
Journal of Nephrology & Renal Therapy Category: Clinical
Type: Research Article
Observation on Clinicopathological Profile of Post-Infectious Glomerulonephritis
*Corresponding Author(s):
Mangal Charan MurmuDepartment Of Paediatrics, SCB Medical College & Hospital, Cuttack, Odisha, India
Tel:+91 9437445041,
Email:mangal74murmu@yahoo.co.in
Received Date: Jun 07, 2017
Accepted Date: Aug 19, 2017
Published Date: Sep 04, 2017
Abstract
Introduction: Post-infectious glomerulonephritis is one of the most important and intriguing conditions in the discipline of paediatric nephrology. Although the eventual outcome is excellent in most cases, Post Streptococcal Glomerulonephritis (PSGN) remains an important cause of acute renal failure and hospitalization for children in both developed and underdeveloped areas.
Methods: It’s a direct prospective study done in the Paediatric Department of SCB Medical college, Cuttack, Odisha, India.
Results: Most common predisposing factor was scabies along with other infected skin lesion. Most patients had diminished complement level.
Conclusion: Post streptococcal glomerulonephritis is the most common type of glomerulonephritis in childhood. Decreased Serum C3 level is the one of predictor of PSGN.
Methods: It’s a direct prospective study done in the Paediatric Department of SCB Medical college, Cuttack, Odisha, India.
Results: Most common predisposing factor was scabies along with other infected skin lesion. Most patients had diminished complement level.
Conclusion: Post streptococcal glomerulonephritis is the most common type of glomerulonephritis in childhood. Decreased Serum C3 level is the one of predictor of PSGN.
Keywords
Acute glomerulonephritis; Antistreptoysin-O; Compliment C3
ABBREVIATIONS
AGN: Acute Glomerulonephritis
ASO: Antistreptoysin-O
CRP: C-Reactive Protein
C3: Complement 3
ESR: Erythrocyte Sedimentation Rate
HSP: Henoch-Schonlein Purpura
PSGN: Post Streptococcal Glomerulonephritis
RBC: Red Blood Cell
SLE: Systemic Lupus Erythematosus
WBC: White Blood Cell
ASO: Antistreptoysin-O
CRP: C-Reactive Protein
C3: Complement 3
ESR: Erythrocyte Sedimentation Rate
HSP: Henoch-Schonlein Purpura
PSGN: Post Streptococcal Glomerulonephritis
RBC: Red Blood Cell
SLE: Systemic Lupus Erythematosus
WBC: White Blood Cell
INTRODUCTION
Presence of oliguria, oedema, hematuria, proteinuria and hypertension are the essential criteria of acute glomerulonephritis [1,2]. Acute glomerulonephritis is an immune complex disease where there is inflammation of glomerulus occurs by proliferations of cellular elements i.e., endocapillary proliferation with mesangial proliferation and neutrophil cell in the capillary of the glomerular tuff through an immunological response as seen in optic microscopy [2,3]. There have been stages of development of our knowledge with regards to etilogy and clinical course of acute glomerulonephritis since Bright’s description [4]. Post-infectious glomerulonephritis is the most common cause constituting 80% cases of post streptococcal glomerulonephritis. Postinfectious glomerulonephritis is results from an antecedent infection of the skin (impetigo) or throat (pharyngitis) caused by nephritogenic strains of group A beta-hemolytic streptococci and is coined as Post Streptococcal Glomerulonepritis (PSGN) [5-7]. In 1941, Seegal and Earl described the concept of nephritogenic strain causing PSGN [8]. The M and T proteins in the bacterial wall have been used for characterizing streptococci. Nephritogenicity is mainly restricted to certain M protein serotypes (i.e., 1, 2, 4, 12, 18, 25, 49, 55, 57 and 60) that have shown nephritogenic potential. These may cause skin or throat infections. The specific M types 49, 55, 57, and 60 are most commonly associated with skin infections. Some strain of Group C streptococci like Streptococcus zooepidemicus have been seen responsible for recent epidemics of APSGN. Hence it can be stated that nephritogenic antigens are present and possibly shared by streptococci from several groups [9]. The Incidence of acute post streptococcal glomerulonephrtis is more common with antecedent history of pharyngitis or pyoderma, [10-12].
CLINICAL CASE REPORT
A 31-year-old primiparous female, at 35 weeks gestational age, presented to obstetrics emergency department complaining of absence of fetal movements for the last 12h before admission.
She had been regularly attending the antenatal consultations with no risk factors identified. Her prenatal laboratory results were unremarkable except for GBS-unknown. She had three normal obstetric ultrasounds (one of each trimester); her blood type was A+. Pregnancy was uneventful with no history of vomiting, blood loss or abdominal trauma.
On admission at the delivery unit, the obstetric ultrasound revealed no fetal movements with the presence of heart beat. The Cardiotocograph (CTG) was not tranquilizing as it showed prolonged deceleration and reduced variability with pathological trace that suggested a sinusoidal pattern and, as a result, an emergent caesarean section was performed (Figure 1).A baby boy was born weighing 2610g. The newborn had a circular of the umbilical cord around the arms. On examination at birth, he was markedly pale and hypotonic with respiratory depression. Orothracheal intubation and connection to mechanical ventilation was immediately performed. He responded well and was extubated 4 minutes after and transferred to the neonatal unit with oxygen directly to his face, for further evaluation and management. The Apgar score was 5/8/8.
