
NHexane Extract of Caryota no Seeds Modulates Survival and Longevity in Drosophila Melanogaster
*Corresponding Author(s):
Chinonye A MaduagwunaDepartment Of Pharmacology And Therapy, University Of Abuja, Abuja, Nigeria
Tel:+234 8037114483,
Email:elchinonye@gmail.com
Abstract
Study background: The current issues with novel infections and established global resistance to already available anti-infective agents make it imperative that more efforts must be put into exploration of plants as sources of medicine and for prolongation of longevity since they are healthy components of diet. The purpose is to investigate the toxic effects of nHexane extract of Caryota No (CN) seeds in Drosophila Melanogaster (DM) and its effect on survival and life span. Experimental design was employed.
Methods: The Lethal Concentration (LC50) was determined by exposing 50 flies in each vial to concentrations ranging from 1 mg to 600 mg per 10 g diet and mortality of flies was scored every 24 hours for 14 days and from the results, five doses were chosen for the next assay. Survival assays were carried out by exposing 50 flies in each vial to the following concentrations: 300 mg, 350 mg, 400 mg, 500 mg and 600 mg of nhexane extracts in 5 replicates for 28 days with daily recording of mortality while the longevity assay was done as in the survival assay but continues until the last fly dies. The control flies received plain diet.
Results: The LC50 value of the nhexane extract was determined to be 4565 mg extract/10g fly food in Drosophila melanogaster. The result of nHexane Caryota no extract showed significantly (P < 0.05) reduced survival compared to the control. The longevity assay revealed that the extract significantly (P < 0.05) increased the life span in Drosophila melanogaster.
Conclusion: The results obtained from evaluating the nhexane extract of Caryota no indicate that the plant is relatively non-toxic since it did not produce any severe toxic effect and maybe safe under acute, subacute and chronic exposures and by extrapolation may not only be safe for human consumption but may also prolong life span.
Keywords
Caryota no; Drosophila Melanogaster; LC50; Longevity; Survival; Toxicity
LIST OF ABBREVIATIONS
DM: Drosophila Melanogaster
CN: Caryota no
SEM: Standard Error of the Mean
LC50: Lethal Concentration at 50% mortality
SOP: Standard Operating Procedures
GLP: Good Laboratory Practices
NEJM: New England Journal of Medicine
BACKGROUND
The current emergence and re-emergence of very virulent species of microbes which cause severe symptoms, death and which are also spreading rapidly as illustrated by the current Corona, Hanter, Zika, virus diseases, the Lassa and Ebola haemorrhagic fevers, there is need for researchers in the developing world (which are expected to be the worst hit in these events) to quickly adapt to current sciences of drug discovery to escape the elimination of races or whole tribes by disease. It is necessary to screen the antioxidant activities of nHexane extract of Caryota no seed since plants have been proved to be reliable sources of potent phytochemicals [1]. The World Health Organization defines a medicinal plant as any plant which possesses in one or more of its organs certain substances that can be used for therapeutic purposes, or which are precursors for chemo-pharmaceutical processes. Such a plant will have its parts like leaves, roots, rhizomes, stems, barks, flowers, fruits, grains or seeds, employed in the control or treatment of a disease conditions and would therefore contain chemical components that are medically active. These non-nutrient plant chemical compounds or bioactive components are often referred to as phytochemicals and are responsible for protecting the plant against microbial infections or infestations by pests [2]. Medicinal herbs have consistently been considered the leading source of pharmaceuticals, employed in the treatment of various human diseases due to their high chemical diversity and broad biological functionality [3]. Traditional medicines are mostly compounded from natural products therefore, there is a likelihood of them being accepted by the body better than synthetic substances [4]. The relatively lower incidence of adverse reactions to plant preparations compared to modern conventional pharmaceuticals, coupled with their reduced cost, is encouraging both the consuming public and national health care institutions to consider plant medicines as alternatives to synthetic drugs [5]. Thus plants are considered as one of the most important and interesting subjects that should be explored for the discovery and development of newer and safer drug candidates. Natural toxicants present in human foods and animal feeds present a potential hazard to health. Saponins are phytochemicals found in variety of plant foods. They exhibit strong foaming ability in aqueous solutions, as well as cytotoxic effect [6], they also demonstrate hemolytic properties [7] which also inhibits the activities of proteases [8]. Standard measures of the toxicity of a plant substance include assays for lethal concentration, effects on survival and longevity on different organisms. Lethal Concentration 50 or LC50 is a standard measure of toxicity to determine how much of a substance is needed to kill half of a group of experimental organisms in a given time [9]. These and other preclinical studies must be undertaken before any biologically active substance must be evaluated clinically for therapy.
