
New Global Challenges for Patients with Immune Thrombocytopenia during the Coronavirus Disease (COVID-19) Pandemic
*Corresponding Author(s):
Jing SunDepartment Of Hematology, Nanfang Hospital, Southern Medical University, No. North 1838, Guangzhou Avenue, Guangzhou 510515, China
Email:jsun_cn@hotmail.com
Jieyu Ye
Department Of Hematology, Nanfang Hospital, Southern Medical University, No. North 1838, Guangzhou Avenue, Guangzhou 510515, China
Email:jieyu_ye@163.com
Abstract
Background: The novel coronavirus disease (COVID-19) outbreak has seriously threatened global health, society, and economy. However, little is known about the secondary disasters of COVID-19, especially in patients with chronic diseases such as immune thrombocytopenia (ITP).
Objectives: To assess the psychological and physical health status of ITP patients since the COVID-19 outbreak and raise societal and government awareness.
Methods: In this cross-sectional study, we investigated the effect of the COVID-19 pandemic on the physical and psychological status changes in 480 ITP patients by administering a questionnaire. Version 2016 of the ITP bleeding scale and SF-36 are included in our survey.
Results: In total, 480 ITP patients completed the questionnaire. The mean scores of SF- 36 subscales during this period were lower than usual and negatively correlated with the bleeding score. Patients with platelet<10G/L and patients with chronic ITP experienced the most marked reduction in the quality of life (QoL) compared with those before the pandemic. Women experienced more significant physical and psychological changes than men, especially those of ages 31-50 years. Furthermore, the higher the income, the less the QoL was affected. Patients’ residence in different grade risk areas were differentially. Psychological health was more severely affected than physical health during the pandemic,
Conclusion: The physical and psychological status of ITP patients are markedly affected by COVID-19, and these impacts may pose new challenges in their treatment. We aim to call on society and governments to focus more on these groups.
Keywords
Immune thrombocytopenia (ITP); COVID-19; Secondary disasters; Quality of life
INTRODUCTION
Immune thrombocytopenia (ITP) is an acquired autoimmune hemorrhagic disease characterized by an isolated decrease in the peripheral blood platelet count (<100×10^9/L) [1,2]. Patients with ITP are at an increased risk of bleeding, which can be life-threatening if it occurs in a vital organ such as the brain [3]. Norgaard et al. conducted a study to analyze the 5-year mortality of chronic ITP patients whose age and gender were matched to healthy individuals. The mortality rate of chronic ITP patients was twice as high as that of healthy subjects. The main causes of death were infection, hemorrhage, and development of the disease [4]. In addition, ITP patients frequently suffer from fear of bleeding, unexplained fatigue, non-specific pain, and decreased emotional health. Although ITP is not a malignant disease, patients often have a very poor quality of life (QoL), even worse than that of cancer patients according to a past study [5].
The major goal for ITP treatment is to provide a safe platelet count (one that prevents major bleeding and thrombosis) rather than correcting the platelet count to normal levels [6]. Treatment for newly diagnosed or severe adult patients is steroids. The initial dose was 1mg/kg methylprednisolone or 40 mg/d dexamethasone. Although up to 80% of ITP patients have an initial response, most of them relapse during dose reduction or after withdrawal of steroids [7,8]. A real-world study showed that more than 50% of patients used high dose of steroids (prednisolone>5mg/d) for more than four months, and 15.6% used it for more than one year [9]. Long-term use of steroids accompanied with many side effects, such as weight gain, abnormal glucose metabolism, osteoporosis, fatigue, nervousness, irritability, sleep disturbance, and decreased immunity [10]. Other treatments such as thrombopoietin receptor agonists, IVIg, anti-D or rituximab, or splenectomy were reported to have at least one side effect, liver function damage, affection, thromboembolism or hemorrhage [11,12]. Not only the disease itself, but also treatment-related side effects can reduce the QoL of ITP patients.
A cluster of novel pneumonia cases were first reported in Wuhan, Hubei province, China in December 2019. Mild patients initially presented with fever, diarrhea, and fatigue, among other symptoms. Severe patients often presented with complications of acute respiratory distress syndrome and myocardial injury [13-16]. The novel coronavirus was isolated from the airway epithelial cells of these patients and its nucleic acid sequence was analyzed. This analysis revealed that the novel coronavirus differed from the previously known human coronaviruses (e.g. SARS, MERS). The new virus was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by the virus was named novel coronavirus disease (COVID-19) [17]. COVID-19 spread across the world and caused countless deaths, and has been declared a global pandemic in February 2020. By June 2020, there were more than7.6 million confirmed cases and 423,000 reported deaths globally. The outbreak of COVID-19 has consumed a tremendous amount of medical resources and caused significant damages to society and economy. Despite ongoing efforts, we still know very little about COVID-19 and there has yet to be an approved drug or vaccine. Studies show that COVID-19 is mainly transmitted through droplets or close person-to-person contact [18,19]. Although SARS-CoV-2 has been detected in feces in some cases, further studies are needed to determine whether it can be spread through the fecal-oral route [20]. The key strategies to prevent the spread of COVID-19 are early discovery, early isolation and early treatment. These strategies have been regarded as priorities by many governments, which have implemented strict measures to control person-to person transmission by prohibiting clustered activities and encouraging people to stay at home, among others.
