
Nurse Practitioner Managed Outpatient Induction onto Buprenorphine/Naloxonein Women with Opioid Use Disorder during Pregnancy: A Retrospective Cohort Analysis
*Corresponding Author(s):
Elisabeth JohnsonUnc Horizons And Department Of Obstetrics And Gynecology, School Of Medicine, University Of North Carolina, Chapel Hill, North Carolina, United States
Tel:+1 9194454646,
Fax:+1 9199669169
Email:elisabeth_johnson@med.unc.edu
Abstract
Objective: To compare maternal, fetal and neonatal clinical characteristics and outcomes of Nurse Practitioner managed outpatient induction and community induced transfers of buprenorphine/naloxone treatment during pregnancy.
Methods: A retrospective cohort analysis of patients managed by nurse practitioner and treated with buprenorphine/naloxone for opioid use disorder during pregnancy and their neonates. N=319 mother-neonate dyads were treated at the University of North Carolina Horizons’ Clinic between January 1, 2014 and May 31, 2018. N=51 mother-neonate dyads that underwent buprenorphine/naloxone induction via the Horizons’ Clinic protocol versus n=44 mother-neonate dyads that underwent community buprenorphine/naloxone induction via an outside provider and transferred to Horizons’ for prenatal care and management of Medications for Addiction Treatment (MAT) during pregnancy were compared. Demographic data; maternal, fetal, and neonatal clinical characteristics; and clinical outcomes were collected via chart review.
Results: No significant differences were found in maternal clinical characteristics between the two groups. Estimated Fetal Weight (EFW) percentile was the only significantly different fetal outcome with the Horizons’ induction group having a significantly higher mean EFW percentile compared to those who underwent community induction (41.8vs. 35.7, P=0.042). Finally, no significant differences in preterm birth or low birth weight were found between Horizons’ induction group and the North Carolina state averages.
Conclusion: The induction protocol utilized by the UNC Horizons prenatal clinic shows similar safety and efficacy in pregnancy outcomes compared to community induced transfers. The outcomes for both groups indicate relative safety and effectiveness of buprenorphine/naloxone during pregnancy. Nurse practitioner prescribing and outpatient protocols could help expand access to MAT by providing more options for patients.
Keywords
Buprenorphine/naloxone induction; Opioid use in pregnancy
INTRODUCTION
Each day, nearly 120 Americans die from opioid overdose [1]. Between 1999 and 2017, opioid overdose deaths among women aged 30-64 increased nearly 5 times with a substantial increase involving synthetic opioids (1,643.0%) and heroin (915.0%) [2]. The increasing number of women of childbearing age reporting regular use of opioids combined with a high rate of unintended pregnancy in this group has led to increased number of opioid exposed pregnancies. This is reflected in the fourfold increase in the national incidence of Neonatal Abstinence Syndrome (NAS) reported between 1999 and 2013 [3]. In addition to NAS, opioid exposure in pregnancy, especially that which is untreated, may increase the risk of fetal growth restriction, placental abruption, fetal death and preterm labor [4].
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines Opioid Use Disorder (OUD) as a problematic pattern of opioid use that leads to serious impairment or distress [5]. In 2017, the American College of Obstetricians and Gynecologists (ACOG) and the Substance Abuse and Mental Health Services Administration (SAMHSA) formally recommended opioid agonist therapy, commonly referred to as Medication Assisted Treatment (MAT), as the treatment of choice for OUD treatment during pregnancy [6]. Compared to MAT, medically supervised withdrawal leads to higher relapse rates and worse pregnancy outcomes [6,7]. Many office-based providers, including obstetricians, do not prescribe MAT due to workload concerns [8]. Thus, a more simple induction protocol may help overcome this issue. Further, under the Comprehensive Addiction and Recovery Act (CARA) of 2016 Nurse Practitioners (NPs) can obtain buprenorphine prescribing waivers from the Drug Enforcement Administration to treat OUD [9]. To date, few data exists documenting the outcomes of nurse practitioners to prescribe buprenorphine to pregnant patients either from the time of starting induction or managing patients that are stable on their medication and transferred to their care [10]. The purpose of this paper is to compare maternal, fetal and neonatal clinical characteristics and outcomes of Nurse Practitioner-managed outpatient induction and community induced transfers of buprenorphine/naloxone treatment during pregnancy.