Caryota No (CN) palm is a native of the Borneo rainforests. The common name is the Giant Fishtail Palm. In habitat, this palm can reach a height of 75 inches and stems measure 18-20 inches in diameter [10]. Caryota species, mostly found in Asia, are used traditionally in the treatment of gastric ulcer, migraine headaches, and snakebite poisoning and also rheumatic swellings by preparing porridge from the flowers [11]. What sets this palm apart from others in the genus is its upright growth habit. Although this palm is considered a giant, its footprint in the landscape is reduced by its fronds growing mostly upward and rarely ever extending horizontally from the stem. This palm would grow successfully anywhere a coconut palm thrives. Caryota no is not wind resistant and actually is one of the least wind resistant palms.
The aim of this work is to do a preliminary screen of nHexane extracts of Caryota no for LC50, and their effects on survival and longevity of Drosophila melanogaster so as to determine toxicity and have a base line for future studies on the plant.
METHODS
Study design and population
Experimental design was used and sample of population used was fifty (50) for each experimental group in a vial.
Chemicals and reagents
All chemicals used were of analytical grade. nHexane and distilled water were obtained from Africa Centre of Excellence in Phytomedicine Research and Development, Jos, Plateau State, Nigeria.
Plant collection and preparation
The plant material was collected from Games Village, Abuja, Nigeria. The plant was identified by a taxonomist in the herbarium of the Federal college of Forestry Jos. The seeds were sorted, air-dried for several days and then pulverized to powder using a commercial grinding machine. The soxhlet extractor was used for extraction of the plant compound using analytical grade nhexane as solvent following a method described by [12]. A rotary evaporator was employed to recover the different solvents. The extracts were further dried in a water bath regulated at 40°C and kept in an airtight container. This yielded the nhexane extract from the CN seeds (2%), which was used in the biological tests.
Fly strains and diet
D. melanogaster (Harwich strain) was obtained from Africa Center of Excellence in Phytomedicine Research and Development, University of Jos and maintained at constant temperature and humidity (23°C; 60 % relative humidity, respectively) under 12 hour dark/light cycle. The flies were cultured by feeding them with a standard medium of the following compositions; 1700 ml of water, 16 g agar, 20 g of baker’s yeast, 100 g of corn flour, and 1 g of methyl paraben dissolved in 5 ml of absolute ethanol, 1700 ml of water [13].
LC50 of nHexane seed extract of CN
The 14- days LC50 was determined following the method described [9] with slight modification. 50 flies (of both genders (1-3 days old) per vial were exposed to the following concentrations; 1 mg, 10 mg, 50 mg, 100 mg, 250 mg, 300 mg and 350 mg of nHexane extract of Caryota no seed per 10 g diet. Young flies 1-3 days old were preferred. To obtain the young flies of known age the culture bottles with pupae were strictly emptied of all flies and the date noted and labeled accordingly. Adult flies of known age were then harvested from the newly hatched population. Mortality of flies was scored every 24 hours for 14 days. During the experimental period, flies were transferred onto new vials containing fresh food every 2 days. Details are stated in the next two sessions.
Survival assay of nHexane seed extract of CN -treated flies
50 flies of both genders (1-3 days old) were exposed to selected concentrations of nHexane extracts of CN seeds (300mg, 350mg, 400mg, 500mg and 600mg prepared in distilled water) in five replicates for 28 days as described by [14,15]. The numbers of live and dead flies were scored daily till the end of the experiment and the survival rate was expressed as percentage of live flies. The data were subsequently analyzed and plotted as cumulative mortality and percentage survival after the treatment period.