History has demonstrated that a pandemic is usually followed by a series of secondary disasters [21-23]. During the epidemic period of COVID-19, strict controls caused a serious impact not only on the treatment but also on the quality of life of patients with emergent and chronic diseases, such as angiocardiopathy, cancer, and ITP. These patients were unable to have regular hospital visits on time. Such inconsistencies in care may have serious consequences, as patients who experience bleeding at any site may ingest large doses of medications in a state of panic, a behavior that may lead to drug toxicities. Other patients may suffer from treatment discontinuation as they have no means of accessing medications or receiving injections. Moreover, acute and severe patients who require urgent treatment and platelet transfusion risk not being able to receive timely medical assistance due to the depletion of medical resources. Studies have shown that COVID-19 patients with severe comorbidities and immunodeficiency are more likely to progress to the severe stage [13,24]. ITP is characterized as an immune deregulated disease. Therefore, ITP patients are susceptible to infection and are more likely to have a severe presentation of COVID-19. These problems not only threaten the physical health of ITP patients, but may also increase their psychological burden and significantly reduce their quality of life.
Current research mainly focuses on the clinical manifestations, diagnosis, treatment, and prevention of COVID-19. However, secondary disasters caused by COVID-19 such as the reduced quality of life and psychological impacts on patients with chronic diseases have not yet been studied. During the epidemic of COVID-19, ITP patients may face a greater risk of bleeding and mental stress than usual due to restrictions on daily activities and a depletion of medical resources. Therefore, in this study, we administered a questionnaire to understand the impacts of the pandemic on the mental and physical status of ITP patients. We aim to raise greater societal awareness of these groups and to establish a better response system for emergency treatment, both physically and psychologically. Furthermore, this study hopes to provide physicians and patients with reliable evidence for better clinical decision-making during the pandemic.
PATIENTS AND METHODS
The study examined 480 ITP patients, all of whom were diagnosed according to the latest guidelines [25]. To be eligible, subjects were ages 10-75 years. Exclusion criteria included the presence of cognitive disorders, psychiatric and malignant diseases. All participating patients had been followed up in the Department of Hematology of Nanfang Hospital, Southern Medical University, for a long time. Patients were informed about the study objectives and provide informed consent before enrolling. Most of the questionnaires were completed online, and a few were completed by telephone. Those aged less than 13 years completed the questionnaire with their parents. All questionnaires were completed no less than 15mins and were instructed by a research assistant.
The pandemic began in late January of 2020 and lasted until mid-April in China. Therefore, the period of disease outbreak was considered January 23, 2020 to April 15, 2020. The first part of the questionnaire pertained to the patient's general information (date of birth, gender, residence), phase of diagnosis, family income, lifestyle, current therapeutic regimen, and the lowest platelet count during this period. The next section consisted of the content found in Version 2016 of the ITP bleeding scale, as well as the Short Form 36 (SF-36). Several other questions were designed to investigate how often patients visit hospitals and how they received medical help during the COVID-19 outbreak. The location of the patient's residence during the pandemic were divided into high (>900 confirmed cases), moderate (300-900 confirmed cases), and low (<300 confirmed cases) risk areas based on the number of confirmed cases of COVID-19.
Version 2016 of ITP bleeding scale
A consensus-based ITP-specific bleeding assessment tool (ITP-BAT) was first proposed by the International Working Group (IWG) in 2013 [26]. However, the data acquisition is time-consuming, which affects its clinical practicability. A novel scale (Version 2016 of the ITP bleeding scale) based on ITP-BAT was recommended by the Chinese Medical Association in 2016 to simplify the evaluation process and to increase applicability in clinical practice [27]. Version 2016 of the ITP bleeding scale has the advantages of concise description, objectivity, ease of administration and good practicality in clinical application. It has also been proven to be highly consistent with ITP-BAT [28]. Version 2016 of the ITP bleeding scale is composed of age and bleeding symptoms. The final result is the sum of the age score and bleeding symptoms score (the highest of all bleeding symptoms) [27]. The higher the score, the greater the risk.
36-Item short-form (SF-36)
The Short-form 36 (SF-36) Questionnaire (the MOS item short form health survey) was developed based on the Medical Outcomes Study Short Form (MOS-SF) and proved to be reliable and valid in different patient groups [29,30]. The content of the SF-36 evaluates eight dimensions, including physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH). Physical health consists of PF, RP, BP and GH, and the rest composes psychological health [31,32]. The final score is the sum of each part and ranges from 0 (worst) to 100 (best). The higher the score, the better the health. SF-36 is very effective in monitoring population health, estimating the burden of different diseases, monitoring clinical practice results and assessing treatment outcomes [33]. However, SF-36 fails to assess for problems specifically caused by bleeding, so some questions were adjusted based on our needs.
ANALYSIS
All analyses were performed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA). Two- sided P values were selected and P <0.05 was considered statistically significant. Changes in SF scores before and after the outbreak of COVID-19 were tested by non-parametric tests of two related samples.
RESULTS
A total of 480 ITP patients were administered the battery of questionnaires and 100% of patients completed them. Among the participants, 62.3% were female and 37.7% were male. Patients aged <=13 years (y), 14-30 y, 30–50 y and >=51y accounted for 30.2%, 27.1%, 33.5% and 9.2%, respectively. Of all participants, 68.3% had chronic ITP with platelet count less than 100 G/L. Newly diagnosed ITP, persistent ITP, and chronic ITP with the number of platelets more than 100G/L accounted for 1.5%, 15%, and 15.2% respectively. The lowest number of platelets during the COVID-19 pandemic<10G/L, 11- 20G/L, 21-50G/L and >=51G/L accounted for 16.9%, 14.0%, 25.6% and 24.3% respectively. Regarding areas of residence, 15.6% of the patients lived in low-risk areas, 44.0% in moderate-risk areas and 40.4% in high-risk areas. During the outbreak, 28.1% of subjects changed their treatment regimen while 71.9% maintained the same management plan. There was one case of infection with SARS-CoV-2. Patients who lived with families accounted for 91.5%. The monthly disposable income was less than $705 for 63.3%,
$706-1409 for 20.2% and over $1410 for 9% of the patients. Patients have regular hospital visits once a month, twice a month, once every two or three months and never visits account for 29.2%, 15.4%, 20.6% and 16.5% before the pandemic. After the outbreak of COVID-19, patients have regular hospital visits once a month, twice a month, once every two or three months and never visits account for 15.8%, 10.8%, 18.3% and 38.3% respectively (Table 1).