She had been regularly attending the antenatal consultations with no risk factors identified. Her prenatal laboratory results were unremarkable except for GBS-unknown. She had three normal obstetric ultrasounds (one of each trimester); her blood type was A+. Pregnancy was uneventful with no history of vomiting, blood loss or abdominal trauma.
On admission at the delivery unit, the obstetric ultrasound revealed no fetal movements with the presence of heart beat. The Cardiotocograph (CTG) was not tranquilizing as it showed prolonged deceleration and reduced variability with pathological trace that suggested a sinusoidal pattern and, as a result, an emergent caesarean section was performed (Figure 1).A baby boy was born weighing 2610g. The newborn had a circular of the umbilical cord around the arms. On examination at birth, he was markedly pale and hypotonic with respiratory depression. Orothracheal intubation and connection to mechanical ventilation was immediately performed. He responded well and was extubated 4 minutes after and transferred to the neonatal unit with oxygen directly to his face, for further evaluation and management. The Apgar score was 5/8/8.
Initial blood gas from the umbilical cord revealed pH 7.27, pCO2 50.6 mm Hg, Hemoglobin 4.4, g/dL, bicarbonate 21.9 mmol/L and lactates 5.8 mmol/L. Laboratory exams revealed 4.0 g/dL of hemoglobin, white blood cell count of 47.700/10 EXP 9/L with 22.7% neutrophils (10.800), platelets count 183.000/10 EXP 9/L, DHL 680 UI/L, CK 190 UI/L. Further laboratory evaluation was unchanged (bilirubin, cardiac enzymes and C reactive protein). Coombs test and viral serology for Parvovirus B19 and Cytomegalovirus were negative. Hemoglobin electrophoresis showed a presence of 5% fetal hemoglobin on mother’s blood. Kleihauer-Betke test was performed, since it is a more specific exam and quantifies the amount of blood transfusion. It revealed 17.8% of fetal red cells in maternal circulation, which corresponds to a volume of approximately 890 mL of fetal blood based on the formula: (% of fetal cells determined by Kleihauer-Betke test/100) X 5000 mL = volume of FMH (in mL) [3] and also according to the fact that 1% of fetal erythrocytes in maternal circulation is equivalent to a fetal hemorrhage of 50mL [4].
Two red blood cell transfusions were made and at 12 hours of life his hemoglobin was 13.3 g/dL, white blood cells count of 10.100/uL (Neutrophils: 64.4%), platelets count of 219.000/uL and erythroblasts 87/100 leucocytes.
The outcome was favorable with hemodynamic and respiratory stability and absence of abnormal movements. Cranial ultrasonography showed, in the 3rd day of life, frontal bilateral parenchymal hyperechogenicity, was not present on 11th day of life as the ultrasounds were made by two different physicians. The authors admit that the hyperechogenicity have not been valorized by the second physician.
Follow-up at 2 and 4 months revealed a normal physical and neurological examination.
OBJECTIVES
The objectives of the study is to know the clinicopathological profile of PSGN and its association with sore throat and pyoderma, clinical presentations, various laboratory parameters including Serum C3 level and course of illnesses.
Method
This study was done in the Department of Paediatrics, SCB medical college, Cuttack, a tertiary care referral hospital from October 2014 to September 2016 after obtaining clearance from institutional ethical committee. Total number of case study were 56, who were admitted to indoor of Paediatrics ward of SCB Medical college hospital, Cuttack with the features of PSGN.
Inclusion criteria
The child who satisfy the criteria of PSGN were taken into study. The criteria taken in our study were sudden onset of oliguria, hematuria and proteinuria with presence of hypertension, edema and impaired renal function [2,13].
Exclusion criteria
The child having similar presentation with other systemic disease like secondary nephrotic syndrome, Ascitis of different origin, Congestive Cardiac Failure were excluded with allied investigations.
Methods
It was a direct prospective study. After the children were admitted, a detailed history of present illness, past history and through clinical examination were done in a prescribed performa to rule out any bias. All the investigation are done in the central laboratory, pathology department and biochemistry department of SCB Medical College Hospital, Cuttack. The following examination was being done. 1) Urine: routine and microscopic ( including Ph, specific gravity, Benedict’s Qualitative Glucose Test, heat coagulation test & culture) 2) Blood: Hb, DC, TLC, ESR, blood urea, serum creatinine, serum electrolyte, serum cholesterol, serum comlpement-3 (nephelometry method) and ASO Titre (ortho ASO slide test). 3) Throat swab/skin swab for grams stain and culture-sensitivity. 4) X-ray chest, Ultrasound of abdoman (KUB) and 5) Kidney biopsy (in selected case of renal failure).
Observation
The observations were recorded and tabulated (Tables 1-7, Figures 1-3).
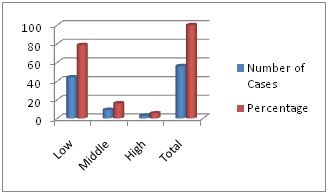
Figure 1: Socioeconomic status of parents of patients of PSGN.