The flies were divided into six groups containing 50 flies each. Control group was placed on normal diet alone while groups II-IV were placed on basal diet containing nhexane seed extract of CN at various concentrations of diet as shown thus;
Control group Basal diet
300 mg group Basal diet + 300mg CN nhexane seed extract/10g fly food
350 mg group Basal diet + 350mg CN nhexane seed extract/10g fly food
400 mg group Basal diet + 400mg CN nhexane seed extract/10g fly food
500 mg group Basal diet + 500mg CN nhexane seed extract/10g fly food
600 mg group Basal diet + 600mg CN nhexane seed extract/10g fly food
During the experimental period, flies were transferred onto new vials containing fresh food every 2 days. The flies were exposed to these treatments for 28 days, and the vials containing flies were maintained at room temperature. All experiments were carried out in triplicate (each experimental group was carried out in five independent vials). Survival analyses were calculated based on the number of deaths recorded and evaluated by the log-rank Mantel-Cox test.
Longevity assay of nHexane seed extract of CN-treated flies
Longevity assay proceeded as a continuum from the survival assay as described by [16,17], such that after 28 days, the daily recording of number of deaths continued until the last fly dies. Survival analyses were calculated based on the number of deaths recorded and evaluated by the log-rank Mantel-Cox test. The vials containing fresh food were made to be at room temperature for each transfer. During the experimental period, flies were transferred onto new vials containing fresh food every 2 days. This step will ensure that the feeding environment for young females is not disrupted by the presence of larvae. This transfer was completed without anesthesia, which can induce acute mortality, particularly in older flies.
During each vial transfer, the dead flies in the old vial were counted, and the dead flies that are carried to the new vial also noted. This information was recorded separately in two columns in a spreadsheet. This will ensure that the carried flies are not double-counted. The total number of deaths (dead + carried) should at least equal the number of carried flies from the previous transfer. Subtract the number of previously carried flies from the total number of deaths to determine the number of new deaths. A fly is considered right-censored if it left the experiment prior to natural death through escape or accidental death. Animals exiting the experiment in this way were entered into a separate column on the day that the fly exited the experiment. Censored flies are not recorded as dead.
These transfer steps were continuously repeated until the last survivor is dead. As the flies age, some flies may lie on their back and appear dead due to their inactiveness. Therefore when counting carried (dead) flies, the side of the vials were tapped to determine if there are leg movements. If so, these flies are still alive. In the case where flies remain stuck to the food in the old vial but alive, they should not be counted as dead but were rescued by further tapping of the vial to dislodge the fly. Censoring such flies should be used with caution as it may result in experimental bias.
Statistical analysis
Analysis of the data was done on for the determination of the LC50 of Caryota no on adult D. melanogaster. The data was expressed as mean ± SEM (standard Error of the Mean) of five parallel measurements, and the statistical analysis was carried out using one-way Analysis of Variance (ANOVA) using the software, Graphpad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). The results were considered statistically significant at P <0.05.
RESULTS
LC50 of nHexane seed extract of CN
Exposure to nhexane extract of CN seeds resulted in LC50 level of 4565mg/10g fly food in DM. The concentration that can kill 50% of the test organism, LC50 is shown in the result, (Figure 1). The LD50 of the methanolic extract in swiss rats was also determined by this researcher to be > 5000mg/kg by the oral route and >1000mg /kg by the intraperitoneal route (unpublished). This high level of LC50 suggests the safety of this extract and also serves as a baseline for selecting the concentrations; 350 mg, 400 mg and 500 mg per 10 g diet for 28-days survival study. It can therefore be inferred that the nhexane extract of CN seeds is relatively safe.
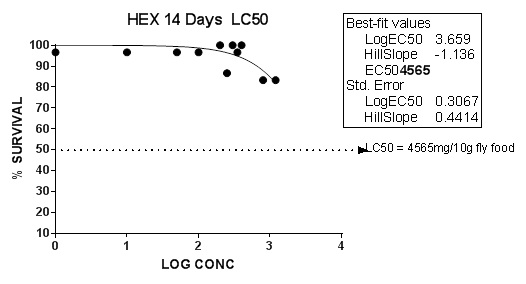 Figure 1: LC50 of nHexane extract of CN seeds on Drosophila melanogaster. The scattered dots representing the expected percentage of fly survival are joined to form a trend line which help determine the log concentration of the extracts corresponding to 50% fly death. The LC50 of CN in log10 scale is between 1- 3.079 after 14 days. The Hillslope value for the LC50 in 95% confidence interval is found to be in the range -0.489 to 0.176 mg/10g food. Data presented as mean ± SEM of five (5) independent biological replicates for each extract concentration (n = 50). P <0.05 vs control.