|
|
|
Frequency |
Percent (%) |
|
Sex |
|
|
|
|
|
men |
181 |
37.7 |
|
|
female |
299 |
62.3 |
|
Age |
|
|
|
|
|
<=13y |
145 |
30.2 |
|
|
14-30y |
130 |
27.1 |
|
|
31-50y |
161 |
33.5 |
|
|
>=51y |
44 |
9.2 |
|
Location |
|
|
|
|
|
high |
194 |
40.4 |
|
|
moderate |
211 |
44 |
|
|
low |
75 |
15.6 |
|
Phase of ITP |
|
|
|
|
|
7 |
1.5 |
|
|
|
3-12 months |
72 |
15 |
|
|
>12 months with PLT>=100G/L |
73 |
15.2 |
|
|
>12 months with PLT<100G/L |
328 |
68.3 |
|
|
the lowest platelet counts during the pandemic |
|
|
|
|
<10G/L |
81 |
16.9 |
|
|
11-20G/L |
67 |
14 |
|
|
21-50G/L |
123 |
25.6 |
|
|
>=51G/L |
116 |
24.2 |
|
|
whether adjusted the treatment during pandemic |
|
|
|
|
NO |
345 |
71.9 |
|
|
YES |
135 |
28.1 |
|
|
Get infected with SARS CoV SARS -2 or not |
|
|
|
|
YES |
1 |
2 |
|
|
NO |
479 |
99.8 |
|
|
Income (dollars/month) |
|
|
|
|
<=705 |
304 |
63.3 |
|
|
706-1409 |
97 |
20.2 |
|
|
>=1410 |
43 |
9 |
|
|
Hospital visits frequency (Before pandemic) |
|
|
|
|
Never |
79 |
16.5 |
|
|
1/2-3 Months |
99 |
20.6 |
|
|
once in a month |
140 |
29.2 |
|
|
Twice in a Month |
74 |
15.4 |
|
|
>=Three times in a Month |
88 |
18.3 |
|
|
Hospital visits frequency (After pandemic) |
|
|
|
|
Never |
184 |
38.3 |
|
|
1/2-3 Months |
88 |
18.3 |
|
|
once in a month |
76 |
15.8 |
|
|
Twice in a Month |
52 |
10.8 |
|
|
>=Three times in a Month |
88 |
16.8 |
Table 1: Demographic and clinical characteristics.
Due to the restriction of the SF-36 form, the score of role-functioning physical (RP) subscale prior to the COVID-19 pandemic could not be obtained, so our study mainly focused on the other seven subscales. Mean scores of SF-36 including physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH) are shown in Figure 1. All subjects scored significantly lower on the SF-36 after the outbreak of COVID-19 compared to before the pandemic.
The phase of disease was found to be associated with the decline in QoL after the outbreak of COVOD- 19, especially in patients with chronic disease. Chronic ITP patients with platelet counts below 100 G/L were greatly affected in all dimensions. Chronic ITP patients with platelet counts of over 100 G/L reported significant changes in their PF, RE, and MH subscales. Persistent ITP was experienced in three dimensions (PF, p=0.04; RE, p < 0.001 SF, p=0.044). It is worth noting that there were no significant changes in either physical or psychological health among the newly diagnosed ITP patients (Figure 2).
We found that the QoL of patients varied with the number of platelets during the COVID-19 outbreak (Figure 3). Patients in whom the number of platelets was less than 10 G/L reported significant changes across all seven subscales (p<0.05). Patients with the number of platelets between 11-20 G/L reported decreases in PF, RE, and SF. Patients with the number of platelets between 20-50 G/L were greatly affected in five (PF, RE, BP, SF and MH) of seven subscales (p<0.05). Patients with the number of platelets more than 50 G/L scored significantly less in six (PF, VT, RE, BP, SF and MH) of seven subscales compared to prior to the outbreak (p<0.05). As shown in Table 2, the age score and the highest of bleeding scores were added to obtain the final bleeding score. Pearson correlation was used to find that the final score of SF-36 after the pandemic was negatively correlated with the bleeding score (r=-0.325; p=0.000).
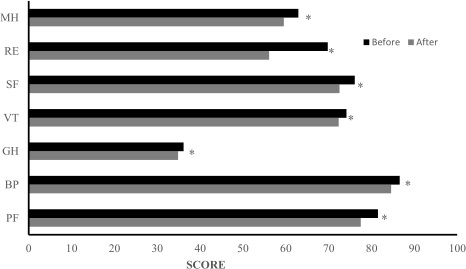 Figure 1: All subjects scored significantly lower on the SF-36 after the outbreak of COVID-19 compared to before the pandemic. *P<0.05.
Figure 1: All subjects scored significantly lower on the SF-36 after the outbreak of COVID-19 compared to before the pandemic. *P<0.05.
