
Optimization of Ultrasonic Assisted Extraction of Polysaccharides Extract from Aloe vera Using Response Surface Methodology
*Corresponding Author(s):
Chiramet AuranwiwatExpert Centre Of Innovative Herbal Products, Thailand Institute Of Scientific And Technological Research, Pathum Thani, Thailand
Email:chiramet@tistr.or.th
Abstract
This study investigates the extraction of aloe vera using ultrasonic extraction technology. The key extraction parameters examined include extraction time, the ratio of raw material to solvent, and extraction temperature. The optimal extraction conditions were designed using the Box-Behnken Design, resulting in 17 experimental conditions. The extraction yield ranging between 63.47 and 92.45 %w/w. The obtained polysaccharide extract was analyzed for total sugar content, which was found to be in the range of 64.89% to 74.34% by weight. Subsequently, the results were analyzed using ANOVA to determine the optimal conditions for ultrasonic extraction of Aloe vera. The optimal extraction conditions were found to be a temperature of 45 °C, a raw material to solvent ratio of 1:7.5, and an extraction time of 17 min and 30 sec. This research finding suggests the beneficial opportunities for ultrasound-assisted extraction for the production of polysaccharide extract from aloe vera leading to apply in functional food industry.
Keywords
Aloe vera, Polysaccharide, Response Surface Methodology, Ultrasonic Extraction
Introduction
Aloe vera (Aloe vera (L.) Burm.f.) is well-known as a drought resisting and medicinal plant that belongs to Liliaceae family and widely spreads in Africa, Asia, Europe and America. It is a succulent plant that consists of characteristic fleshy, triangular, and green to grey thick leaves and tubular flowers range in color from yellow to orange [1]. The compositions of aloe vera have been investigated and revealed containing a large quantity of water (96%) along with 4% of dry matter such as polysaccharides, minerals, proteins, amino acids, fatty acids, vitamins and phenolic compounds [2,3]. According to historical utilization, aloe vera has been employed in many industries such as food supplements, cosmetics, and therapeutics. Particularly in medicinal applications, aloe vera has shown notable properties for instance promotion of wound healing and dressing, anti-inflammatory properties anticancer properties, antidiabetic effect, digestive diseases protection, bone regeneration and protection, and protective medication [3,4].
The bioactive polysaccharides that were mainly found from aloe vera gel and powders have been published their pharmacological activities numerously, especially acemannan (acetylated mannan). Acemannan, a major polysaccharide, exhibited immunomodulatory effect to induce IL-6 and -8 expression and NF-κB/DNA binding in human gingival fibroblasts and increased TLR5/NF-κB-dependent signaling pathway [5]. In case of dental protection, acemannan stimulated denin regeneration in teeth with reversible pulpitis [6,7], a treatment of oral aphthous ulceration [8], and bone regeneration [9]. In addition to polysaccharide mixture, it showed remarkable activity of antioxidant, antidiabetic activity, antibacterial activity, antiviral activity, and antipsoriatic activity [10,11]. Hence, extraction techniques and separation conditions play important factors that can cause the quality and quantity of polysaccharide from aloe vera powders.
Extraction approaches such as ethanol precipitation, hot water, enzyme-assisted, ultrasound-assisted, microwave-assisted, and supercritical fluid extraction have been applied in many polysaccharide sources. In 2018, Souleymane and co-workers have reported the study of aloe vera extraction with hot and cold water, and boiled ethanol, the extraction with cold water (pH 5.3, 25 °C for 4 h) showed the best polysaccharide yields of 69.4 ± 0.1% [12]. Meanwhile, acemannan precipitation with ethanol solvent (40 °C for 14 h) has been investigated in 2021 by Redjeki and co-workers containing highest yield at 515.18 mg/g glucose content [13]. Response surface methodology (RSM) has been applied in aloe vera extract for enhance aloin A and aloin B which optimized conditions including extraction time, concentration of EtOH and sonication power. The results showed extraction yield as 9.43% and aloin A (4.33 mg/g extract) and aloin B (4.46 mg/g extract) [14]. Our previous study, we reported the investigation of extraction method using enzymatic extraction and the modified encapsulation properties [15].
Herein, Ultrasonic-Assisted Extraction (UAE) has been involved for alternative green extraction that can decrease the organic solvent consumption, costs, and extraction time. To enhance extraction efficiency of aloe vera powders, this work focusses on three factors of extraction using UAE experiment, including temperature, extraction time, and raw materials to solvent ratio together with the combination of ethanol precipitation technique. All parameters of extraction were designed and generated the extraction conditions using respond surface methodology.