Figure 1: LC50 of nHexane extract of CN seeds on Drosophila melanogaster. The scattered dots representing the expected percentage of fly survival are joined to form a trend line which help determine the log concentration of the extracts corresponding to 50% fly death. The LC50 of CN in log10 scale is between 1- 3.079 after 14 days. The Hillslope value for the LC50 in 95% confidence interval is found to be in the range -0.489 to 0.176 mg/10g food. Data presented as mean ± SEM of five (5) independent biological replicates for each extract concentration (n = 50). P <0.05 vs control.
Survival assay of nHexane seed extract of CN -treated flies
Exposure to nhexane extract of CN caused significant (P = 0.0002 ***) effect on survival in DM (Figure 2). By the 28th day, the survival proportions for the control, 300, 350, 400, 500 and 600 mg/10g diet groups were 41.2, 16.4, 28.4, 24.8, 29.5 and 23.0 percent respectively. Also, the number of subjects at risk in the same order at the 28th day of the assay was 107, 44, 73, 63, 75 and 60. For the summary of the data, the median survival is 26, 23, 23, 23, 23 and 23 for the groups. The lowest extract dose recorded the least percentage survival proportion and the least number of subjects at risk. The higher extract doses caused improvement in these parameters but not as well as the control. It can therefore be inferred that the nhexane extract of CN significantly decreased survival of DM adult fly after 28 days oral exposure.
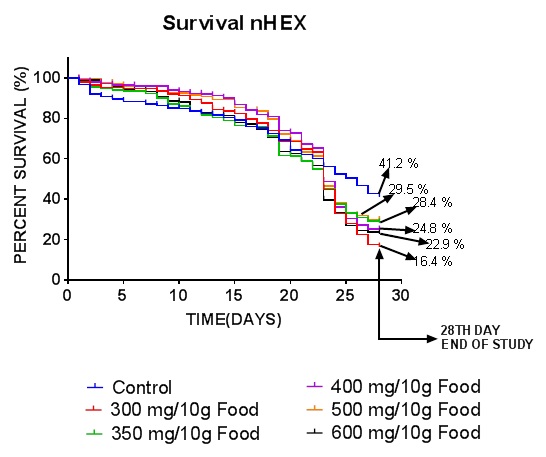 Figure 2: Effect of nhexane extract of CN on survival of Drosophila melanogaster. Data presented as mean ± SEM of five (5) independent biological replicates for each extract concentration (n = 50). P <.05 vs control.
Figure 2: Effect of nhexane extract of CN on survival of Drosophila melanogaster. Data presented as mean ± SEM of five (5) independent biological replicates for each extract concentration (n = 50). P <.05 vs control.
Percentage death of flies treated with nHexane seed extract of CN
The survival result (Figures 3 and 4) for the nhexane extract of CN show statistically significant (P = 0.013 *) increase in death by the treatment groups compared to the control. The lowest extract dose recorded the highest number of deaths. The higher doses lowered the percentage death. There was rather a significant difference (P = 0.0059 **) between the lowest extract dose (300 mg/10g food) and the control. There was no difference between the highest extract dose and the control. It can therefore be inferred that exposure to nhexane extract of CN especially at extreme doses caused significant (P<0.05) increase in percentage deaths in DM.
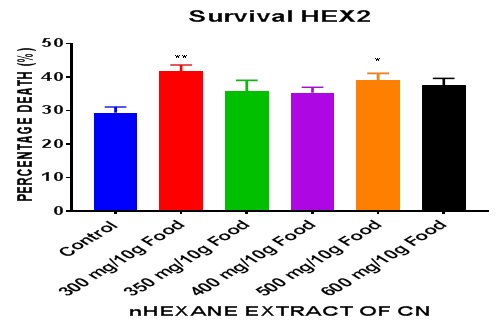 Figure 3: Day 28 percentage death of Drosophila melanogaster after treatment with nhexane extract of CN Seed. Data presented as mean ± SEM of five (5) independent biological replicates of each extract concentration (n = 50). Extracts: significant from control * P < 0.05; ** P < 0.001; *** P = 0.0002 - 0.0004; **** P < 0.0001.