METHODS
This retrospective study consisted of a chart review of pregnant patients with opioid use disorder seen at the University of North Carolina Horizons’ Clinic between January 1, 2014 and May 31, 2018. From January 2014-May 2017 patients were prescribed buprenorphine/naloxone by a waivered physician and managed by the Nurse Practitioner. From May 2017 - May 2018 the waived Nurse Practitioner then both prescribed and managed the patients. The Institutional Review Board of the University of North Carolina approved this study. The Horizons Program at the University of North Carolina at Chapel Hill’s is a trauma-informed substance use disorder treatment program for women of childbearing age, including pregnant and parenting women. Since its founding in 1993 Horizons has served thousands of women from over half of North Carolina’s 100 counties. Horizons provides residential and outpatient treatment, including a specialized OB/GYN clinic and serves approximately 250 women each year. For additional information about the Horizons Program see Jones et al., [11].
The Horizons’ OB/GYN clinic offers health screenings, general physicals, family planning services, medical evaluations, pap smears, screening and treatment for Sexually Transmitted Infections (STIs), prenatal and postpartum care, and MAT with buprenorphine to patients with opioid use disorders, including patients who are pregnant. The clinic model is based on the premise that patients in this high-risk population are best engaged in both prenatal care and opioid use disorder treatment by providing a continuum of services at one site with consistent providers. At the clinic, patients see the same staff members, including the Horizons’ Physician and Family Nurse Practitioner (FNP), a licensed therapist and a peer support specialist, at each visit. Patients are generally scheduled for weekly or bi-weekly prenatal visits throughout pregnancy.
Participants
A total of 319 UNC Horizons’ Clinic patients were reviewed. Of these, 95 met the study inclusion criteria. Of the 224 patients that did not meet the study criteria, 155 were not prescribed buprenorphine/naloxone, 24 were not being seen at the Horizons’ clinic for prenatal care, 6 had buprenorphine/naloxone managed by an outside provider and 39 did not deliver at UNC Hospital.
Procedures
The 95 patients that met study inclusion criteria were broken into two groups based on the type of buprenorphine/naloxone induction that they had undergone. 51 patients had undergone induction following the Horizons’ protocol (Figure 1), while the remaining 44 had undergone community induction, outside of the UNC Horizons clinic and were then transferred to UNC Horizons for prenatal care and buprenorphine/naloxone management during pregnancy. Many of these patients were transferred to UNC Horizons by their previous buprenorphine/naloxone provider when they became pregnant due to provider discomfort. Of the 44 patients who underwent community induction, 17 (38.0%) initiated induction inpatient at UNC hospital and the remaining 27 (62.0%) underwent induction at an outside clinic. Those with a history of illicit buprenorphine use, but no recent prescription history, were included with Horizons’ inductions due to the lack of consistency and stability of treatment.
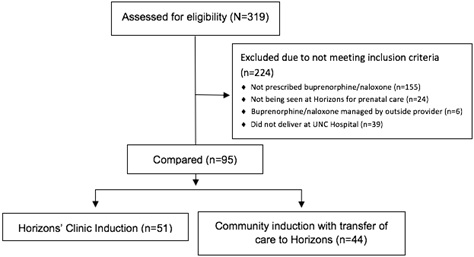 Figure 1: Study CONSORT diagram.
Figure 1: Study CONSORT diagram.
Data extracted from the electronic medical records (EPIC system) came from both maternal and newborn charts. The following data were collected: Maternal: race (white, black, other); age at delivery (years); parity (number of prior births and current pregnancy); enrollment in Horizons’ residential program at the time of pregnancy (yes or no); opioid type at entry to prenatal care (determined by patient disclosure at initial visit); psychiatric diagnosis (yes or no; including: anxiety, depression, bipolar disorder 1 and 2, and PTSD); tobacco smoking status (yes or no); toxicology screening for THC, cocaine, benzodiazepines, heroin and any opioid including buprenorphine (yes or no; determined via urine toxicology screen at initial prenatal visit); total number of gynecology, obstetric and post-partum visits with Horizons’ buprenorphine certified provider; Fetal: estimated gestational age at the time of initial prenatal visit (weeks); ultrasound results (including EFW percentile [adjusted for gestational age] if ultrasound was completed at 20 weeks or greater gestation); fetal heart rate tracings in beats per minute from every prenatal visit; Neonatal: gestational age at birth (weeks); place of delivery (UNC or other); mode of delivery (caesarean section vs vaginal delivery); infant birth weight (grams); APGAR scores (1 and 5 minute); days in nursery; and whether medication was needed for Neonatal Abstinence Syndrome (NAS) treatment (yes or no).
