
Overcoming Barriers to Reproductive Health and Rights. Introduction of Assisted Reproductive Technology with Egg/Sperm Donation in the Public Health Services in Italy
*Corresponding Author(s):
Francesca RizzelloAssisted Reproductive Technology Center, Careggi Hospital, University Of Florence, Largo Brambilla 3, 50134 Florence, Italy
Tel:+39 0557946219,
Email:francesca.rizzello@gmail.com
Abstract
The aim of this paper is to discuss the reintroduction of Assisted Reproductive Technology (ART) with egg/sperm donation in Italy, by retracing the steps taken by the first Italian public health center who offered this technique within the National Healthcare System. In Italy, the history of medically assisted reproduction was marked by Law no. 40 - approved on March 19, 2004 - which forbade the assisted reproductive technology (ART) treatments using donated human gametes. Consequently, Italian couples were forced to turn to foreign assisted procreation centers, triggering the complex phenomenon of procreative tourism. After 30 years, heterologous ART was reintroduced in Italy at Careggi University Hospital center, which, as the first public center experiencing this technique, had to face great difficulties; above all the presence of an ambiguous legislation, the great demand, and the lack of donors. In order to offer the couples a chance of pregnancy comparable to other centers, the only possible way was to import gametes from foreign biobanks. The model adopted by the first Italian hospital offering heterologous ART is represented by the process of ‘two countries-two centres’, a new form of collaborative embryo-lab process. Nowadays, the model is followed by other centres with good results.
Keywords
Assisted reproduction; Gamete donation; Heterologous procreation; Italian legislation; Reproductive tourism
Introduction
In Italy, the Assisted Reproductive Technology (ART) treatments using donated human gametes, i.e., sperm and egg cells (oocytes), have been forbidden since Law no. 40 was approved on March 19, 2004. This led to procreative tourism with all the implications of people going to foreign countries. The objective of this paper is to present the great difficulty in reintroducing the technique in Italy and the experience of the first centre, which had immediately faced enormous issues such as the absence of donors.
Gamete Donation – A Well-Established Technique Worldwide
‘Gamete donation’ refers to using male gametes, female gametes, and both female and male gametes by another person (donor), in order to help intended parents to have a pregnancy by the process of assisted reproduction [1]. Nowadays, gamete donation has become a well-established technique with thousands of children born worldwide through this process. According to data collected from the 15 annual reports published in Human Reproduction, the proportion of Egg Donation (ED) during 1997-2011 increased from 2 to 5% [2]. Due to an ever-increasing prevalence of age-related infertility, the proportion of cycles with ED is likely to increase further.
The 17th ESHRE report on ART for Europe demonstrated that altogether 686271 treatment cycles started in 2013 (data from 38 countries), 7.2% more than in 2012. Cycles with ED were 40244, corresponding to 5.9% of all cycles. A total of 43785 cycles were performed with insemination with donor sperm (20% of total IUI). Fresh cycles, IVF-ICSI cycles executed with donor semen were a figure of 5.5% of the total [3]. A total of 231936 cycles were performed in fertility clinics with 9.1% ED cycles, as reported by the 2015 ART Fertility Clinic Success Rates Report, published by the Centers for Disease Control and Prevention (CDC), in the United States. There were no data available regarding male donors [4].
According to Gerkowicz et al., the donor sperm usage in ART has increased up to 6 % in USA, in all the assisted reproductive cycles conducted in 2014 [5]. The Italian summary ART register monitoring the activity and outcomes of Italian ART centers in 2017, reported 743 IUI with sperm donation (29 more than in 2016), 6771 second-level cycles performed with eggs and sperm donation (with an increment of 4.1% and 22% respectively than in 2016). Overall, by this technique, 1610 children were born, 57 of which by a double donation [6]. Additionally, regarding the Italy case, the data are certainly underestimated as procreative tourism remains and not all couples declare having carried out the treatment abroad at the time of birth.
Evolving Italian Law and Procreative Tourism
In Italy, the heterologous ART was never allowed in the public hospitals since the introduction of the ministerial circular in 1985 by the Minister for Health, Costante Degan, while it was allowed in private centers. In 2004, the Law 40/2004 on ART confirmed the prohibition of ART with eggs and sperm donation in both private and public sectors. These restrictions pave the way for procreative tourism which refers to the notion of couples, or an individual, seeking assisted reproduction in a country other than the one they reside in [7]. A study conducted in 2010 by Shenfield et al., provided a picture of quantity and reasons for seeking cross border reproductive care. The authors pointed out that in Europe, Italy had the highest number of patients who travelled to another country for the sake of ART, with the most popular destinations being Spain, Belgium, and Switzerland. Among the cross-border patients participating, 31.8% came from Italy, making Italy the first place for ‘cross-border reproduction care’ [8]. Another study conducted in 2012 indicates that only in 2011, at least 4000 Italian couples travelled abroad for ART, half of whom left the country for IVF treatments via gamete donation [9].