Figure 3: Day 28 percentage death of Drosophila melanogaster after treatment with nhexane extract of CN Seed. Data presented as mean ± SEM of five (5) independent biological replicates of each extract concentration (n = 50). Extracts: significant from control * P < 0.05; ** P < 0.001; *** P = 0.0002 - 0.0004; **** P < 0.0001.
 Figure 4: Combined cumulative mortality and percentage survival after treatment with nhexane extract of CN Seed. Data presented as mean ± SEM of five (5) independent biological replicates of each extract concentration (n = 50). Extracts: significant from control * P < 0.05; ** P < 0.001; *** P = 0.0002 - 0.0004; **** P < 0.0001.
Figure 4: Combined cumulative mortality and percentage survival after treatment with nhexane extract of CN Seed. Data presented as mean ± SEM of five (5) independent biological replicates of each extract concentration (n = 50). Extracts: significant from control * P < 0.05; ** P < 0.001; *** P = 0.0002 - 0.0004; **** P < 0.0001.
Longevity assay of nHexane seed extract of CN -treated flies
The graph (Figure 5) illustrates that the nhexane extract caused a significantly (P< 0.0002 ***) prolonged life span in D. melanogaster between the groups and the control. By the 59th day, the survival proportions for the control, 300, 350, 400, 500, and 600 mg/10g diet groups were 68, 59, 65, 68, 68, 70 % respectively. Also, the number of subjects at risk in the same order at the 59th day of the assay was 41, 2, 32, 6, 21 and 16. The lowest extract dose also recorded the least survival proportion value. The higher doses were better tolerated. The nhexane extract elongated the fly life span in comparison to the control (P< 0.05). The control flies all died by day 69 while most of the treatment groups also survived to that day and beyond. A particular group (600 mg/10g diet) all died by day 70 and this is a prolongation of life span on chronic exposure in comparison to the control.
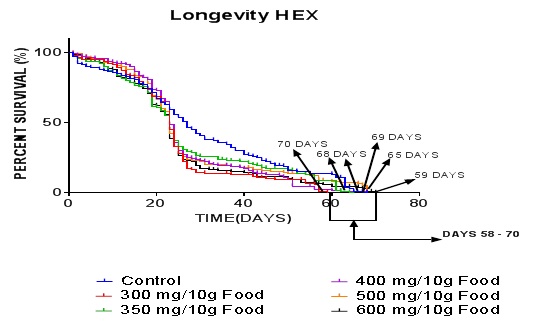 Figure 5: Effect of nhexane extract of CN on lifespan of Drosophila melanogaster. Data presented as mean ± SEM of five (5) independent biological replicates for each extract concentration (n = 50). P <0.05 vs control.
Figure 5: Effect of nhexane extract of CN on lifespan of Drosophila melanogaster. Data presented as mean ± SEM of five (5) independent biological replicates for each extract concentration (n = 50). P <0.05 vs control.
DISCUSSION
The concentration that can kill 50% of the test organism, LC50 shown in the result, (Figure 1), the 14- days LC50 of C. no nhexane extract in D. melanogaster was determined to be 4565 mg/10g diet. It was reported [18] that methanol and dichloromethane extracts of Dendranthema grandiflorum leaves showed LC50 values of 5.02 and 5.93 pm, respectively while another group [19] demonstrated that the petroleum ether extract of the bark of Mirabilis jalapa showed significant cytotoxic activity with the LC50 value 8.12 µg/ml compared to vincristine sulphate (LC50 0.33 µg/ml). These were done at acute, subacute and chronic durations. A lower concentration of any chemical might be more effectively toxic when applied for a longer duration and revealed that exposure duration to any chemical is a very important and deciding factor in the process of LC50 determination. The very high level of LC50 of the index study after subacute exposure imply the safety of this extract and also serves as a baseline for selecting the concentrations further study.