The Horizons’ clinic induction protocol is outlined in appendix 1. Day 1 occurs at the medical care provider’s office, staff administers the Clinical Opiate Withdrawal Scale (COWS) to the patient and when scores are at least 8 (indicating that they are in adequate withdrawal); buprenorphine/naloxone is then administered to the patient. The patient is then sent home with detailed instructions and the remainder of the induction occurs outside of clinic. Each subsequent day, the patient should begin with a dosage equivalent to the cumulative doses from the previous day. All patients in the Horizons’ clinic received education about the medication, how it works, how to take the medication, its risks and side effects and how to safely store it. Regardless of benzodiazepine use, patients were warned about the risks of combined opioid and benzodiazepine use at every visit. Patients taking benzodiazepines at their initial visit were given education materials and the risk of overdose and death were discussed. For every patient on a benzodiazepine, previous diagnosis and prescriptions were verified and strategies for benzodiazepine discontinuation were discussed. If possible, the benzodiazepines were then tapered and discontinued. For some patients, this was not practical: benefits of maintaining them on a stable treatment course outweighed potential risks associated with stopping treatment.
Data for preterm birth and low birth weight for both the Horizons’ induction group (n=51) and the total study sample (n=95) were also compared to North Carolina state averages from 2016.
Data-Analysis
Descriptive statistics [mean and standard deviation for continuous variables, frequency (n) and percentage for categorical variables] were calculated. Variances for all variables were calculated to determine best comparison test. If variances between groups differed by more than twofold that outcome variable was considered to have unequal variance. Standard, unpaired two-sample t-tests were used to measure association between continuous variables with equal variance. Welch’s two-sample t-tests were used to measure association between continuous variables with unequal variance. Pearson’s chi-squared tests were used to test for association between all categorical variables. One-sample t-tests were used to measure association between study data and North Carolina state averages. All statistical analyses were done using StataSE 15.
RESULTS
A total of 95 pregnant patients and 95 infants were included in the chart review. Eighty-six percent of the study population was white with an average age at delivery of 27.8 years. Twenty-five percent of the participants were enrolled in Horizons’ residential treatment program. The average gestational age at the first prenatal visit (with Horizons) was 21.7 weeks, with an average of 8 prenatal visits and 1 postpartum visit per patient. Maternal demographic and clinical characteristics can be found in table 1. Outcomes were compared based on 12 variables. Table 2 lists the twelve outcome variables, mean (SD) or N (%) (based on variable type), t-scores or x2 (based on variable type) and p values. All comparisons produced p values >0.25 except for Estimated Fetal Weight (EFW) percentile, which had a p value of <0.05.
|
|
Community Induction (n=44) |
Horizons' Induction (n=51) |
All Participants (N=95) |
t or |
p |
|||
|
|
Mean (SD) |
N (%) |
Mean (SD) |
N (%) |
Mean (SD) |
N (%) |
||
|
Age at Delivery |
28.6 (4.6) |
27.1 (4.7) |
27.8 (4.7) |
1.5 |
0.14 |
|||
|
Race |
3.27 |
0.07 |
||||||
|
Black |
2 (4.5) |
7 (13.7) |
9 (9.5) |
|||||
|
White |
41 (93.2) |
41 (80.4) |
82 (86.3) |
|||||
|
Other |
1 (2.3) |
3 (5.9) |
4 (4.2) |
|||||
|
Parity* |
2.7 (1.2) |
2.7 (1.5) |
2.7 (1.4) |
-0.16 |
0.88 |
|||
|
Total prenatal visits |
7.9 (3.8) |
8.2. (3.3) |
8.1 (3.5) |
-0.48 |
0.63 |
|||
|
Total postpartum visits |
1.1 (0.88) |
1.3 (0.86) |
1.2 (0.87) |
-1.24 |
0.22 |
|||
|
Residential Program? |
12 (27) |
12 (24) |
24 (25.3) |
0.18 |
0.68 |
|||
|
Maternal Tobacco Use |
37 (84) |
45(88) |
82 (86.3) |
0.34 |
0.56 |
|||
|
Initial Prenatal Visits Unine Drug Screen? |
||||||||
|
THC |
17 (39) |
18 (35) |
35 (36.8) |
0.11 |
0.74 |
|||
|
Cocaine |
9 (20) |
13 (25) |
22 (23.2) |
0.34 |
0.56 |
|||
|
Benzodiazepine |
8 (18) |
7 (14) |
15 (15.8) |
0.35 |
0.55 |
|||
|
Heroin |
5 (11) |
6 (12) |
11 (11.6) |
0.004 |
0.95 |
|||
|
Any Opioid§ |
37 (84) |
45 (88) |
82(86.3) |
0.34 |
0.56 |
|||
|
Psychiatric Diagnosis# |
36 (82) |
29 (57) |
65(68.4) |
6.81 |
0.01 |
|||
|
EGA at initial prenatal |
21.5 (9.7) |
21.9 (7.3) |
21.7 (8.5) |
-0.21 |
0.83 |
|||
Table 1: The demographic and other characteristics of women receiving buprenorphine/naloxone during pregnancy in an integrated obstetrical and behavioral health outpatient setting.