PF: Physical functioning); BP: Bodily pain; GH: General health; VT: Vitality; SF: Social functioning; RE: Role emotional; MH: Mental health.
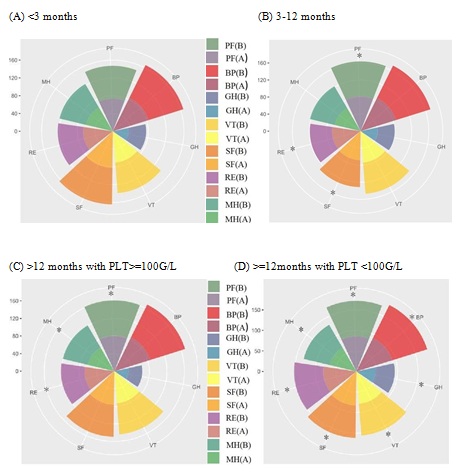 Figure 2: Comparison of SF-36 subscale scores of patients in different phases of ITP before and after COVID-19 outbreak. (A) Newly diagnosed ITP (<3montns) scored no difference in all subscales. (B) Persistent ITP was experienced in PF, RE and SF subscales. (C) Chronic ITP patients with platelet counts of over 100 G/L reported significant changes in their PF, RE, and MH subscales. (D) Chronic ITP patients with platelet counts below 100 G/L were greatly affected in all dimensions. *P < 0.05.
Figure 2: Comparison of SF-36 subscale scores of patients in different phases of ITP before and after COVID-19 outbreak. (A) Newly diagnosed ITP (<3montns) scored no difference in all subscales. (B) Persistent ITP was experienced in PF, RE and SF subscales. (C) Chronic ITP patients with platelet counts of over 100 G/L reported significant changes in their PF, RE, and MH subscales. (D) Chronic ITP patients with platelet counts below 100 G/L were greatly affected in all dimensions. *P < 0.05.
PF (B): mean score of physical functioning before COVID-19 outbreak, PF (A): mean score physical functioning after COVID-19 outbreak, BP (B): mean score of bodily pain before COVID- 19 outbreak, BP (A): mean score of bodily pain after COVID-19 outbreak, GH (B): mean score of general health before COVID-19 outbreak, GH (A): mean score of general health after COVID-19 outbreak, VT(B): mean score of vitality before COVID-19 outbreak, VT(A): mean score of vitality after COVID-19 outbreak, SF(B): mean score of social functioning before COVID-19 outbreak, SF(A): mean score of social functioning after COVID-19 outbreak, RE (B): mean score of role emotional before COVID-19 outbreak, RE (A): mean score of role emotional after COVID-19 outbreak, MH (B): mean score of mental health before COVID-19 outbreak, MH (A): mean score of mental health after COVID-19 outbreak.
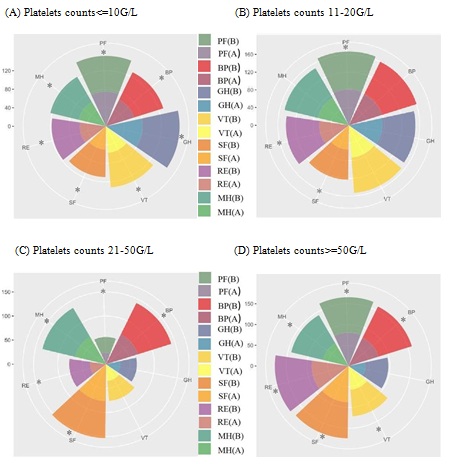 Figure 3: Comparison of SF-36 subscale scores of patients with platelet counts before and after COVID-19 outbreak. (A) Chronic ITP patients with platelet counts below 100 G/L were greatly affected in all dimensions. (B) Patients with the number of platelets between 11–20 G/L reported decreases in PF, RE, and SF. (C) Patients with the number of platelets between 20–50 G/L were greatly affected PF, RE, BP, SF and MH. (D) Patients with the number of platelets more than 50 G/L scored significantly less in PF, VT, RE, BP, SF and MH.*P < 0.05.
Figure 3: Comparison of SF-36 subscale scores of patients with platelet counts before and after COVID-19 outbreak. (A) Chronic ITP patients with platelet counts below 100 G/L were greatly affected in all dimensions. (B) Patients with the number of platelets between 11–20 G/L reported decreases in PF, RE, and SF. (C) Patients with the number of platelets between 20–50 G/L were greatly affected PF, RE, BP, SF and MH. (D) Patients with the number of platelets more than 50 G/L scored significantly less in PF, VT, RE, BP, SF and MH.*P < 0.05.
PF (B): mean score of physical functioning before COVID-19 outbreak, PF (A): mean score physical functioning after COVID-19 outbreak, BP (B): mean score of bodily pain before COVID- 19 outbreak, BP (A): mean score of bodily pain after COVID-19 outbreak, GH (B): mean score of general health before COVID-19 outbreak, GH (A): mean score of general health after COVID-19 outbreak, VT(B): mean score of vitality before COVID-19 outbreak, VT(A): mean score of vitality after COVID-19 outbreak, SF(B): mean score of social functioning before COVID-19 outbreak, SF(A): mean score of social functioning after COVID-19 outbreak, RE (B): mean score of role emotional before COVID-19 outbreak, RE (A): mean score of role emotional after COVID-19 outbreak, MH (B): mean score of mental health before COVID-19 outbreak, MH (A): mean score of mental health after COVID-19 outbreak.
|
|
|
SF-36 Score |
Bleeding Score |
|
SF-36 Score |
Pearson Correlation |
1 |
0.325** |
|
|
Sig.(2-Tailed) |
|
0 |
|
|
N |
480 |
480 |
|
Bleeding score |
Pearson Correlation |
0.325** |
1 |
|
|
Sig.(2-Tailed) |
0 |
|
|
|
N |
480 |
480 |
**Correlation is significant at the 0.01 level (2-tailed)
Table 2: The bleeding score is negative correlation with the SF-36 score during the pandemic.