The survival result (Figure 2) revealed significantly (P<.05) decreased survival proportion in the nhexane extract treated groups compared to control. In comparison of the curves, the P value by Log-rank or Mantel-cox test is 0.0002 - *** showing that the curves are significantly different. Further observation of the survival results by looking at percentage death (Figure 3) showed the difference between the lowest extract dose (300 mg/10g food) and the control to be P = 0.0059 (**) while between the control and the 500 mg/10g food dose was (P = 0.048) slight (*). There was however no statistically significant (P> 0.05) difference between the control and the two middle doses and the highest extract dose (350, 400, 600 mg/10g food). The result from this survival assay is corroborated by the already proven fact that a certain proportion of fat enhances survival kinetics in drosophila and also implies that a moderate dose of the nhexane extract enhances fly survival. The nhexane extract which is basically oil was better tolerated by the flies at moderate doses. A review [20] which refers to another work [21] has proven that the fruit fly tolerates only up to 1% of fat in the diet. It was also observed [15] that higher dietary inclusions of Garcinia kola seed reduced the survival rate of D. melanogaster more significantly compared to control flies. This agrees with what was observed by this researcher that different doses of dietary inclusions could increase or reduce survival. Another study reported the larvicidal activity of saponins extracted from Quillaja saponaria against the mosquitos’ larvae of two species Aedes aegypti and Culex pipiens; their results showed 100% mortality using 1,000 mg/L during 5 days [22]. The observed high abundance of saponin in Garcinia kola seed could be one of the major reasons for the mortality and impairment in locomotor performance in flies. This effect observed with the nhexane extract may be due to the fact that plant extracts possess various phyto-constituents which may enhance or retard survival of animal life depending also on their levels. The graph in figure 4 illustrates succinctly the relationship between the two parameters investigated for survival. As the percentage death increases, the survival percentage decreases for each extract dose or the control.
The graph (Figure 5) illustrates that the nhexane extract caused a significantly (P < 0.05) increased life span in D. melanogaster compared to the control. In comparison of the curves, the P value by Log-rank or Mantel-cox test is 0.0002 - *** showing that the curves are significantly different. Although a research work [21] reported by another [16] already established that the flies thrive well on about 1% fat in diet which is illustrated in the survival assay buta different researcher [23] concluded that diets rich in excess cholesterol tend to lower the life span of flies. Linford and colleagues [21] also stated by their work that adult female flies on a less concentrated diet (5% Sucrose and Yeast -SY) typically live significantly longer than those on a more concentrated diet (15% SY). A certain group of researchers also proved that the root extract of Rhodiola rosea could extend the lifespan of Drosophila melanogaster [24]. The oxidative stress hypothesis of aging has been well reported in various animal models [25]. Specifically, there have been several reports implicating ROS generation and oxidative stress in reduced life span in DM [26].
Nutrition is in addition to obesity, tobacco smoking and physical activity still considered as one of the major driving forces influencing life expectancy and the occurrence of chronic diseases in a population [27]. The Physicians Committee for Responsible Medicine (PCRM), a prevention-driven non-profit organization of plant-based medical professionals, organized a gathering of doctors in front of the White House in Washington, D.C., in 2018 to send America a message: “Ditch Dairy” and “Go Vegan.” The goal is to share essential knowledge about plant-based and preventative medical care with a focus on nutrition. Medical students were offered opportunity to learn more about the plant-based lifestyle and how it can be the first line of defense for their future patients - a concept that has not been highly promoted in traditional medical school [28]. The so-called Western diet, high in fat and sugar, is contributing to the rise in obesity, type 2 diabetes, hypertension and high cholesterol among Americans. And all these conditions, in turn, exacerbate risk for the most deadly genetic diseases such as heart disease, cancer, Alzheimer’s disease and kidney disease [29]. In the NEJM study, [30] the researchers culled factors from the strategic goals of the American Heart Association: no current smoking, body-mass index of less than 30, physical activity at least once weekly, and a healthy diet involving higher consumption of fruits, nuts, vegetables, whole grains, fish and dairy products and a reduced amount of refined grains, processed meats, unprocessed red meats and sugar-sweetened beverages. From the same patients studied above, some researchers measured the activity of an enzyme produced by genes, telomerase, believed to be involved in slowing the aging process. At five years, the age-related decrease in telomerase activity was much less in the plant-based group than a control group and their telomeres were longer, suggesting a slowing of the aging process. Telomeres prevent chromosomes from fraying and altering the genetic codes they contain. This is crucial since genetic material within our cells shortens with age, but does so much more slowly in those that eat a plant diet. One measure of longevity often cited in research is telomere length. In a nutshell, telomeres are caps found at the ends of chromosomes that protect DNA. When they become too short, a cell becomes old or dysfunctional. This is why shorter telomeres are associated with a lower life expectancy and an increased risk of developing chronic diseases [31]. Research has shown that a greater adherence to a Mediterranean diet (fruits, vegetables and nuts) is linked to longevity through maintaining longer telomere length. A plant-based diet promotes health and longevity better than any other diet or lifestyle choice known to the medical field.