|
Outcome |
Community Induction (n=44) |
Horizons' Induction (n=51) |
t or |
p |
||
|
|
Mean (SD) |
N (%) |
Mean (SD) |
N (%) |
||
|
Normal Anatomy US |
40 (90.9) |
47 (92.2) |
0.048 |
0.83 |
||
|
Estimated Fetal Weight Percentile* |
35.7 (13.6) |
41.8 (12.8) |
-2.07 |
0.042 |
||
|
Fetal Heart Rate Within Normal Limit |
0.996 (0.021) |
0.989 (0.039) |
0.97 |
0.33 |
||
|
Gestational Age at Birth |
38.6 (2.4) |
39.0 (2.0) |
-0.91 |
0.36 |
||
|
Preterm Birth (<37wks) |
4 (9.1) |
6 (11.8) |
0.18 |
0.67 |
||
|
Birth Weight (grams) |
3010 (574) |
3134 (510) |
-1.11 |
0.27 |
||
|
Low Birth Weight (<2500g) |
6 (13.6) |
5 (9.8) |
0.34 |
0.56 |
||
|
Cesarean Rate |
15 (34.1) |
16 (31.4) |
0.079 |
0.78 |
||
|
1 Minute AP GAR |
7.9 (1.1) |
7.9 (1.5) |
-0.034 |
0.97 |
||
|
5 Minute AP GAR? |
8.7 (0.9) |
8.6 (1.4) |
0.39 |
0.70 |
||
|
Days in nursery? |
7.4 (10.2) |
6.2 (5.4) |
0.73 |
0.47 |
||
|
Medication for NAS§ |
12 (27.3) |
16 (31.4) |
0.19 |
0.66 |
||
Table 2: The fetal, maternal and birth outcomes of women and their children receiving buprenorphine/naloxone during pregnancy in an integrated obstetrical and behavioral health outpatient setting.
The prevalence of preterm birth and infants born with LBW were compared to the NC state averages for both the Horizons’ induction group (n=51) and the total study population (n=95). Among all North Carolina resident live births, 10.4% deliver preterm and 9.2% deliver infants with LBW. Of the 51 mothers who underwent the Horizons’ induction, 6 (11.8%) delivered preterm and 5 (9.8 %) had infants deliver with LBW (11.8 vs. 10.4, p=0.77 & 9.8 vs. 9.2, p=0.89). Of the 95 patients who received prenatal care at Horizon’s during pregnancy, 10 (10.5%) delivered preterm and 11 (11.6%) had infants deliver with LBW (10.5 vs. 10.4, p=0.97 & 11.6 vs. 9.2, p=0.47).
DISCUSSION
To our knowledge, this is the first study to examine the relative safety and efficacy of buprenorphine/naloxone induction during pregnancy as well as the first to focus on Nurse Practitioner patient induction and management. This retrospective cohort study examined and compared the maternal, fetal and neonatal clinical characteristics and outcomes for 95 pregnant patients with OUD treated with buprenorphine/naloxone at the UNC Horizons’ Clinic between January 1, 2014 and May 31, 2018. Of the 95 participants, 51 underwent induction via the Horizons’ clinic protocol while 44 underwent community induction with transfer of care to Horizons for prenatal and MAT management during pregnancy. All patients were managed successfully by Horizons’ Nurse Practitioner and physician.