Most cases belong to low socioeconomic group (78.57%). The middle income groups were 16.07% and high income group’s were 5.35 %.
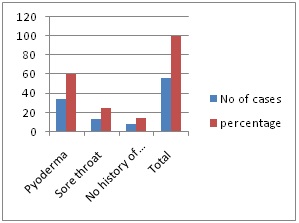
Figure 2: History of preceding infection in PSGN.
In 85.71% cases of PSGN there was history suggestive of infection, out of which history of Pyoderma was 60.71%, Sore throat was 25.00%.

Figure 3: Treatment course and outcome of PSGN.
Complete recovery was seen in 89.28% of cases of PSGN, 5 patients (8.92%) were discharged on request (latteron followed up), one patient died (1.78%) in hospital (after all investigation were done) due to associated sepsis.
| Age in years | Male | Female | Total | Percentage |
| Less than 3 years | 2 | 1 | 3 | 5.35 |
| 3 to 8 years | 24 | 10 | 34 | 60.71 |
| 8-14 years | 10 | 9 | 19 | 33.92 |
| Total | 36(64.28) | 20(35.71) | 56 | 100 |
AGN is most common in between 3-8 years age group, male were more affected than female. Average ratio is M:F =1.8:1.
| Latent period | Sore throat | Pyoderma | Total | |||
| No | % | No | % | No | % | |
| Less than 7 days | - | - | - | - | - | - |
| 8-14 days | 13 | 27.08 | 5 | 10.41 | 18 | 37.50 |
| 15-21 days | 1 | 2.08 | 22 | 45.83 | 23 | 47.91 |
| More than 21 days | - | - | 8 | 14.58 | 7 | 14.58 |
The majority of patients have latent period between 8 to 21 days (85.41%). Patient with sore throat have latent period of 8 to 14 days in majority cases (27.08%). The patient with pyoderma has latent period of 15 to 21 days in majority of cases (45.83%)
| Symptoms | Number of cases | Percentage |
| Oliguria | 53 | 94.64 |
| Puffiness of face | 53 | 94.64 |
| Pedal edema | 34 | 60.71 |
| Hematuria | 32 | 57.14 |
| Fever | 24 | 42.05 |
| Breathlessness | 16 | 28.57 |
| Headache and vomiting | 14 | 25.00 |
| Palpitation | 8 | 14.28 |
| Convulsion | 5 | 8.92 |
| Altered sensorium | 4 | 7.14 |
The most common presentation was oliguria, and puffiness of face in 94.64 % cases, pedal oedema in 60.71% cases, hematuria in 57.14% cases, fever in 42.05% cases and convulsion in 8.92% of cases.
| Presenting signs | Number of cases | Percentage |
| Hypertension | 42 | 75.00 |
| Oedema | 34 | 60.71 |
| Congestive cardiac failure | 7 | 12.50 |
| Ascitis | 7 | 12.50 |
| Pulmonary oedema | 5 | 8.92 |
| Acute renal failure | 3 | 5.35 |
| Papiloedema | 2 | 3.57 |
The most common sign in PSGN in our study was hypertension (75%) followed by oedema (60.71%) congestive cardiac failure was seen in 12.50% of cases.
| Urine examination finding | Number of cases | Percentage |
| Albuminuria Trace + ++ +++ ++++ | 42415103 | 7.1442.8526.7817.855.35 |
| Heamaturia Gross Microscopic | 3256 | 57.14100 |
| Pus cell(more than 5/HPF) | 18 | 32.14 |
| CastRBC CastGranular castHyaline cast | 18129 | 32.1421.4216.07 |
| Urine Culture Positive KlebsillaE. ColiAcenetobacter | 9531 | 16.078.925.351.78 |
All cases had albuminuria and microscopic hematuria (100%) in our study. Gross hematuria was seen in 54.14% cases. Pyoderma was found in 32.14% of cases. RBC cast was present in 32.14% of cases. Urine culture was positivein 16.075 of cases and most of which was infection with Klebsiella in 12.85% of cases.
| Examination finding | Number of cases | Percentage |
| Raised ESR | 49 | 87.50 |
| Raised CRP | 8 | 14.28 |
| ASO TiterPositive (Total)PharyngitisPyodermaNo infection | 261376 | 46.4250.0026.9223.07 |
| Low Complement 3 levelPositive Negative | 497 | 87.5012.50 |
The ESR was raised in 87.50% of cases; CRP was raised in 14.28% of cases. The ASO titer was positive in 26 (40.42%) patients out of which 13 patients (50%) had pharyngitis, 7 patients (26.92%) had pyoderma. Out of 14 patients with pharyngitis, 13 patients had ASO Positive and out of 34 patients with pyoderma only 7 (26.92%) patients had ASO Positive. Low Complement 3 level was positive in 49 patients (87.50%) and negative in 12.50% patients. Only 5 patients could be followed up after 8 weeks and out of which 4 patients (80%) had complement level came back to normal level.
| Organism isolated | Number of cases | Percentage |
| Throat SwabNo growthGroup A Beta haemolyticStreptococciKlebsiella | 65 3 | 42.8535.71 21.42 |
| Total | 14 | 100 |
| Skin swabNo growthGroup A Beta haemolyticStreptococci Staphyllococcus aureus | 1511 8 | 44.1132.35 23.52 |
| Total | 34 | 100 |
Throat swab was taken from 14 patients with preceding history of sore throat shows no growth in 42.85% of cases. Group-A Beta haemolytic streptococci were seen in 35.71% of cases. Klebsiella wasfound in 21.42% cases. Skin swab was taken from 34 patients with preceding history of pyoderma shows no growth in 44.11% of cases. Group-A Beta haemolytic streptococci were seen in 32.35% of cases. Staphyllococcus aureus was in 23.52% cases.