In this case, it can be safely hypothesized that the fatty acids contained in nhexane extract of CN maybe the safe type (parallels of high density lipoprotein - HDL in humans) since instead of shortening the life span, it rather prolonged longevity in DM. The phytochemistry showed that this extract contained a lot of steroids, moderate amounts of phenols, terpenoids and cardiac glycosides (unpublished) which may globally help in antioxidation and anti- infectivity and would ultimately modulate survival. The choice of diet alone can greatly influence lifespan and can interact with genetic factors to produce diet-specific effects on longevity. The major limitation to this study was constant supply of electrical energy which has been a major setback in the country but this was overcome by the installation of an independent source of power in the laboratory. Also, the need for close control of temperature for the fly room was ensured by provision of air-conditioning system, room heaters, room thermometer and a resident staff. There was also a regular rehearsal and strict adherence to the Standard Operating Procedures (SOP) by all staff in order to maintain Good Laboratory Practices (GLP) since this is reference centre for drosophila studies.
CONCLUSION
From the findings it can be concluded that nHexane extract of Caryota no seeds has very high LC50 = 4565 mg/10 g diet and showed significantly reduced survival compared to the control in DM. The longevity assay revealed that the extract significantly increased the life span of the nhexane extract-treated flies. This implies that there are indeed bioactive substances present in this extract requiring further pharmacological evaluation of the extract which would help to validate or abrogate the claims of the folkloric usage of the plant. Further studies on this extract would also help to extrapolate its effect of significantly reducing survival in DM to public health.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study does not involve any human or animal testing that requires ethical approval.
AVAILABILITY OF DATA AND MATERIAL
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
Self - sponsorship
AUTHORS’ CONTRIBUTION
MCA designed the study, performed the statistical analysis, wrote the protocol, and with EMA wrote the first draft of the manuscript. GSS and OS managed the analyses of the study and the literature searches. All authors read and approved the final manuscript.
ACKNOWLEDGEMENT
Our appreciation goes to all the staff of the ACEPRD (African Centre of Excellence for Phytomedicine Research and Development), University of Jos, Jos, Nigeria for all their individual support.
REFERENCES
- Maduagwuna CA, Omale S, Etuh MA, Gyang SS (2020) Antioxidant Activity of nHexane Extract of Caryota no Seed Using Drosophila melanogaster JABB 23: 39-47.
- Hamuel JD, Human IS, Benade S, Ndakidemi P (2009) Phytochemicals as chemotherapeutic agents and antioxidants: Possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. Journal of medicinal plant research 3: 839-848.
- Therasa SV, Thirumalai T, Tamilselvan N, David E (2014) In-vivo and ex-vivo inhibition of intestinal glucose uptake: A scope for antihyperglycemia. J Acute Dis 3: 36-40.
- Egua M, Etuk E, Bello S, Hassan S (2015) Isolation and Structural Characterization of the Most Active Antidiabetic Fraction of Corchorus olitorius Seed Extract. J Adv Med Pharm Sci 2: 75-88.
- Nair R, Kalariyat T, Chanda S (2005) Antibacterial activity of some selected Indian medicinal flora. Turk J Biol 29: 41-47.
- Haridas V, Arntzen CJ, Gutterman JU (2001) Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-kappaB by inhibiting both its nuclear localization and ability to bind DNA. Proc Natl Acad Sci USA 98: 11557-11562.
- Takechi M, Doi K, Wakayama W (2003) Biological activities of synthetic saponins and cardiac glycosides. Phytotherapy Research 17: 83-85.
- Wierenga JM, Hollingworth RM (1992) Inhibition of insect acetylcholinesterase by the potato glycoalkaloid α?chaconine. Natural Toxins 1: 96-99.
- Podder S, Roy S (2014) Exposure-dependent variation in cryolite induced lethality in the non-target insect, Drosophila melanogaster. Interdiscip Toxicol 7: 17-22.
- https://wcsp.science.kew.org/prepareChecklist.do?checklist=selected_families%40%40255110920200628383.
- Kavitha S (2017) A study on the bioefficacy of Caryota urens World journal of pharmaceutical research.