In addition, gender and age were identified to be important factors. Female patients scored much lower than men during the COVID-19 pandemic period, especially those aged 31-50 y (Figure 4A). Female patients were more likely to score lower in the seven subscales of SF-36, but male patients only experienced significant decrease across four subscales, which were PF (p<0.001), RE (p<0.001), SF (p=0.008), and MH (p<0.001) (Figure 4B, 4C). After the outbreak, patients over the age of 51 reported significant changes in their VT, PF, RE, BP, and MH scores, while subjects aged 14-30 y reported decreases in PF, RE, BP and MH scores. Pediatric patients were found to have lower scores in PF, RE, SF and MH. It is worth noting that patients aged 31-50 y reported significant changes in all subscales other than GH (P=0.077). Female patients aged 31-50 y reported changes in six subscales (VT, p=0.002; PF, p<0.001; RE, p=0.000; BP, p<0.001; SF, p<0.001; MH, p<0.001) while male patients only had documented changes pertaining to PF (p=0.042) and RE (p=0.041). No similar findings were noted in other age groups using the same method (Figures 5 and 6).
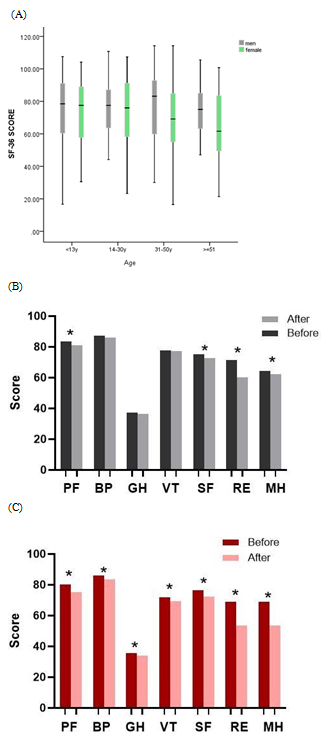 Figure 4: (A) Female patients scored much lower than men during the COVID-19 pandemic period, especially those aged 31-50y. (B) Male patients experienced significant decrease across PF, RE, SF and MH after COVID-19 outbreak. (C) Female patients experienced significant decrease in all subscales. *P < 0.05.
Figure 4: (A) Female patients scored much lower than men during the COVID-19 pandemic period, especially those aged 31-50y. (B) Male patients experienced significant decrease across PF, RE, SF and MH after COVID-19 outbreak. (C) Female patients experienced significant decrease in all subscales. *P < 0.05.
PF (physical functioning), BP (bodily pain), GH (general health), VT (vitality), SF (social functioning), RE (role emotional) and MH (mental health).
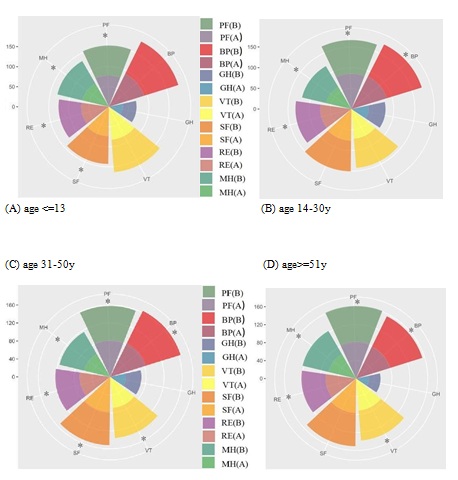 Figure 5: Comparison of seven subscales of patients in different age groups. (A) Pediatric patients were found to have lower scores in PF, RE, SF and MH. (B) Subjects aged 14–30 y reported decreases in PF, RE, BP and MH scores. (C) Patients aged 31– 50 y reported significant changes in all subscales other than GH. (D) Patients aged over 51 reported significant changes in their VT, PF, RE, BP, and MH scores. *P < 0.05.
Figure 5: Comparison of seven subscales of patients in different age groups. (A) Pediatric patients were found to have lower scores in PF, RE, SF and MH. (B) Subjects aged 14–30 y reported decreases in PF, RE, BP and MH scores. (C) Patients aged 31– 50 y reported significant changes in all subscales other than GH. (D) Patients aged over 51 reported significant changes in their VT, PF, RE, BP, and MH scores. *P < 0.05.
PF (B): mean score of physical functioning before COVID-19 outbreak, PF (A): mean score physical functioning after COVID-19 outbreak, BP (B): mean score of bodily pain before COVID- 19 outbreak, BP (A): mean score of bodily pain after COVID-19 outbreak, GH (B): mean score of general health before COVID-19 outbreak, GH (A): mean score of general health after COVID-19 outbreak, VT(B): mean score of vitality before COVID-19 outbreak, VT(A): mean score of vitality after COVID-19 outbreak, SF(B): mean score of social functioning before COVID-19 outbreak, SF(A): mean score of social functioning after COVID-19 outbreak, RE (B): mean score of role emotional before COVID-19 outbreak, RE (A): mean score of role emotional after COVID-19 outbreak, MH (B): mean score of mental health before COVID-19 outbreak, MH (A): mean score of mental health after COVID-19 outbreak.