DISCUSSION
In our study commonest presentation of PSGN is between 3-8 years age group (60.71%), male (64.28%) were more affected than female (35.71%), ratio was 1.8:1. Sarkissian et al., [14] found similar type of study. We have found in 60.97% cases of PSGN there was history suggestive of infection, most common being the scabies, out of which pyoderma was 60.71%, sore throat was 25%. Popovic- Rolovic et al., [15] found preceding infection of respiratory tract in 82%, skin infection in 10 % and no infection in 8% of cases. Puri RK et al., [16] found 67.4% having history of skin infection and 8.9% with respiratory tract infection. Manhans et al., [17] found 60 % cases with history of pyoderma and 20% with sore throat. Dillon et al., [18] have reported pyoderma as the leading cause of PSGN in 66% cases. The finding is consistent with our results. Out of 56 patient have 41 patients latent period between 8 to 21 days (85.41%). Patient with sore throat have latent period of 8 to 14 days in majority cases (27.08%). The patient with pyoderma has latent period of 15 to 21 days in majority of cases (45.83%). Rajajee et al., [19] had reported that the latent period in AGN after streptococcal pharyngitis is 7 to 14 days and that after skin infection is 14 to 21 days. Similar finding about the latent period were reported by Hingalis et al., [20] and Lewy et al., [21]. The most common presentation was oliguria, and puffiness of face in 94.64 % cases, followed by pedal edema (60.71%), hematuria in 57.14% cases and fever in 42.71% cases. This finding was consistent with study done by Puri et al., [16] (85.7%) and Manhas et al., [17] (90%) respectively. Lewy et al., [21] reported edema in 61.1%. Hingalis et al., [20] observed gross hematuria in 77.8% cases. Puri et al., [16] reported 55.7 % cases PSGN with hematuria. Berry et al., observed association of hypertension in 69.1% cases. Puri et al., [16] found hypertension in 74.6% of cases. Our finding is consistent with them. Hypertension was found in 49% of cases by Meharban Singh et al., [22]. Fever, vomiting & headache are encountered in 55.71% & 24.28% respectively. Puri et al., [16] and Manhas et al., [17] have found similar type of presentation.
Atypical presentation like hypertensive encephalopathy was seen in 2.85% of cases where as Puri et al., [16] have encountered 8.8% of cases having hypertensive encephalopathy. We have seen 7.14% of patient having convulsion where as Strauss and welt et al., [23] reported 5% of PSGN cases having convulsion. Acute renal failure was observed in 5.35% of cases with PSGN in our study. Our finding is consistent with the finding of Earle DP et al., [24] and Sharma BK et al., [25], who found prolonged oliguria in 5-13% of cases but differ from the study done by Manhas et al., [17], who have reported 1.4% cases with anuria. Puri et al., [16] have reported 14.3% cases with ARF. Chugh et al., [26] reported 9.8% incidence of ARF due to PSGN. Papiloedema was seen in 2.85% of cases of PSGN. These cases were associated with convulsion and hypertensive encephalopathy. Similar finding was observed by Manhas et al., [17].
In our study ESR was raised in 87.50% of cases. Manhas et al., [17] reported raised ESR in 56.6% of cases of PSGN. Puri et al., [16] and Berry et al., [27] found raised ESR in about 95% of cases of PSGN. This is at par with our finding. CRP was raised in 17.14% of cases. Valyasevi A et al., [28] reported raised CRP in 15.38% of PSGN. Puri et al., [16] reported it to be raised in much higher percentages of cases.
In our study 46.42% of cases of PSGN had ASO positive. Out of which 50% had pharyngitis and 26.92% had pyoderma. Leung DT et al., [29] in Hong Kong had shown that 78% of PSGN in children had elevated ASO titer more than 200IU/L. Puri et al., [16] have found a raised titer in 75% cases. Rajajee et al., [19] have not found significant raised in ASO titer like our study with pyoderma. Wannamaker LW et al., [30] have found feeble ASO response in patients with pyoderma induced nephritis.
Complement 3 level was positive in 49 patients (87.50%). Popovic-Rolovic M et al., [15] had found low complement C3 level. Shroff KJ et al., [31] found low complement C3 level in 88% of patients. Federic strife et al., [32] and Vijaykumar M et al., [33] found hypocomplementemia in about 90% of patient with PSGN. Complete recovery was seen in 89.28% of cases of PSGN, 5 patients (8.92%) were discharged on request (latter on followed up), one patient died (1.78%) in hospital (after all investigation were done) due to associated sepsis with hypertensive encephalopathy. Puri et al., [16] found mortality rate of AGN to be 1.4%. Devid Charles et al., [34] and Berry S et al., [27] found mortality to be 1%. Our finding is consistent with their findings. Renal biopsy was done in all 3 cases who developed renal failure, it showed the futures of PSGN.
