine H2 receptor (-17.556).
Serotonin the other major inhibitory neurotransmitter is deemed to be the master neurotransmitter. The imbalance is one of the most often cited contributors to depression and other mood disorders. It is also intimately tied to many biological processes such as sleep, appetite, pain, digestion, and generalized well-being.
Histamine is most commonly known for its role in allergic reactions but it is also involved in neurotransmission and can affect your emotions and behavior as well. Histamine helps control the sleep-wake cycle and promotes the release of epinephrine and norepinephrine.
Serotonin and histamine were the brain monoamines which play a vital role in cognition, emotions, pathophysiology, and treatment of mental disorders. In the current study revealed that the neurotransmitters structure and transportation by mathematical models and in silico molecular docking results strongly shows the Echinacoside is a potent inhibitor in some neurological disorder associated with serotonin and histamine. Further, extend methods adapt to study the mechanism and pathway level interactions between the natural compounds with the disease
Conflict of Interest
We have no conflict of interests to disclose and the manuscript has been read and approved by all named authors.
Acknowledgement
This work was supported by the Philosophical and Social Sciences Research Project of Hubei Education Department (19Y049), and the Staring Research Foundation for the Ph.D. of Hubei University of Technology (BSQD2019054), Hubei Province, China.
References
- Hyman SE (2005) Neurotransmitters. Curr Biol 15: 154-158.
- Masland RH (2004) Neuronal cell types. Curr Biol 14: 497-500.
- Nestler EJ, Hyman SE, Malenka RJ (2001) Molecular Neuropharmacology: Foundation for Clinical Neuroscience. New York: McGraw Hill.
- Pestana M, Jardim H, Correia F, Vieira-Coelho MA, Soares-da-Silva P (2001) Renal dopaminergic mechanisms in renal parenchymal diseases and hypertension. Nephrol Dial Transplant 1: 53-59.
- Marko AM, Gerrard JW, Buchan DJ (1960) Glutamic acid derivatives in adult celiac disease. II. Urinary total glutamic acid excretion. Can Med Assoc J 83: 1324-1325.
- Bélanger R, Chandramohan N, Misbin R, Rivlin RS (1972) Tyrosine and glutamic acid in plasma and urine of patients with altered thyroid function. Metabolism 21: 855-865.
- Ragginer C, Lechner A, Bernecker C, Horejsi R, Möller R (2012) Reduced urinary glutamate levels are associated with the frequency of migraine attacks in females. Eur J Neurol 19: 1146-1150.
- Field T, Diego M, Hernandez-Reif M (2010) Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behav Dev 33: 23-29.
- Ghaddar A, Omar KH, Dokmak M, Kansour NA, Jbara Z (2014) Work-related stress and urinary catecholamines among laboratory technicians. J Occup Health 55: 398-404.
- Liu L, Li Q, Li N, Ling J, Liu R (2011) Simultaneous determination of catecholamines and their metabolites related to Alzheimer’s disease in human urine. J Sep Sci 34: 1198-1204.
- Paine NJ, Watkins LL, Blumenthal JA, Kuhn CM (2015) Sherwood A Blumenthal, Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure, Psychosom Med 77: 136-44.
- Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A (2004) Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res 57: 353-358.
- Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I (1983) CSF and urinary biogenic amines and metabolites in depression and mania. A controlled, univariate analysis. Arch Gen Psychiatry 40: 999-1010.
- Nicholson-Guthrie CS, Guthrie GD, Sutton GP, Baenziger JC (2001) Baenziger, Urine GABA levels in ovarian cancer patients: Elevated GABA in malignancy. Cancer Lett 162: 27-30.
- Nichkova MI, Huisman H, Wynveen PM, Marc DT, Olson KL (2012) Evaluation of a novel ELISA for serotonin: Urinary serotonin as a potential biomarker for depression. Anal Bioanal Chem 402:1593-600.
- Aruoma OI, Bahorun T, Jen LS (2003) Neuroprotection by bioactive components in medicinal and food plant extracts, Mutation Research 544: 203-215.
- Bin Zhao, Diraviyam T, Zhang X (2015) A bio-mathematical approach: Speculations to construct virtual placenta. Applied Mathematics and Computation 256: 344-351.
- Cumpson PS, Naoko (2013) Stability of reference masses V: UV/ozone treatment of gold and platinum surfaces, Metrologia 50: 27-36.
- Ganesh S, Jannet JV, Annie MA (2013) Effects of Phytochemicals extracted from Acanthus Ilicifolius against Staphylococcus aureus: An In-silico approach, American Journal of Drug Discovery and Development 3: 293-297.
- Wang T, Zhang X, Xie X, Deserticola YB, Ma C (2012) Desert Ginseng: A Review, The American Journal of Chinese Medicine 40: 1123-1141.
- Meraj MK, Mahto NB, Christina N, Desai S, Shahbazi S, et al. (2012) Molecular modeling, docking and ADMET studies towards development of novel Disopyramide analogs for potential inhibition of human voltage gated sodium channel proteins. Bioinformation 8: 1139-1146.