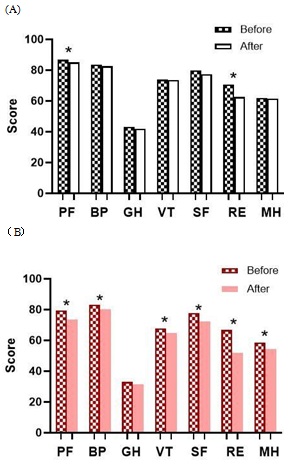 Figure 6: (A) Male patients aged 31-50y had documented changes pertaining to PF and RE. (B) Female patients aged 31–50 y reported changes in six subscales other than GH.*P
Figure 6: (A) Male patients aged 31-50y had documented changes pertaining to PF and RE. (B) Female patients aged 31–50 y reported changes in six subscales other than GH.*P
PF (physical functioning), BP (bodily pain), GH (general health), VT (vitality), SF (social functioning), RE (role emotional) and MH (mental health).
We also found that family income affected QoL. Patients with a monthly family income of more than $1410 experienced changes in their PF, RE, and BP scores. Patients with family income between $706 and $1409 showed differences in VT (p=0.030), PF (p=0.01), RE (p<0.001), SF (p=0.021), and MH (p=0.003). Subjects with a family income of less than $705 experienced significant changes in six (VT, p<0.001; PF, p<0.001; RE, p<0.001; BP, p<0.001; SF, p<0.001; MH, p<0.001) of seven subscales (Figure 7).
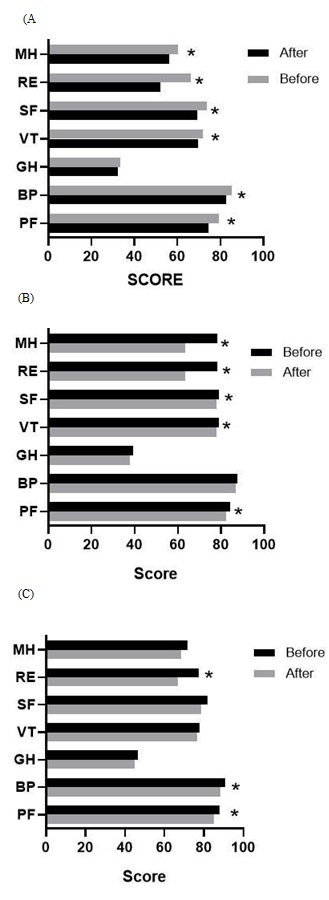 Figure 7: Comparison of SF-36 subscale scores of patients based on family income.
Figure 7: Comparison of SF-36 subscale scores of patients based on family income.
(A) Subjects with a family income of less than $705 experienced significant changes in six subscales other than GH. (B) Patients with family income between $706 and $1409 showed differences in VT, PF, RE, SF, and MH. (C) Patients with a monthly family income more than $1410 experienced changes in their PF, RE, and BP scores. *P < 0.05.
PF (physical functioning), BP (bodily pain), GH (general health), VT (vitality), SF (social functioning), RE (role emotional) and MH (mental health).
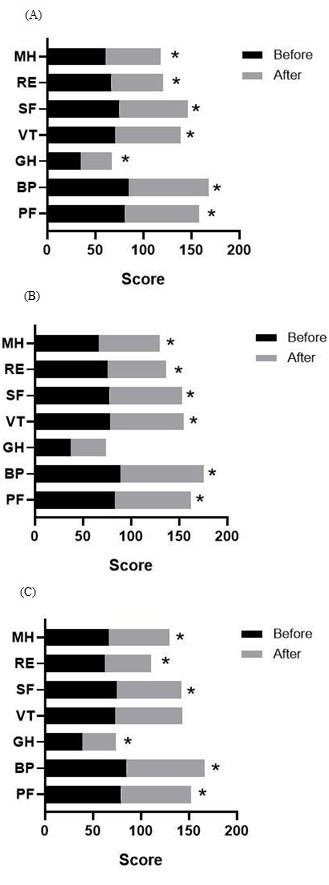 Figure 8: Comparison of SF-36 subscale scores of patients living in different areas.
Figure 8: Comparison of SF-36 subscale scores of patients living in different areas.
(A) Patients residing in high risk areas experienced significant decreases in the all subscales. (B) Patients living in moderate risk areas reported no changes in the GH subscale after the outbreak of COVID-19. (C) Patients residing in low risk areas experienced significant decreases in the all subscales. *P < 0.05.
PF (physical functioning), BP (bodily pain), GH (general health), VT (vitality), SF (social functioning), RE (role emotional) and MH (mental health).
As described in Figure 8, patients residing in high and low risk areas experienced significant decreases in the all subscales (p<0.05). Patients living in moderate risk areas reported no changes in the GH subscale after the outbreak of COVID-19.
DISCUSSION
This is the first study to evaluate the psychological and physical impact of COVID-19 outbreak on ITP patients. We found that both physical and psychological health have been threatened by the pandemic, with more marked effects on the psychological than the physical health of these patients. The QoL of ITP patients in the chronic phase, those with platelet count<10 G/L, female patients (especially with ages 31-50 y) and those with lower family income were the most severely affected (Figure 9).
 Figure 9: Factors affecting the quality of life during the pandemic.
Figure 9: Factors affecting the quality of life during the pandemic.