Atypical presentation like hypertensive encephalopathy was seen in 2.85% of cases where as Puri et al., [16] have encountered 8.8% of cases having hypertensive encephalopathy. We have seen 7.14% of patient having convulsion where as Strauss and welt et al., [23] reported 5% of PSGN cases having convulsion. Acute renal failure was observed in 5.35% of cases with PSGN in our study. Our finding is consistent with the finding of Earle DP et al., [24] and Sharma BK et al., [25], who found prolonged oliguria in 5-13% of cases but differ from the study done by Manhas et al., [17], who have reported 1.4% cases with anuria. Puri et al., [16] have reported 14.3% cases with ARF. Chugh et al., [26] reported 9.8% incidence of ARF due to PSGN. Papiloedema was seen in 2.85% of cases of PSGN. These cases were associated with convulsion and hypertensive encephalopathy. Similar finding was observed by Manhas et al., [17].
In our study ESR was raised in 87.50% of cases. Manhas et al., [17] reported raised ESR in 56.6% of cases of PSGN. Puri et al., [16] and Berry et al., [27] found raised ESR in about 95% of cases of PSGN. This is at par with our finding. CRP was raised in 17.14% of cases. Valyasevi A et al., [28] reported raised CRP in 15.38% of PSGN. Puri et al., [16] reported it to be raised in much higher percentages of cases.
In our study 46.42% of cases of PSGN had ASO positive. Out of which 50% had pharyngitis and 26.92% had pyoderma. Leung DT et al., [29] in Hong Kong had shown that 78% of PSGN in children had elevated ASO titer more than 200IU/L. Puri et al., [16] have found a raised titer in 75% cases. Rajajee et al., [19] have not found significant raised in ASO titer like our study with pyoderma. Wannamaker LW et al., [30] have found feeble ASO response in patients with pyoderma induced nephritis.
Complement 3 level was positive in 49 patients (87.50%). Popovic-Rolovic M et al., [15] had found low complement C3 level. Shroff KJ et al., [31] found low complement C3 level in 88% of patients. Federic strife et al., [32] and Vijaykumar M et al., [33] found hypocomplementemia in about 90% of patient with PSGN. Complete recovery was seen in 89.28% of cases of PSGN, 5 patients (8.92%) were discharged on request (latter on followed up), one patient died (1.78%) in hospital (after all investigation were done) due to associated sepsis with hypertensive encephalopathy. Puri et al., [16] found mortality rate of AGN to be 1.4%. Devid Charles et al., [34] and Berry S et al., [27] found mortality to be 1%. Our finding is consistent with their findings. Renal biopsy was done in all 3 cases who developed renal failure, it showed the futures of PSGN.
SUMMARY
This work comprised of prospective study of post infectious glomerulonephritis from October 2014 to September 2016. In this study an attempt was made to know the various predisposing factors, presentations, course and outcome of post infectious glomerulonephritis. The numbers of patient taken in our study were 56.
PSGN in children was presented more in males than females. Most common age group was 3 to 8 years. Most common preceding infection was infective scabies along with other infected skin lesions. Preceding history of Pharyngitis was found in fewer cases. Latent period between preceding infection and onset of disease varies from 8 to 30 days. The patients with sore throat had shorter latent period than that with skin lesions. Oliguria, facial puffiness, pedal edema, hematuria and hypertension were the most common mode of presentation of the disease, observed in this study. Microscopic hematuria and proteinuria were invariably present in all cases. RBC cast in urine was found in about 1/3rd of cases. Most of the patients with PSGN had diminished complement 3 levels, majority of which return to normal on follow-up after 8 weeks. Beta-hemolytic streptococci were isolated from either skin or throat swab in 1/3rd of patient studied. All most all cases recovered completely within two to three weeks of admission. Mortality from the disease was very low.
PSGN in children was presented more in males than females. Most common age group was 3 to 8 years. Most common preceding infection was infective scabies along with other infected skin lesions. Preceding history of Pharyngitis was found in fewer cases. Latent period between preceding infection and onset of disease varies from 8 to 30 days. The patients with sore throat had shorter latent period than that with skin lesions. Oliguria, facial puffiness, pedal edema, hematuria and hypertension were the most common mode of presentation of the disease, observed in this study. Microscopic hematuria and proteinuria were invariably present in all cases. RBC cast in urine was found in about 1/3rd of cases. Most of the patients with PSGN had diminished complement 3 levels, majority of which return to normal on follow-up after 8 weeks. Beta-hemolytic streptococci were isolated from either skin or throat swab in 1/3rd of patient studied. All most all cases recovered completely within two to three weeks of admission. Mortality from the disease was very low.