Spain is undisputedly the leader and ranked the highest in Europe for infertility treatment to foreigners or non-nationals according to ESHRE data. Studies have shown that almost 50% of all the egg donations in Europe are made in Spain, a major portion to overseas patients. Spanish registry data shows that 8.5 % of all the fertility treatments are done in foreigners (10). Spain is the main destination for infertility treatment because of its flexible law (access to treatment is allowed over 18 years regardless of marital status or sexual orientation), anonymous donors, effective treatment, and higher success rate [10-12].
For some authors, ‘procreative tourism’ should be revaluated as a reproductive ‘exile’ where the infertile couple feel left out from gaining access to ART in their home country [7,11]. This cross-border movement raises medical, social, and ethical concerns of disparity. First, only wealthy couples can access treatment abroad. Second, there is uncertainty about the safety of unfamiliar clinics and treatments. Third, language barriers may exist which can lead to misunderstandings or lack of a clear consent. Fourth, an economic burden exists, due to the absence from work, the travel expenses and treatment itself. Finally, couples may be confronted with different and contrasting laws from Italy that may be uncomfortable.
In the judgment of April 2014, the Constitutional Court (n. 162/2014) ruled that the sections of Law n. 40 of 19 February 2004 (Judgment n. 162/2014) - which prohibited heterologous fertilization – was unconstitutional, making the procedure legitimate in Italy. Prior to that, it was a sort of fencing match with rulings that contradicted each other. Essentially, the Constitutional Court declared that the ban on gamete donation amounted to a discriminatory treatment for couples affected by the most serious pathologies and not having suitable gametes to conceive an embryo. After the Constitutional Court decision, Law 40 remained in force without returning to Parliament. The following requirements persisted: couples should be married or living together, medically certified infertile or sterile, both alive, and of different genders at a potentially fertile age.
The regional Health Department of Tuscany published a resolution on July 28, 2014 (Regional Document N 650, July 28, 2014) authorizing heterologous ART in Tuscany and opening the treatment to all Italian couples. ‘Subjective requirements of couples of patients who can take advantage of gamete donation’ were specified in the document: the heterologous ART was allowed only in cases of irreversible ascertained and certified pathologies causing sterility or infertility and prohibited for illegitimate eugenic purposes.
In detail, regarding the donation of female gametes, the indications are all medical or iatrogenic situations of proven sterility in which the woman cannot have her own valid competent oocytes. For male gametes, the indications to donate are all the medical or iatrogenic situations of proven sterility that determine the unavailability of usable spermatozoa. In both cases, adequate information and psychological support should be guaranteed and available for the receiving couple.
Indications And Modalities
Following Tuscany Region, all Italian regions, moved to a round table and drafted a technical document. Considering that the Government had decided not to intervene with its own legislative provision in a subject so delicate for its ethical implications, the Conference of Regions and Autonomous Provinces (regulations of Conference Regions and Autonomous Provinces 14/109/CR02/C7SAN, September 4, 2014), defined operational guidelines in order to homogenize the access to heterologous procedures at a national level. This agreement was implemented by resolution of the regional council or with specific regional provisions and had a transitional value’ (Table 1). The document was signed by councilors and governors from all Italian Regions but was deliberate and active only by some regions.
|
Clinical indications for heterologous assisted reproduction with egg donation |
Clinical indications for heterologous assisted reproduction with sperm donation |
|
· Women with hypergonadotropic hypogonadism · Women in advanced reproductive age but still in a potentially fertile age · Women with diminished ovarian reserve after failure of homologous fertilization · Women who know they are affected or have a significant genetic defect or a family history of a condition from which the carrier status cannot be determined · Women with poor quality of oocytes and/or embryos or repeated failed attempts of conception using Assisted Reproduction Techniques (ART) · Women with iatrogenic infertility factor |
· Male partner with proven severe male factor infertility (azoospermia and severe oligo-astheno-teratozoospermia or failure to fertilize after intracytoplasmic sperm injection (ICSI)) · Male partner with incurable ejaculatory dysfunction · Men who know they are affected or have a significant genetic or a family history of a condition from which the carrier status cannot be determined · Male partner with a sexually transmitted infection that cannot be eliminated · Men with iatrogenic factor · The female partner is Rh-negative and severely isoimmunised, and the male partner is Rh-positivev |
Table 1: Indications heterologous procedures according to regulations of Conference Regions and Autonomous Provinces 14/109/CR02/C7SAN, September 4, 2014.