Chosen outcome variables were meant to demonstrate safety and effectiveness for mother, fetus and neonate before, during and after pregnancy. Findings suggest that rates of normal anatomy ultrasound screenings, percentage of FHR within normal limits, gestational age at birth, birth weight in grams, 1 and 5 minute APGAR scores, newborn days in nursery, and rates of NAS requiring medication intervention during pregnancy do not differ significantly between patients who underwent the Horizons’ induction and those who underwent community induction. Remarkably, the only significant difference in outcomes was positive: patients in the Horizons’ induction group had significantly higher EFW percentile compared to those who underwent community induction (41.8 vs. 35.7, P=0.042). EFW is a measure of normal fetal growth and can be a predictor for LBW. This is an exciting finding due to the high incidence of low birth weight among patients with OUD. This study shows that buprenorphine/naloxone has relative safety and effectiveness during pregnancy regardless of induction in the clinic or in the community. With the exception of EFW percentile, these data do not provide evidence that outcomes are significantly different among pregnant patients who underwent community induction by an outside provider and those who underwent induction via the Horizons’ protocol. In addition, though untreated opioid use disorder puts patients at a higher risk of preterm birth and Low Birth Weight (LBW), these data provide evidence that when OUD is treated with buprenorphine/naloxone, the outcomes are not significantly different than the NC state averages.
While this study is relatively small, and larger trials are needed, this study provides a basis of evidence to support the effectiveness of Nurse Practitioners successfully treating pregnant patients with buprenorphine for OUD. Both group’s data showed comparable safety and efficacy and should be reassuring to providers that not only is buprenorphine/naloxone safe during pregnancy but it is safe and effective regardless of induction protocol. This study has several limitations. Data were collected retrospectively and data on EFW were not available for each patient. Next, the small Horizons’ sample size may not be representative of the overall population of people with opioid use disorder who receive buprenorphine/naloxone in pregnancy. We did not conduct systematic fetal evaluations during induction, so fetal withdrawal is possible and deserving of future systematic study. Roughly, 12.0% of patients were excluded because they did not deliver at UNC Hospital and it is unclear how this may have affected outcomes. Finally, given that it is unethical to have an untreated OUD comparison group, there was not a true control comparison group. As such, our design does not allow for a direct measure of the benefits of buprenorphine/naloxone versus no medication.
This study also has several strengths. There is currently limited data examining the use of buprenorphine/naloxone during pregnancy and even less data regarding the induction process. This study included maternal, fetal and neonatal results and provides a real-world clinical sample that should have good generalizability to other such samples.
CONCLUSION
In conclusion, there are several barriers that patients with opioid use disorder face when accessing healthcare and specifically MAT such as buprenorphine/naloxone. These barriers only increase when a person becomes pregnant. Women are often turned away or dropped from treatment when they become pregnant due to provider discomfort. This study supports existing evidence that buprenorphine and specifically buprenorphine/naloxone use during pregnancy is safe and effective. Further, it fills gaps in existing research regarding buprenorphine/naloxone induction during pregnancy. Induction for buprenorphine and buprenorphine/naloxone is seen as a barrier by both patients and providers due to its cumbersome protocol. This study demonstrates that the unobserved Horizons induction protocol is safe and effective while also reducing the number of required clinic visits during the induction period, improving access to MAT. Additionally, successful Nurse Practitioner induction management indicates mid-level providers, often underutilized, can play an important role in increasing MAT accessibility. Providers can benefit from the reassurance that pregnancy is not a contraindication to buprenorphine/naloxone use and that in fact preterm birth and LBW outcomes do not differ significantly from the North Carolina averages.
FINANCIAL SUPPORT
R01DA047867 NIDA PI HE Jones
CONFLICTS OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Centers for Disease Control and Prevention (2018) Understanding the Epidemic. CDC, Georgia, USA.
- VanHouten JP, Rudd RA, Ballesteros MF, Mack KA (2019) Drug Overdose Deaths Among Women Aged 30-64 Years - United States, 1999-2017. MMWR Morb Mortal Wkly Rep 68: 1-5.
- Patrick SW, Davis MM, Lehmann CU, Cooper WO (2015) Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 35: 650-655.
- Center for Substance Abuse Treatment (2005) Medication-assisted treatment for opioid addiction during pregnancy. In: Substance Abuse and Mental Health Services Administration (eds.). Medication-assisted treatment for opioid addiction in opioid treatment programs. A Treatment Improvement Protocol TIP. SAMHSA, Maryland, USA.
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5thedn). American Psychiatric Association, Arlington, USA.
- American College of Obstetricians and Gynecologists (2017) Opioid Use and Opioid Use Disorder in Pregnancy. Obstet Gynecol 130: 81-94.
- Substance Abuse and Mental Health Services Administration (2018) Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. Substance Abuse and Mental Health Services Administration, Rockville, Maryland, USA. Pg no: 165.
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, et al. (2009) Integrating buprenorphine treatment into office-based practice: a qualitative study. J Gen Intern Med 24: 218-225.