CONCLUSION
Post streptococcal acute glomerulonephritis is the most common type of acute glomerulonephritis in childhood. In this part of India infected scabies (pyoderma) is the major preceding infection. Serum C3 level can be a useful tool in diagnosing PSGN. Serum C3 level is more sensitive than ASO estimation for diagnosing PSGN. Early and appropriate treatment of scabies and pharyngitis, improvement of socio-economic status and general hygiene can prevent development of PSGN. Early diagnosis of PSGN by clinical and laboratory investigations, timely and appropriate treatment can result in complete cure.
REFERENCES
- ison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ (2011) Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol 26: 165-180.
- Rodríguez-Iturbe B, Batsford S (2007) Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int 71: 1094-1104.
- Avner ED, Davis ID (2004) Acute post-streptococcal glomerulonephritis. In: Behrman RE, Kliegman RM, Jenson HB (eds.). Nelson Textbook of Pediatrics (17thedn). Elsevier Science, Amsterdam, Netherlands.
- Bright R (1836) Cases and observations illustrative of renal disease accompanied with the secretion of albuminous urine. In: Johnson HJ (ed.). The Medico-chirurgical Review, S Highley Publishers, University of Michigan, USA. PG no: 338-379.
- Blyth CC, Robertson PW, Rosenberg AR (2007) Post-streptococcal glomerulonephritis in Sydney: a 16-year retrospective review. J Paediatr Child Health 43: 446-450.
- Sanjad S, Tolaymat A, Whitworth J, Levin S (1997) Acute glomerulonephritis in children: a review of 153 cases. South Med J 70: 1202-1206.
- Sagel I, Treser G, Ty A, Yoshizawa N, Kleinberger H, et al., (1973) Occurrence and nature of glomerular lesions after group A streptococci infections in children. Ann Intern Med 79: 492-499.
- Kimmelstiel P (1965) The hump-a lesion of glomerulonephritis.Bull Pathol 6: 187.
- Blue Star (2012) Acute Glomerulonephritis (AGN). Pocket Atlas of Human Anatomy (4thEdn). Blue star.
- Kleinman H (1954) Epidemic AGN at salt Lake. Minn Med 37: 479.
- Dillon HC (1967) Pyoderma and Nephritis. Annual Review of Medicine 18: 207-218.
- García-Fuentes M, Arias M (1981) Poststreptococcal glomerulonephritis. An Esp Pediatr 15: 166-181.
- Parmar MS (2016) Acute Glomerulonephritis Updated. Medscape, New York, USA.
- Sarkissian A, Papazian M, Azatian G, Arikiants N, Babloyan A, et al., (1997) An epidemic of acute postinfectious glomerulonephritis in Armenia. Arch Dis Child 77: 342-344.
- Popovic-Rolovic M (1973) Serum C3 levels in acute glomerulonephritis and postnephritic children. Arch Dis Child 48: 622-626.
- Puri RK, Khanna KK, Raghu MB (1976) Acute glomerulonephritis in children. Indian Pediatr 13: 707.
- Manhas RS, Patwari A, Raina C, Singh A (1979) Acute nephritis in Kashmiri children-A clinical and epidemiological profile (A study of 350 cases). Indian Pediatr 16: 1015-1021.
- Dillon HC, Dillon MS (1974) New Streptococcal Serotypes Causing Pyoderma and Acute Glomerulonephritis Types 59, 60, and 61. Infect Immun 9: 1070-1078.
- Rajajee S (1990) Post-streptococcal acute glomerulonephritis : A clinical, bacteriological and serological study. Indian J Pediatr 57: 775-780.
- Hingalis N (1974) Long term prognosis of AGN. Am J Med 56: 52-60.
- Lewy JE, Salinas-Madrigal L, Herdson PB, Pirani CL, Metcoff J (1971) Clinico-pathologic correlations in acute poststreptococcal glomerulonephritis. A correlation between renal functions, morphologic damage and clinical course of 46 children with acute poststreptococcal glomerulonephritis. Medicine (Baltimore) 50: 453-501.
- Singh M, Azizi E, Qureshi MA, Arya LS (1984) Clinical profile of acute glomerulonephritis in children. Indian J Pediatr 51: 553-557.
- Strauss MB, Welt LG (1971) Diseases of the Kidney (2ndedn). Little Brown Publishers, Boston, USA.
- Earle DP, Farber SJ, Alexander JD, Pellegrino ED (1951) Renal Function and Electrolyte Metabolism in Acute Glomerulonephritis. J Clin Invest 30: 421-433.
- Sharma BK, Mahakur AC, Datta BN, Mathew MT, Bansal VK (1974). Acute oliguric glomerulonephritis. J Assoc Physicians India 22: 581-588.
- Chugh KS, Narang A, Kumar L, Sakhuja V, Unni VN, et al., (1987) Acute renal failure amongst children in a tropical environment. Int J Artif Organs 10: 97-101.
- Berry S, Prakash K, Srivastava G, Gupta S (1971) Acute glomerulonephritis in children. Indian Pediatr 8: 198.
- Valyasevi A, Sloan JM, Barness LA (1960) C-reactive protein in the serum in nephrosis and acute glomerulonephritis. Pediatrics 25: 106-111.
- Leung DT, Tseng RY, Go SH, French GL, Lam CW (1987) Poststreptococcal glomerulonephritis in Hong Kong. Arch Dis Child 62: 1075-1076.