- Virot M, Tomao V, Ginies C, Chemat F (2008) Total Lipid Extraction of Food Using d-Limonene as an Alternative to n-Hexane. Chromatographia 68: 311-313.
- Etuh MA, Aguiyi JC, Ochala SO, Simeon O, Oyeniran OI, Debola OO, et al. (2019) The In vivo Antioxidant Protective Activity of Mangifera indica Cold Aqueous Leaf Extract in Drosophila Melanogaster. J Adv Biol Biotechnol 22: 1-7.
- Adedara IA, Abolaji AO, Rocha JBT, Farombi EO (2016) Diphenyl Diselenide Protects Against Mortality, Locomotor Deficits and Oxidative Stress in Drosophila melanogaster Model of Manganese-Induced Neurotoxicity. Neurochem Res 41: 1430-1438.
- Oboh G, Ogunsuyi OB, Ojelade MT, Akomolafe SF (2018) Effect of dietary inclusions of bitter kola seed on geotactic behavior and oxidative stress markers in Drosophila melanogaster. Food Sci Nutr 6: 2177-2187.
- Abolaji AO, Olaiya CO, Oluwadahunsi OJ, Farombi EO (2017) Dietary consumption of monosodium L-glutamate induces adaptive response and reduction in the life span of Drosophila melanogaster. Cell Biochem Funct 35: 164-170.
- Linford NJ, Bilgir C, Ro J, Pletcher SD (2013) Measurement of lifespan in Drosophila melanogaster. J Vis Exp 71: 50068.
- Spindola KCVW, Simas NK, dos Santos CE, da Silva AG, Romão W, et al. (2016) Dendranthema grandiflorum, a hybrid ornamental plant, is a source of larvicidal compounds against Aedes aegypti larvae. Revista Brasileira de Farmacognosia 26: 342-346.
- Rumzhum N, Rahman MM, Islam MS, Chowdhury SA, Sultana R, et al. (1970) Cytotoxicity and Antioxidant Activity of Extractives from Mirabilis jalapa. Stamford Journal of Pharmaceutical Sciences 1: 85-88.
- Abolaji AO, Kamdem JP, Lugokenski TH, Nascimento TK, Waczuk EP, et al. (2014) Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic Biol Med 71: 99-108.
- Miquel J, Fleming J, Economos AC (1982) Antioxidants, metabolic rate and aging in Drosophila. Arch Gerontol Geriatr 1: 159-165.
- Ikbal C, Monia BHK, Hamouda MHB (2007) Development perturbation of cotton leave noctuid with green cestrum extracts. Journal of Entomology 4: 121-128.
- Hirth F (2012) Drosophila melanogaster in the Study of Human Neurodegeneration. CNS Neurol Disord Drug Targets 9: 504-523.
- Schriner SE, Lee K, Truong S, Salvadora KT, Maler S, et al. (2013) Extension of Drosophila Lifespan by Rhodiola rosea through a Mechanism Independent from Dietary Restriction. PLoS One 8: 63886.
- Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M (2009) Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal 11: 3071-3109.
- Lozinsky OV, Lushchak OV, Kryshchuk NI, Shchypanska NY, Riabkina AH, et al. (2013) S-nitrosoglutathione-induced toxicity in Drosophila melanogaster: Delayed pupation and induced mild oxidative/nitrosative stress in eclosed flies. Comp Biochem Physiol A Mol Integr Physiol 164: 162-170.
- Boeing H (2013) Nutritional epidemiology: New perspectives for understanding the diet-disease relationship? Eur J Clin Nutr 67: 424-429.
- Tanya Flink (2018) 100S of doctors tell America to ‘ditch dairy’ and ‘go vegan’ in front of the white house. Food & Health.
- Peterson JDA (2017) Exploring the diet-life span connection. The Jackson Laboratory.
- Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, et al. (2016) Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med 375: 2349-2358.
- Vidacek NŠ, Nanic L, Ravlic S, Sopta M, Geric M, et al. (2018) Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J Gerontol A Biol Sci Med Sci 73: 39-47.
Citation: Maduagwuna CA, Omale S, Etuh MA, Gyang SS (2020) nHexane Extract of Caryota no Seeds Modulates Survival and Longevity in Drosophila Melanogaster. J Gerontol Geriatr Med 6: 069.
Copyright: © 2020 Steven S Gyang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