Long after, in July 2015, the ministerial guidelines were drafted and allowed the possibility to access heterologous treatment solely in two situations:
- In a couple, one or both partners should be proven sterile, without competent gametes
- Wherein the female partner should be Rh-negative and seriously isoimmunized, and the male partner should be Rh-positive
Consequently, a discordance existed between the regulations of Conference of Regions and Autonomous Provinces, which contained all the details of the procedures opposed to the ministerial guidelines which gave just two indications without including what was previously covered in the first document (Figure 1). Thus, the regulations of Conference of Regions and Autonomous Provinces still contains the more complete version of indications and modalities and is most commonly followed by the operators.
The latest guidelines, published in Official Journal 107/2024, nine years later, a six-year delay, replace the previous ones published in 2015 and take into account, in addition to technical and scientific developments in the sector, the amendments introduced by several Constitutional Court rulings that repeal some prohibitions in the original law. However, these maintain the same guidelines for access to procedures involving sperm and egg donation.
 Figure 1: Introduction and implementation of egg/sperm donation in Italy.
Figure 1: Introduction and implementation of egg/sperm donation in Italy.
Making it Happen- the First Public Centre
Tuscany, through Governor of Tuscany and his Health Department, was the first Region in Italy to provide ART treatments with egg/sperm donation in a public health centre offered by Health Region Public System, (Regional Document N 650 of July 28, 2014). After almost 30 years, the Careggi University Hospital in Florence became the first Public health center in Italy to offer ART with sperm/egg donation within the national healthcare system.
As a result, the demand from couples from all over the Italy, was immediately very huge with thousands of calls for appointment that the hospital call center went haywire. In just a few weeks, there was a waiting list of more than two years for the first appointment. A Task force was created and soon a request for more than 5 days/week of consultation was organized. The reasons for the long waiting list were not only due to the access of the procedure in Italy, but also to the great trust Tuscany users had in the public health system. Moreover, Italian patients could avail the service with a ticket payment of only 500 euros versus 7000–9000 euros in private clinics.
Enormous Demand: A New Challenge
In the regulations of Conference Regions and Autonomous Provinces 14/109/CR02/C7SAN three modalities to obtain the gametes were described:
- Men/women who spontaneously and altruistically decide to donate their gametes and are not in treatment for ART
- Men/women undergoing ART cycles (sperm/egg sharing)
- Men/women who donate their prior frozen gametes
Gametes available through the modalities expressed in the points 2 and 3 may be used if they meet the requirements for selecting donors, including the results of genetic, psychological, and infectious investigations, according to European Union directives [13]. Although a dedicated call center was organized in the hospital of Careggi, voluntary donors were very few. The reasons might be due to the poor culture for donation, socio-religious background, and the lack of an ‘expense reimbursement’ or a sort of supportive contribution for lost days’ time.
Unfortunately, gametes donated through the egg and sperm sharing way, very often did not respond to quality requirements and those available were quite inferior to the demand. Especially, egg sharing was not ideal because of the infertility of female donors; low numbers of oocyte donated as compared to demand; mean age of women undergoing IVF/ICSI, which in Italian centres is 36.7 years, the highest age in comparison to the rest of Europe [6]. Regarding sperm donation, the reason accounting for few donors might be related to both a cultural background (negative attitude by catholic church, moral. or ethical reasons) and strict criteria of selection for male donors due to the worsening of seminal fluid quality in young men.
Previously frozen gametes could not be utilized as men and women treated did not undergo all clinical, genetic, psychological, and other exams necessary to donate gametes. Another cause might be the limited number of live births by a donor, which is 10 for Italy (according to the Document of Regions and Autonomous Provinces Conference number 4/209/CR02/C7SAN) as compared to 25 per population of 800,000 for USA [14].
Import Of Gametes: A Solution
Faced with this great demand, in order to offer couples a chance of pregnancy similar to other centers, the biobanks were the only possible way. Through the biobanks it would have been possible to find oocytes from young women, the number of gametes would have been greater, and the donors would have carried out the examinations that the European legislation had provided. According to the European commission, biobanks are organized collection of biological samples and related information. They focus on collection, storage, genome research, and personalized medicine. Biobanks store oocytes through vitrification, thus improving the effectiveness, efficiency, and reliability of oocyte donation [15]. The banking of the male gametes is based on cryopreservation of spermatozoa.
European Tissues and Cell Directive 2004/23/EC allows the transfer of gametes between European countries and includes all the necessary rules for enhanced safety and security measures for import and export of gametes (safety of samples, quarantine, and testing of donors). It also invites its member states to include the principles of voluntary and unpaid donation in their national legislation. A donor must sign a consent form and must undergo a clinical examination. Donors may receive compensation but in those cases member states define the situations in which compensation may be granted, according to Directive 2004/23/EC of the European Commission [16].