- Wannamaker LW (1976) Differences between Streptococcal Infections of the Throat and of the Skin. N Engl J Med 282: 23-31.
- Shroff KJ, Ravichandran R, Acharya VN (1984) ASO titre and serum complement (C3) in post-streptococcal glomerulonephritis. J Postgrad Med 30: 27-32.
- Strife CF, Forristal TJ, Forristal J (1994) Serum complement levels before and after the onset of acute post-streptococcal glomerulonephritis. A case report. Pediatric Nephrology 8: 214-215.
- Vijayakumar M (2002) Acute and crescentic glomerulonephritis. Indian J Pediatr 69: 1071-1075.
- Florey DV, Kessner DM, Kashgarian M, Senter MG (1971) Mortality trends for chronic nephritis and infections of the kidney: A clinical and statistical comparison between mortality in New Haven, Connecticut and the United States, 1950-1960. J Chronic Dis 24: 71-77.
SUPPLEMENTARY FILES
| Cast | Culture | Hb(gm/dl) | TLC/cmm of blood | ESR mm fall in !st hour | ASO | CRP | Blood urea (mg/dl) | Serum creartinine (mg/dl) | Serum cholesterol (mg/dl) | Serum Sodium (meq/l) | Serum Potassium(meq/l) | Serum C3 level (mg/dl) | Throat swab/skin swab culture | CXR/Xray KUB | Outcome |
| 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 |
| - | - | 8 | 10000 | 96 | + | - | 59 | 1.3 | 149 | 142 | 5.7 | 15 | - | N | R |
| - | - | 14.43 | 15800 | 32 | + | + | 53 | 1.4 | 187 | 133 | 3.3 | 41.6 | GAS | A | R |
| R | KLEB | 7.6 | 8600 | 80 | + | - | 58 | 1.9 | 160 | 153 | 4.8 | 14.12 | GAS | N | R |
| GR | KLEB | 9.9 | 10800 | 45 | - | - | 160 | 1.8 | 211 | 130 | 5.8 | 26.8 | GAS | N | R |
| GR | - | 8 | 8600 | 22 | - | - | 190 | 4.8 | 130 | 130 | 4.9 | 118.8 | SA | N | R |
| HY | - | 11 | 16000 | 26 | + | - | 104 | 4.2 | 171 | 140 | 4.2 | 45 | GAS | N | R |
| R | - | 9.9 | 8800 | 8 | - | - | 62 | 2.2 | 146 | 136 | 5.6 | 45 | GAS | N | R |
| GR | - | 10.12 | 10400 | 24 | + | - | 29 | 0.8 | 136 | 136 | 3.6 | 17 | KLEB | N | R |
| - | - | 13 | 7800 | 35 | - | - | 40 | 1 | 166 | 139 | 3.9 | 30 | GAS | A | LA |
| R | E.C. | 4.8 | 10000 | 48 | + | - | 37 | 1.2 | 152 | 132 | 5.4 | 17 | - | N | R |
| - | - | 9.62 | 15000 | 28 | - | - | 72 | 1.4 | 212 | 132 | 5.4 | 15 | GAS | N | R |
| GR | - | 10 | 9800 | 67 | - | - | 30 | 0.8 | 236 | 130 | 5.3 | 103.6 | - | N | R |
| R | - | 9.2 | 10800 | 26 | + | + | 40 | 0.9 | 232 | 138 | 5.2 | 11 | GAS | N | R |
| - | - | 10.2 | 6200 | 50 | - | - | 46 | 1.3 | 152 | 136 | 5.6 | 79.5 | - | N | R |
| R | CAN | 9 | 8100 | 50 | - | - | 84 | 2.5 | 178 | 141 | 3.9 | 25 | GAS | A | R |
| R | KLEB | 7.16 | 12000 | 90 | + | - | 63 | 1.5 | 215 | 143 | 3.9 | 132 | - | N | R |
| - | - | 14.9 | 12450 | 10 | + | - | 31 | 0.4 | 492 | 140 | 5.6 | 67 | GAS | N | R |
| HY | - | 11 | 16500 | 46 | - | - | 40 | 1.4 | 188 | 141 | 4.8 | 14.4 | GAS | N | R |
| HY | - | 11 | 9800 | 90 | + | + | 21 | 0.7 | 116 | 134 | 4.6 | 36.6 | GAS | N | LA |
| R | - | 11 | 14000 | 26 | - | - | 26 | 0.7 | 154 | 134 | 3.3 | 38.4 | SA | N | R |
| GR | - | 13.69 | 15000 | 48 | - | - | 54 | 1.7 | 156 | 137 | 3.5 | 15.6 | GAS | A | D |
| R | EC | 8.2 | 12400 | 48 | - | - | 78 | 1.7 | 178 | 132 | 5.8 | 17.82 | - | N | R |