In our study, 90.8% of participants were less than 50 years old and 83.54% of them suffered from chronic ITP [34]. There was only one case of infection with SARS-CoV-2, which indicates that patients with ITP are not necessarily at greater risk of infection. ITP patients scored much lower on the SF-36 than the healthy population [35]. Prolonged fatigue, fear of bleeding, and limitations in emotional and functional health, work life, social and leisure activities are the reasons for poor quality of life in patients with chronic ITP [36]. After the outbreak of COVID-19, many governments and hospitals implemented a series of policies to control the spread of the virus, including placing a lockdown policy, severely restricting outpatient services except emergencies, limiting the number of visitors, stopping certain examinations with the potential to spread the virus, and diverting medical resources to treat patients with the SARS-CoV-2 infection. These policies further worsen the QoL of ITP patients, particularly those living in high-risk regions.
Furthermore, chronic ITP patients are usually poorly responsive to several kinds of drugs and need to adjust their treatment regularly. Suffering from side effects due to long-term treatment also have important implications. Steroids is the first-line treatment for newly diagnosed ITP, as well as acute and severe ITP [25,37,38]. The efficacy is as high as 70-80%, which may be a reason why the QoL was not as noticeably affected in patients with newly diagnosed ITP. However, long-term and over-dose usage of steroids can aggravate the mental and physical health of patients, causing weight gain, insomnia, high blood pressure, and hyperglycemia [39]. Hence, patients taking steroids should have their medication dosage and treatment regimen adjusted regularly by a health professional. Although treatment with thrombopoietin receptor agonists (TPO-Ras) in adults with persistent or chronic ITP for an extended period of time has proven to effectively improve QoL, increase platelet counts and reduce bleeding/bruising, alleviate fatigue, and improve physical function, especially in responders [40], it may cause headache, liver damage, deep vein thrombosis and myocardial infarctions if not adjusted in a timely manner [40-42]. Therefore, the uneven distribution of medical resources, which results in the unavailability of timely medical consultation and treatments, combined with the limited ability to monitor side effects of the drugs, may have led to the reduction of these patients’ QoL.
Our results suggest that women presented with more significant deterioration of both physical and psychological health than men, especially women with ages 30-50 y. Patients of this group are likely to have many more family and social responsibilities than other groups. We also found that the QoL of patients who lived with their family during the COVID-19 pandemic is worse than others. Several studies have shown that the mental status of pediatric ITP patients positively correlates with that of their parents [41,43], but the relationship between the psychological health status of adult patients and that of their families has not been studied. Our findings indicate that the care and anxiety of family members may be a psychological burden to patients. The psychological status of family members should be taken into consideration in making clinical decisions, especially in these unprecedented times of a pandemic. In addition, family income also affected the QoL of ITP patients, which is consistent with the study by Z. Zhou [44]. The higher family income, the less the patients were affected by the pandemic. This trend may be explained by the fact that these patient groups may have more access to medical consultation during the epidemic and higher health literacy, especially pertaining to their own disease.
Our findings indicate that a series of secondary disasters may be caused by the COVID-19 pandemic. Although the spread and the direct impacts of the pandemic appear to be slowing, the uneven distribution of medical resources and the secondary disasters caused by the pandemic will last for a long time. We should not only pay attention to the patients with SARS-CoV-2 infection, but also to patients with chronic diseases, such as ITP. These chronic patients lead a poorer quality of life owing to the unstable status of their diseases and have no access to timely medical help in these difficult times. Our study aims to call social and government attention to this population and establish a better response system to reduce secondary disasters. It is necessary to give priority for matters concerning these chronic patients, especially for acute and severe cases. At the same time, physicians need to allow more time to follow up on these patients in different ways, such as through internet consultations. Furthermore, efforts should be made in health education to increase awareness of these diseases, with a particular focus on psychological health, which is likely to suffer more than physical health and necessitates the help of psychiatry, social workers, and family members of patients.
Our study has the following limitations: (1) our study was a cross-sectional design, which means it was impossible to obtain the further information about the patients QoL companying with the progress of the pandemic. (2) The relationship between treatments regime and QoL was not studied.
ACKNOWLEDGEMENT
The authors would like to thank Editage (www.editage.cn) for English language editing and the language editor Michelle Chae-min.
FUNDING
This work was supported by the Natural Science Foundation of Guangdong, Education and Research of Guangdong Province (2017A030313767 [to J.Y.Y]).
CODE AVAILABILITY
SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA)
AUTHORS’ CONTRIBUTIONS
Z.P.L and X.Y.Z contribute equally to this work. Z.P.L., X.Y.Z and J.Y.Y conducted the experiments and collected data. Z.P.L. and X.Y.Z. performed the data and statistical analyses. Z.P.L. drafted the manuscript. Y.Y.J. and Y.J.Z. collected the statistics and helped patients to complete the questionnaire. Z.P.L. and J.Y.Y analyzed data and revised this manuscript repeatedly.
REFERENCES
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, et al. (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 113; 2386-2393.
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, et al. (2010) International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 115: 168-186.
- Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B (2000) The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med 160: 1630-1638.
- Nørgaard M, Jensen A, Engebjerg MC, Farkas DK, Thomsen RW, et al. (2011) Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: A Danish population-based cohort study. Blood 117: 3514-3520.
- McMillan R, Bussel JB, George JN, Lalla D, Nichol JL (2008) Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol 83: 150-154.
- Arnold DM, Kelton JG (2007) Current options for the treatment of idiopathic thrombocytopenic purpura. Semin Hematol 44: S12-S23.
- Mithoowani S, Arnold DM (2019) First-Line Therapy for Immune Thrombocytopenia. Hamostaseologie 39: 259-265.
- Wei Y, Ji XB, Wang YW, Wang JX, Yang EQ, et al. (2016) High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: A prospective multicenter randomized trial. Blood 127: 296-302.