Materials and Methods
General experimental procedures
The ultrasonic extractor was 10 mm diameter of horn and frequency 20 kHz (Biobase, China). The spectrophotometer was determined on multimode microplate reader (Thermo Scientific, USA). Field Emission Scanning Electron Microscope (FE-SEM) was JEOL JSM7800F, Japan. D-glucose standard was purchased from Acros organics, Belgium. Phenol was purchased from Carlo Erba, Italy. Ethanol and sulfuric acid were analytical grade from Merck, Germany. The dry gel powder of A. vera were purchased from Subio Co., Ltd., Thailand.
Ultrasonic assisted extraction (UAE)
The extraction method was modified from our previous report [15]. The powder of A. vera were extract using water under ultrasonic conditions. There are 17 conditions was designed by Design-Expert which investigated on three factors including extraction time (5-30 min), temperature (30-60 °C) and material and liquid ration (1:5-1:10) as showed in table 1. The extract was centrifuged at 8,000 rpm at 4 °C for 30 min and then collected supernatant. EtOH was added to supernatant solution with the ratio 2:1 and kept at 4 °C for 18 h. The solution centrifuged at 8,000 rpm at 4 °C for 30 min and then collected the precipitate to be dry by freeze dryer. The extraction yield of A. vera was calculated on dry basis.
|
Factor |
Symbol |
Levels |
||
|
-1 |
0 |
1 |
||
|
Time (min) |
A |
5 |
17.5 |
30 |
|
material and liquid ratio (g/mL) |
B |
1:5 |
1:7.5 |
1:10 |
|
Temperature (°C) |
C |
30 |
45 |
60 |
Table 1: Experimental levels of the process variables.
Determination of total carbohydrate
The A. vera extract was determined using Phenol-Sulfuric acid method [16]. D-glucose standard was prepared as standard solutions in the range of 10 - 100 µg/mL. The extract 10 mg was dissolved water and adjusted to volumetric flask 10 mL and then diluted to have a concentration at 500 µg/mL. One milliliter of diluted solution (500 mg/mL) was mixed with 1 mL of 5% phenol before 5 mL of conc. sulfuric acid was added. The reaction mixture was left for 10 min before mixing and continue left for 30 min. Finally, the solutions were measured absorbance with wavelength at 490 nm by spectrophotometer.
Field Emission Scanning Electron Microscopy (FE-SEM)
The physical property of A. vera extract using UAE was determined on Field Emission Scanning Electron Microscope. The sample was coated with gold in a sputter coater. The experiment was performed under high vacuum conditions at an accelerating voltage of 2 kV, with image magnifications ranging from 200-1000x.
Statistical analysis
The experiments were conducted in triplicate. The Box Behnken Design (BBD) was generated from Design-Expert program. The statistical analysis was performed one-way ANOVA for multivariate analysis.
Results And Discussion
Ultrasonic assisted extraction (UAE) of A. vera
There were three factors consisted of extract time, temperature and material to liquid ratio which different level. The results of extraction yield and total carbohydrate were showed in table 2. Furthermore, the regression coefficient showed in table 3 and equation of formulate regression model (equation (1) and (2)) were generated to predict values. The invested models were significant suitable for F-values of 4.39 and 11.33 for yield and total carbohydrate, respectively. The relation coefficient values of R2 at 0.7250 and 0.6140 showed a significant model. The response surface of yield and total carbohydrate showed in 3D plot to present the correlation of extraction factors (Figure 1).