| - | - | 9.2 | 10800 | 82 | - | - | 23 | 0.7 | 132 | 154 | 3.8 | 74.32 | SA | A | R |
| HY | - | 9.24 | 12400 | 29 | + | - | 47 | 1.5 | 176 | 142 | 4.6 | 108.6 | - | N | LA |
| R | - | 7.4 | 8400 | 7 | + | + | 66 | 1.8 | 136 | 136 | 3.6 | 84.6 | GAS | N | LA |
| GR | - | 9.6 | 10000 | 32 | + | - | 172 | 3 | 239 | 132 | 3.8 | 98.4 | - | N | R |
| GR | - | 8 | 7000 | 140 | - | - | 22 | 0.9 | 160 | 126 | 4.2 | 10.62 | SA | N | R |
| R | - | 8.2 | 8200 | 42 | - | - | 40 | 1.1 | 310 | 138 | 1.6 | 17.42 | - | N | R |
| R | - | 7.8 | 7800 | 46 | - | - | 48 | 1.3 | 210 | 138 | 3.5 | 22.24 | - | N | R |
| R | - | 10.4 | 5600 | 52 | + | - | 36 | 0.8 | 184 | 142 | 4.2 | 22.84 | - | N | R |
| GR | KLEB | 10.2 | 10200 | 45 | + | + | 46 | 1.3 | 186 | 141 | 4.6 | 24.8 | - | N | R |
| HY | - | 10.8 | 6800 | 9 | + | - | 68 | 1.6 | 130 | 136 | 5.2 | 66.4 | GAS | N | R |
| GR | - | 9.6 | 9200 | 56 | - | - | 102 | 1.8 | 212 | 134 | 4.8 | 76.42 | SA | A | R |
| HY | - | 9.8 | 8600 | 33 | + | - | 96 | 1.8 | 186 | 128 | 3.8 | 28.42 | - | N | R |
| - | - | 10.6 | 14200 | 110 | - | - | 82 | 1.6 | 126 | 132 | 5.6 | 82 | - | N | R |
| HY | - | 12.6 | 11200 | 26 | - | - | 48 | 1.4 | 148 | 142 | 5.2 | 28.42 | GAS | N | R |
| - | - | 9.6 | 6500 | 19 | - | - | 52 | 1.3 | 184 | 141 | 4.4 | 82 | - | N | R |
| R | - | 7.6 | 7200 | 18 | - | - | 32 | 0.6 | 180 | 140 | 3.8 | 46.44 | - | N | LA |
| R | - | 9.4 | 8600 | 76 | - | - | 18 | 0.4 | 202 | 138 | 3.7 | 32.1 | - | N | R |
| - | - | 10.2 | 10400 | 74 | + | - | 56 | 1.4 | 154 | 138 | 4.2 | 24.06 | GAS | N | R |
| - | - | 9.4 | 9300 | 56 | - | - | 12 | 0.4 | 148 | 136 | 4.9 | 36.8 | - | N | R |
| R | - | 7.4 | 7800 | 10 | - | - | 52 | 1.4 | 148 | 134 | 4 | 24.06 | SA | A | R |
| - | - | 8 | 8000 | 54 | + | - | 54 | 1.8 | 256 | 156 | 5.6 | 14.14 | GAS | N | R |
| GR | EC | 12 | 6500 | 48 | + | + | 106 | 2.2 | 146 | 136 | 4.2 | 15 | - | N | R |
| R | - | 9.6 | 5600 | 47 | + | - | 108 | 2.1 | 180 | 138 | 4.9 | 110 | GAS | A | R |
| HY | - | 7.6 | 4800 | 46 | + | - | 112 | 2 | 310 | 142 | 3.4 | 16 | SA | N | R |
| R | - | 9.6 | 10000 | 6 | - | - | 20 | 0.8 | 200 | 140 | 5.2 | 28.6 | - | N | R |
| R | KLEB | 10.4 | 9200 | 54 | - | - | 30 | 1.3 | 210 | 128 | 5.8 | 140 | - | N | R |
| GR | - | 10.6 | 9000 | 33 | + | + | 32 | 0.4 | 206 | 138 | 4.8 | 42.4 | KLEB | N | R |
| GR | - | 9.4 | 4800 | 31 | - | - | 132 | 2.4 | 206 | 146 | 3.8 | 56.8 | SA | N | R |
| R | - | 13 | 6200 | 29 | - | - | 84 | 1.6 | 201 | 145 | 3.6 | 17.42 | - | N | R |
| - | - | 9.4 | 7200 | 24 | + | + | 34 | 0.6 | 184 | 138 | 5.9 | 22.22 | GAS | N | R |
| HY | - | 9.6 | 8400 | 31 | - | - | 24 | 1 | 186 | 140 | 3.9 | 38.4 | - | N | R |
| - | - | 10.4 | 6400 | 92 | - | - | 84 | 1.6 | 174 | 146 | 5.2 | 15 | - | N | R |
| - | - | 10.6 | 10000 | 42 | - | - | 20 | 0.8 | 210 | 144 | 4.8 | 17 | - | N | R |
| - | - | 11 | 7200 | 28 | - | - | 36 | 0.6 | 176 | 142 | 3.9 | 38.6 | SA | N | R |
Citation: Murmu MC, Swain A, Satpathy SK (2017) Observation on Clinicopathological Profile of Post-Infectious Glomerulonephritis. J Nephrol Renal Ther 3: 014.
Copyright: © 2017 Mangal Charan Murmu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