- Wang L, Xu L, Hao H, Jansen AJG, Liu G, et al. (2020) First line treatment of adult patients with primary immune thrombocytopenia: a real- world study. Platelets 31: 55-61.
- Buttgereit F, Burmester GR, Lipworth BJ (2005) Optimised glucocorticoid therapy: The sharpening of an old spear. The Lancet 365: 801-803.
- Trotter P, Hill QA (2018) Immune thrombocytopenia: improving quality of life and patient outcomes. Patient Relat Outcome Meas 9: 369-384.
- Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, et al. (2011) Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. The Lancet 377: 393-402.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395: 497-506.
- Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, et al. (2020) Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol 30: 313-324.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382: 1708-1720.
- Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109: 102433.
- Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol 94: 7.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, et al. (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 7: 11.
- Gu J, Han B, Wang J (2020) COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology 158: 1518-1519.
- Qiu W, Chu C, Mao A, Wu J (2018) The Impacts on Health, Society, and Economy of SARS and H7N9 Outbreaks in China: A Case Comparison Study. J Environ Public Health 2018: 2710185.
- Lee AM, Wong JG, McAlonan GM, Cheung V, Cheung C, et al. (2007) Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry 52: 233-240.
- Bai Y, Lin CC, Lin CY, Chen JY, Chue CM, et al. (2004) Survey of stress reactions among health care workers involved with the SARS outbreak. Psychiatr Serv 55: 1055-1057.
- Liang W, Guan W, Chen R, Wang W, Li J, et al. (2020) Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. The Lancet Oncology 21: 335-337.
- Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, et al. (2019) Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv 3: 3780-3817.
- Rodeghiero F, Michel M, Gernsheimer T, Ruggeri M, Blanchette V, et al. (2013) Standardization of bleeding assessment in immune thrombocytopenia: Report from the International Working Group. Blood 121: 2596-2606.
- Thrombosis and Hemostasis Group, Hematology Society, Chinese Medical Association (2016) [Consensus of Chinese experts on diagnosis and treatment of adult primary immune thrombocytopenia (version 2016)]. Zhonghua Xue Ye Xue Za Zhi 37: 89-93.
- Xiao S, Liu Q, Hou M (2017) [Comparative study between two bleeding grading systems of primary immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi 38: 394-398.
- Newnham EA, Harwood KE, Page AC (2007) Evaluating the clinical significance of responses by psychiatric inpatients to the mental health subscales of the SF-36. J Affect Disord 98: 91-97.
- Brigden A, Parslow RM, Gaunt D, Collin SM, Jones A, et al. (2018) Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: triangulation using three different methods. Health Qual Life Outcomes 16: 202.
- Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30: 473-483.
- Yang R, Yao H, Lin L, Ji JM, Shen Q (2020) Health-Related Quality of Life and Burden of Fatigue in Chinese Patients with Immune Thrombocytopenia: A Cross-Sectional Study. Indian J Hematol Blood Transfus 36: 104-111.
- Li L, Wang HM, Shen Y (2003) Chinese SF-36 Health Survey: Translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health 57: 259-263.
- Segal JB, Powe NR (2006) Prevalence of immune thrombocytopenia: Analyses of administrative data. J Thromb Haemost 4: 2377-2383.
- Sestol HG, Trangbaek SM, Bussel JB, Frederiksen H (2018) Health-related quality of life in adult primary immune thrombocytopenia. Expert Rev Hematol 11: 975-985.
- Mathias SD, Gao SK, Miller KL, Cella D, Snyder C, et al. (2008) Impact of chronic Immune Thrombocytopenic Purpura (ITP) on health-related quality of life: a conceptual model starting with the patient perspective. Health Qual Life Outcomes 6: 13.
- Lambert MP, Gernsheimer TB (2017) Clinical updates in adult immune thrombocytopenia. Blood 129: 2829-2835.
- Cines DB, Bussel JB (2005) How I treat idiopathic thrombocytopenic purpura (ITP). Blood 106: 2244-2251.
- Brown TM, Horblyuk RV, Grotzinger KM, Matzdorff AC, Pashos CL (2012) Patient- reported treatment burden of chronic immune thrombocytopenia therapies. BMC Blood Disord 12: 2.
- Khelif A, Saleh MN, Salama A, Portella M, Duh MS, et al. (2019) Changes in health-related quality of life with long-term eltrombopag treatment in adults with persistent/chronic immune thrombocytopenia: Findings from the EXTEND study. Am J Hematol 94: 200-208.
- Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, et al. (2017) Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: Final results of the EXTEND study. Blood 130: 2527-2536.
- Neunert CE (2019) Thrombopoietin Receptor Agonist Use for Immune Thrombocytopaenia. Hamostaseologie 39: 272-278.
- Zhang H, Wang L, Quan M, Huang J, Wu P, et al. (2016) Health-related quality of life in children with chronic immune thrombocytopenia in China. Health Qual Life Outcomes 14: 45.
- Zhou Z, Yang L, Chen Z, Chen X, Guo Y, et al. (2007) Health-related quality of life measured by the Short Form 36 in immune thrombocytopenic purpura: A cross-sectional survey in China. Eur J Haematol 78:518-523.
Citation: Li ZP, Zeng XY, Jiao YY, Zhang Y, Sun J, et al. (2020) New Global Challenges for Patients with Immune Thrombocytopenia during the Coronavirus Disease (COVID-19) Pandemic. J Clin Immunol Immunother 6: 040.
Copyright: © 2020 Zong Peng Li, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