|
No. |
A |
B |
C |
yield (%w/w) |
Total carbohydrate (µg/ml) |
|
1 |
17.5 |
5 |
30 |
88.55 |
74.34 |
|
2 |
30 |
7.5 |
30 |
78.25 |
70.92 |
|
3 |
17.5 |
7.5 |
45 |
79.25 |
65.72 |
|
4 |
30 |
10 |
45 |
68.70 |
65.73 |
|
5 |
17.5 |
7.5 |
45 |
70.20 |
65.34 |
|
6 |
5 |
7.5 |
30 |
69.70 |
69.05 |
|
7 |
17.5 |
5 |
60 |
75.55 |
71.036 |
|
8 |
17.5 |
10 |
30 |
63.47 |
71.18 |
|
9 |
5 |
7.5 |
60 |
49.98 |
70.67 |
|
10 |
5 |
10 |
45 |
66.90 |
65.27 |
|
11 |
30 |
5 |
45 |
78.86 |
65.99 |
|
12 |
17.5 |
10 |
60 |
79.90 |
71.92 |
|
13 |
17.5 |
7.5 |
45 |
79.10 |
65.59 |
|
14 |
5 |
5 |
45 |
72.05 |
66.47 |
|
15 |
17.5 |
7.5 |
45 |
74.35 |
66.21 |
|
16 |
17.5 |
7.5 |
45 |
79.85 |
68.52 |
|
17 |
30 |
7.5 |
60 |
92.45 |
64.89 |
Table 2: The results of BBD design and respond values.
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
p-value |
|
Model |
1114.81 |
6 |
185.8 |
4.39 |
0.0197 |
|
A |
471.71 |
1 |
471.71 |
11.16 |
0.0075 |
|
B |
162.36 |
1 |
162.36 |
3.84 |
0.0785 |
|
C |
0.0105 |
1 |
0.0105 |
0.0002 |
0.9877 |
|
AB |
6.28 |
1 |
6.28 |
0.1484 |
0.7081 |
|
AC |
257.92 |
1 |
257.92 |
6.1 |
0.0331 |
|
BC |
216.53 |
1 |
216.53 |
5.12 |
0.0471 |
|
Residual |
422.76 |
10 |
42.28 |
|
|
|
Lack of Fit |
352.92 |
6 |
58.82 |
3.37 |
0.1299 |
|
Pure Error |
69.84 |
4 |
17.46 |
|
|
|
Cor Total |
1537.57 |
16 |
|
|
|
|
Std. Dev. |
6.5 |
|
|
|
|
|
Mean |
74.43 |
|
|
|
|
|
C.V. % |
8.74 |
|
|
|
|
|
R² |
0.725 |
|
|
|
|
|
Adjusted R² |
0.5601 |
|
|
|
|
|
Predicted R² |
-0.172 |
|
|
|
|
|
Adeq Precision |
7.5301 |
|
|
|
|
Table 3: Analysis of the variance of the fitted second-order polynomial models for extraction yield.
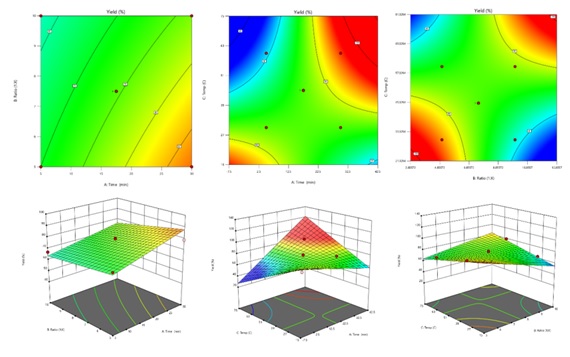 Figure 1: The 3D response surface plots of interaction between two factors on extraction yield: time and material and liquid ratio (A), time and temperature (B) and material and liquid ratio and temperature (C).
Figure 1: The 3D response surface plots of interaction between two factors on extraction yield: time and material and liquid ratio (A), time and temperature (B) and material and liquid ratio and temperature (C).
YYield = 7.67875A - 4.505B - 0.03625C - 1.2525AB + 8.03AC + 7.3575BC + 74.43 (1)
YSugar = -0.4906A - 0.4650B - 0.8722C + 0.2317AB - 1.91AC + 1.01BC - 1.82A2 +1.41B2 + 4.43C2 + 66.28 (2)
The highest point of prediction and optimal condition was for 17.5 min, material and liquid ratio of 1:7.5 and temperature at 45 °C. The extraction time showed highly significant with p value at 0.0075 which effect to yield. Then, the combination of extraction time and temperature exhibited effect on yield and total carbohydrate content [17-19]. Furthermore, the exponential of each factor presents the influenced to carbohydrate content in extract (Tables 3 & 4) [20]. This model was validated using the optimal condition for three times and results showed the extraction yield and total carbohydrate as 72.65 %w/w and 71.88 µg/mL which closed to prediction. The results indicated was effective and successfully designed for A. vera using RSM (Figures 2 & 3).