- American Society of Addiction Medicine (2016) FAQs About the Final Rule Raising the Buprenorphine Patient Limit. ASAM, Maryland, USA.
- Andrilla CHA, Patterson DG, Moore TE, Coulthard C, Larson EH (2018) Projected Contributions of Nurse Practitioners and Physicians Assistants to Buprenorphine Treatment Services for Opioid Use Disorder in Rural Areas. Med Care Res Rev.
- Jones H, Andringa K, Carroll S, Horton E, Johnson E, et al. (2015) Making a Difference in the Lives of Substance-using Pregnant Women: UNC Horizons and a Comprehensive Care Model. Couns Mag Addict 16: 50-55.
APPENDIX: UNC HORIZONS INDUCTION PROTOCOL FOR WOMEN DURING PREGNANCY
Buprenorphine/naloxone Outpatient Induction/Maintenance Protocol
Purpose: Patients opting for opioid agonist treatment will be inducted onto and maintained on buprenorphine HCl/naloxone HCl dehydrate that is formulated in a ratio of 4:1 milligrams and taken by the sublingual route. The medication will be used to safely and quickly manage opioid withdrawal.
Readiness for induction
- To help prevent precipitated withdrawal, induction onto buprenorphine/naloxone will not begin until withdrawal symptoms are present (COWS ≥8)
- Based on provider discretion/needs of the patient, induction will be scheduled
Induction: Day 1
- Confirm COWS ≥ 8 and take baseline vital signs (HR, BP, RR, Temp)
- Administer 4mg buprenorphine/naloxone (sublingual route; SL)
- Assess for precipitated withdrawal: conduct COWS and take vital signs every 30 minutes for the first 2 hours
- If precipitated withdrawal is suspected (increase in withdrawal symptoms), consult provider
- Patient may receive a PRN dose of buprenorphine/naloxone (4 mg SL) if COWS remains ≥8 after 1-2 hours. Patients are instructed not to exceed 8mg buprenorphine/naloxone on day 1
Induction: Day 2
- AM Dose: total dose from Day 1 (i.e., if patient received a total of 8 mg buprenorphine/naloxone on day 1, patient will take 8mg Buprenorphine/naloxone in the AM of day 2)
- Assess patient 1-2 hours later: conduct SOWS
- If SOWS ≥11, patient may receive a PRN dose of Buprenorphine/naloxone (4 mg SL)
- Continue to reassess patient every 1-2 hours until SOWS <11
- Patients may receive 1 additional PRN dose of Buprenorphine/naloxone (4mg SL), not to exceed 16mg
Induction: Day 3
- Day 3 dose: total dose from Day 2 which can be taken in its entirety in the AM or split into two doses based on the needs of the patient (i.e., if patient received a total of 16 mg Buprenorphine/naloxone on day 2, patient can either take 16 mg in the AM or day 3 or 8 mg in the AM and 8 mg in the PM)
- If patient receives 16 mg Buprenorphine/naloxone and continues to have a SOWS ≥11 and/or intolerable symptoms, consult provider
Induction: Day 4
- Day 4 dose: total dose from Day 3 which can be taken in its entirety in the AM or split into two doses based on the needs of the patient (i.e., if patient received a total of 16 mg Buprenorphine/naloxone on day 3, patient can either take 16 mg in the AM or day 3 or 8 mg in the AM and 8 mg in the PM)
- Assess patient’s response to day 3 dose
- If patient is stable continue dose for 3-7 days
- If patient has reached 16 mg/day and continues to have a SOWS ≥11 and/or intolerable symptoms, consult provider
- If relief of withdrawal is not achieved after 4 days of titration, schedule appointment with provider
Maintenance
- Once withdrawal symptoms have been relieved for 24 hours, the patient will be instructed to continue that dose
- Patients will be instructed to notify their provider if she experiences any side effects or worsening withdrawal signs or symptoms
- All patients should be assessed in person within 1 week of starting the induction and then every 2 weeks
- At each following visit, Buprenorphine and its metabolites norbuprenorphine must be present in the urine drug screen for a new prescription to be written
- Patients will also be subject to random pill counts to verify non-diversion and ensure medication compliance
Citation: Johnson E, Banasiewicz B, Momand AS, Andringa K, Jones HE (2020) Nurse Practitioner Managed Outpatient Induction onto Buprenorphine/Naloxonein Women with Opioid Use Disorder during Pregnancy: A Retrospective Cohort Analysis. J Reprod Med Gynecol Obstet 5: 036.
Copyright: © 2020 Elisabeth Johnson, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