- William JE (2007) Computational Models for ADME. Annual Reports in Medicinal Chemistry 42: 449-467.
- Pollard H, Schwartz JC (1987) Histamine neuronal pathways and their functions. Trends Neurosci 10: 86-89.
- Schmidt JT (1979) The Laminar organization of optic nerve fibres in the tectum of goldfish. Proc R Soc London Ser 205: 287-306.
- Schmidt JT, Freeman JA (1980) Optic nerve transmitters in lower vertebrate species. Brain Res 187: 129-142.
- Strange PG (1987) Dopamine receptors in the brain and periphery: State of the art. Neurochem Intern 10: 27-33.
- Asano TM (1985) Ui N. Ogasawara, J Biol Chem 260: 12653-12658.
- Monaghan DT, Cotman CW (1986) Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes. Proc Natl Acad Sci U S A 83: 7533-7536.
- Bloom FE (1979) The Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. MIT Press 51-58.
- Okoh D, Owolabi O, Ekechukwu C, Folarin O, Arhiwo G, et al. (2016) Babatunde Rabiu, A regional GNSS-VTEC model over Nigeria using neural networks: A novel approach, Geodesy and Geodynamics 7: 19-31.
- Shen J, Tan C, Zhang Y, Li X, Li W, et al. (2010) Discovery of Potent Ligands for Estrogen Receptor β by Structure-Based Virtual Screening. J Med Chem 53: 5361-5365.
- Xiu-ling LI, Jun-jie WEI (2013) Stability and Bifurcation Analysis in a System of Four Coupled Neurons with Multiple Delays. Acta Mathematicae Applicatae Sinica English Series 29: 425-448.
- Chen S, Zhang XJ, Li LX, Wang Y, Zhong RJ, et al. (2015) Histone deacetylase 6 delays motor neuron degeneration by ameliorating the autophagic flux defect in a transgenic mouse model of amyotrophic lateral sclerosis. Neuroscience Bulletin 31: 459-468.
- Urbanska M, Blazejczyk M, Jaworski J (2008) Molecular basis of dendritic arborisation. Acta neurobiologiae experimentalis 68: 264-288.
- Yousefi F, Amoozandeh Z (2016) Statistical mechanics and artificial intelligence to model the thermodynamic properties of pure and mixture of ionic liquids. Chinese Journal of Chemical Engineering 24: 1761-1771.
- Yousefi F, Amoozandeh Z (2017) A new model to predict the densities of nanofluids using statistical mechanics and artificial intelligent plus principal component analysis. Chinese Journal of Chemical Engineering 25: 1273-1281.
- Yong XIE, Yan Mei KANG, Yong LIU, Ying WU (2014) Firing properties and synchronization rate in fractional-order Hindmarsh-Rose model neurons. Science China Technological Sciences 57: 914-922.
- Zhao J, Xu H, Tian X, Hu M, Xiao H (2013) Effect of electroacupuncture on brain-derived neurotrophic factor mRNA expression in mouse hippocampus following cerebral ischemia-reperfusion injury. Journal of Traditional Chinese Medicine 33: 253-257.
- Hong-yan CUI, Chen FENG, Yun-jie LIU (2013) Analysis of prediction performance in wavelet minimum complexity echo state network. The Journal of China Universities of Posts and Telecommunications 20: 59-66.
- Barker JL, McBurney RN (1979) Phenobarbitone Modulation of Postsynaptic GABA Receptor Function on Cultured Mammalian Neurons. Proc R Soc London Ser B 206: 319-327.
- Kosofsky BE, Mollwer ME, Morrison JH, Foote SL (1984) The serotonin and norepinephrine innervation of primary visual cortex in the cynomolgus monkey (Macaca fascicularis ). J Comp Neurol 230: 168-178.
- Brown DA (1986) Neuropharmacology: Acetylcholine and brain cells. Nature (London) 319: 358-359.
- Brown JR, Arbuthnott GW (1983) The electrophysiology of dopamine (D2) receptors: A study of the actions of dopamine on corticostriatal transmission. Neuroscience 10: 349-355.
- Moore RY, Bloom FE (1979) Moore RY, Bloom FE (1979) Annu Rev Neurosci 2: 113-168. Annu Rev Neurosci 2: 113-168.
- Cotman CW, Iversen LL (1987) Excitatory amino acids in the brain - focus on NMDA receptors. Trends Neurosci 10: 263-265.
- Shen J, Tan C, Zhang Y, Li X, Li W, et al . (2010) Discovery of Potent Ligands for Estrogen Receptor β by Structure-Based Virtual Screening. J Med Chem 53: 5361-5365.
- César-Razquin A, Snijder B, Frappier-Brinton T, Isserlin R, Gyimesi G, et al. (2015) A Call for Systematic Research on Solute Carriers. Cell 162: 478-487.
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, et al. (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275: 10767-10771.
Citation: Bin Zhao (2022) Neurotransmitters: A Computational and bio mathematical approach at the time of COVID-19. J Pharmacol Pharmaceut Pharmacovig 6: 032.
Copyright: © 2022 Bin Zhao, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