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
p-value |
|
Model |
132.15 |
9 |
14.68 |
11.33 |
0.0021 |
|
A |
1.93 |
1 |
1.93 |
1.49 |
0.2623 |
|
B |
1.73 |
1 |
1.73 |
1.33 |
0.2859 |
|
C |
6.09 |
1 |
6.09 |
4.7 |
0.0669 |
|
AB |
0.2147 |
1 |
0.2147 |
0.1656 |
0.6962 |
|
AC |
14.61 |
1 |
14.61 |
11.27 |
0.0121 |
|
BC |
4.1 |
1 |
4.1 |
3.16 |
0.1187 |
|
A² |
13.96 |
1 |
13.96 |
10.77 |
0.0135 |
|
B² |
8.4 |
1 |
8.4 |
6.48 |
0.0383 |
|
C² |
82.57 |
1 |
82.57 |
63.71 |
< 0.0001 |
|
Residual |
9.07 |
7 |
1.3 |
|
|
|
Lack of Fit |
2.4 |
3 |
0.7995 |
0.4792 |
0.7141 |
|
Pure Error |
6.67 |
4 |
1.67 |
|
|
|
Cor Total |
141.23 |
16 |
|
|
|
|
Std. Dev. |
4.38 |
|
|
|
|
|
Mean |
64.65 |
|
|
|
|
|
C.V. % |
6.78 |
|
|
|
|
|
R² |
0.614 |
|
|
|
|
|
Adjusted R² |
0.5249 |
|
|
|
|
|
Predicted R² |
0.2217 |
|
|
|
|
|
Adeq Precision |
8.2678 |
|
|
|
|
Table 4: Analysis of the variance of the fitted second-order polynomial models for total carbohydrate.
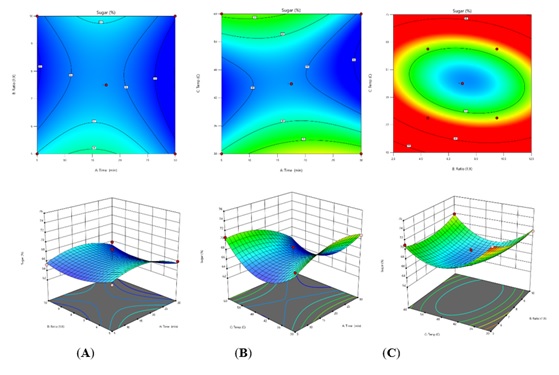 Figure 2: The 3D response surface plots of interaction between two factors on total carbohydrate: time and material and liquid ratio (A), time and temperature (B) and material and liquid ratio and temperature (C).
Figure 2: The 3D response surface plots of interaction between two factors on total carbohydrate: time and material and liquid ratio (A), time and temperature (B) and material and liquid ratio and temperature (C).
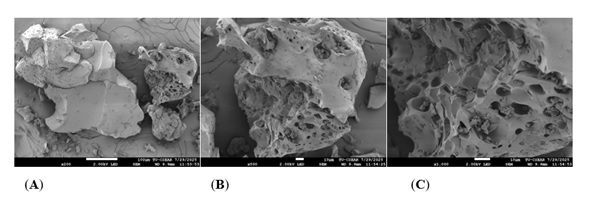
Figure 3: Physical property of A. vera extract by freeze dry: 200x (A), 500x (B) and 1000x (C) under Scanning electron microscopy.
Physical property of A. vera extract
The powder of A. vera extract was investigated surface area by SEM. The material exhibits an irregular morphology characterized by particle fragmentation and porosity, which enhances its potential for applications in substance encapsulation and facilitates efficient mass transfer in chemical reactions.
Conclusion
The optimization of extraction using ultrasonic assisted extraction for enhancing yield and total carbohydrate content exhibited the suitable condition. Temperature was the most effective factor for extraction and the optimal condition were extraction 17.5 min, material and liquid ratio of 1:7.5 and temperature at 45 °C. This model was verified and not significant between prediction and experiment values. The experiment can be applied for enrichment of bioactive compound from natural sources using green extraction technology.
Acknowledgement
The authors thanks Thailand Institute of Scientific and Technological Research (TISTR) for facilities and financial support.
References
- Bai Y, Niu Y, Qin S, Ma G (2023) A new biomaterial derived from aloe vera-acemannan from basic studies to clinical application. Pharmaceutics 15: 1913.
- Bhalang K, Thunyakitpisal P, Rungsirisatean N (2013) Acemannan, a polysaccharide extracted from aloe vera, is effective in the treatment of oral aphthous ulceration. J Altern Complement Med 19: 429-434.
- Bhuvana KB, Hema NG, Patil RT (2014) Review on aloe vera. Int J Adv Res 2: 677-691.
- Kim SH, Shim, KS, Song Y, Kim K, Park CS, et al. (2023) Pharmacological and therapeutic activities of aloe vera and its major active constituent acemannan. Food Suppl Biomater Health 3: 1-13.
- Liu C, Cui Y, Pi F, Cheng Y, Guo Y, et al. (2019) Extraction, purification, structural characteristics, biological activities and pharmacological applications of acemannan, a polysaccharide from aloe vera: A review. Molecules 24: 1554.
- Maan AA, Nazir A, Khan MKI, Ahmad T, Zia R, et al. (2018) The therapeutic properties and applications of aloe vera: a review. J Herb Med 12: 1-10.
- Matei CE, Visan AI, Cristescu R (2025) Aloe vera polysaccharides as therapeutic Agents: benefits versus side effects in biomedical applications. Polysaccharides 6: 36.
- Pal S, Raj M, Singh M, Saurav K, Paliwal C, et al. (2024) The Effect of Aloe vera on skin and its commensals: Contribution of acemannan in curing acne caused by propionibacterium acnes. Microorganisms 12: 2070.
- Redjeki S, Yaqin A, Iriani (2021) Extraction of acemannan polysaccharides active substance from aloe vera flesh with ethanol solvent. J Phys Conf Ser 1899: 012059.
- Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP (2020). Pharmacological update properties of aloe vera and its major active constituents. Molecules 25: 1324.
- Sierra-Garcíaa GD, Castro-Ríosc R, González-Hortaa A, Lara-Ariasb J, Chávez-Montes A (2014) Acemannan, an extracted polysaccharide from aloe vera: A literature review. Nat Prod Commun 9: 1217-1221.
- Souleymane T, Ibourahema C, Edith AA, Gbogouri GA, Kouakou B, et al. (2018) Effect of extraction methods on chemical and physical properties of aloe vera (Aloe Barbadensis Miller) polysaccharides fraction: Liguid gel and powders. Asian J Agri Food Sci 6: 97-108.
- Tabatabaeian M, Esfahanian V (2023) Effect of Acemannan/Aloe vera on bone regeneration Specially in the oral and maxillofacial region: A literature review. J Res Dent Maxillofac Sci 8: 226-235.
- Behaiyn S, Ebrahimi SN, Rahimi M, Behboudi H (2023) Response surface methodology optimization extraction of aloins from Aloe vera leaf skin by ultrasonic horn sonicator and cytotoxicity evaluation. Ind Crops Prod 202:
- Auppasuk P, Ponklang W, Lueachan R, Ploypetchara T, Butseekhot S, et al. (2022) Encapsulation Properties and Digestibility of Encapsulated Aloe vera Bioactive Polysaccharides. TJST 10: 677-690.
- DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350-356.
- Hammi KM, Hammami M, Rihouey C, Cerf DL, Ksouri R, et al. (2016) Optimization extraction of polysaccharide from Tunisian Zizyphus lotus fruit by response surface methodology: Composition and antioxidant activity. Food Chem 212: 467-484.
- Wang Z, Wu S, Wang J, Yang C, Wang Y, et al. (2024) Optimization of Polysaccharide Extraction from Polygonatum cyrtonema Hua by Freeze–Thaw Method Using Response Surface Methodology. Molecules 29: 4879.
- Zhan Q, Zhong H, Yin M, Peng J, Chen M (2022) Optimization of the polysaccharide extraction process from Rosa roxburghii Tratt using Box-Behnken response surface methodology and monosaccharide composition analysis. Food Sci Technol Campinas 42: 86322.
- Pan Y, Liu C, Jiang S, Guan L, Liu X, et al. (2024) Ultrasonic-assisted extraction of a low molecular weight polysaccharide from Nostoc commune Vancher and its structure characterization and immunomodulatory. Ultrasonic sonochemistry 108: 106961.
Citation: Auranwiwat C, Promchai T, Lamphunpong T, Sumsakul W, Sorndech W, et al. (2025) Optimization of Ultrasonic Assisted Extraction of Polysaccharides Extract from Aloe vera Using Response Surface Methodology. HSOA J Altern Complement Integr Med 11: 641.
Copyright: © 2025 Chiramet Auranwiwat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