The Careggi hospital was referred to foreign Biobanks through a European procurement call for tender for biobanks (Exploratory notice for expressions of interest for the gametes acquisition service, used in heterologous ART for the University Hospital of Careggi). This process took 1 year, and, finally, two Institutes from Spain and one from Denmark were selected. The Assisted Reproduction Unit of Careggi was designed as the only center for regional acquisition and constituted the first example and experience in Italy where gametes (sperms or oocytes) were imported from biobanks in another country.
The method of importing gametes from foreign biobanks: two countries-two centers
By following the European tissue and cell directive regulations, the hospital’s process to acquire gametes from abroad may be defined as ‘two countries and two centers (Figure 2). The whole process is divided into the following parts:
- Careggi hospital in Florence identifies the couple’s eligibility for heterologous fertilization with gamete donation
- It forwards a demand for the matching and acquisition of gamete to the foreign banks (Spain and Denmark)
- Biobanks select the donors. In cases of oocyte donation, the female donors are stimulated for retrieval and oocytes are vitrified. In cases of male donors, seminal fluid is collected and frozen. In both situations, gametes are stored to be transported
- Gametes might be imported through different modes of transportation including plane, boat, and land transport, which comprises of transportation via trucks
- In the receiving hospital, endometrium is prepared in the recipient woman, donated oocytes are warmed and fertilized by ICSI, and, finally, embryo transfer is performed. In male donor’s cycles, the female partner undergoes controlled ovarian stimulation, and subsequently treated by IUI or IVF/ICSI with donors’ spermatozoa according to the female indications. Supernumerary embryos are vitrified in both cases
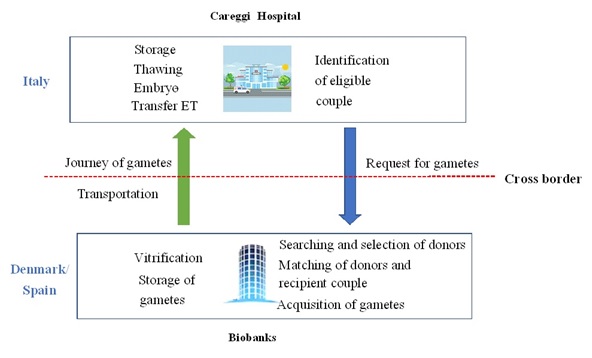
Figure 2: Two Countries, Two Centers. Procurement of gametes.
The first months were pivotal as there was a close collaboration between the two centers with on-site staff training, exchange of know-how, and homogenization of techniques and procedures. The aim of the cooperation was to optimize the methods in order to guarantee high survival rate of the gametes, since the freezing/vitrification and thawing/warming procedures were carried out in two different centers. In this close collaboration, we immediately verified that a decisive step was the transport and in fact, only after two months, plane transport was replaced by the international courier. Since then, ‘door to door by land’ directly from the biobank has been adopted, in order to maintain the control of the entire process and to avoid other intermediaries. This means of transport, even nowadays, represents the gold standard.
A Step Forward/ Conclusion
Heterologous ART was reintroduced in Italy after 30 years. The process ‘two countries-two centers’ represent a new form of collaborative embryo-lab process which was never done before. It was adopted as the first Italian experience of heterologous ART in a public hospital. At Careggi center, from the introduction of heterologous ART till September 2019, 803 cycles with oocyte donation, 368 cycles with sperm donation (specifically, 104 IUI cycles and 264 IVF-ICSI cycles) and 42 cycles using double donation were performed, with good results in terms of pregnancy rate and safety.
The good communication, and exchange of expertise and technology between the two centers of 2 countries are the basis for the success of the entire process. In our case, biologists went to the biobanks center in order to standardize the vitrification/warming technique and to have the best quality surviving gamete rate. In order to pursue continuous improvement, the results are constantly monitored. In some cases, control has led to modification of the process (such as the gametes shipping system). Furthermore, over the years, new security requirements have led to request for different assessments of donors by biobanks.
Nowadays, the example of Careggi is being followed by other Italian centers with good results [6]. Some problems remain unaddressed though. Among all, the first is the lack of donors and the need to import gametes from foreign banks. This could open the broader question of national identity, since the genetically born children are not Italian. In fact, we should consider the fact that Italy is one of the countries with the lowest natality. According to data from the last Italian Report on ART procedures, for 2022, 16,718 live births were achieved through all ART techniques. This represents 4.3% of the total live births in Italy that year. Of these, 3,805 children were born using donated gametes [17].
Author Roles
MEC and FR contributed significantly in design and interpretation of data, along with drafting the article.
Funding
None.
Conflict of Interest
The authors report no conflict of interest.
References
- Ethics Committee of the American Society for Reproductive Medicine (2019) Interests, obligations, and rights in gamete and embryo donation: an Ethics Committee opinion. Fertil Steril 111: 664-670.
- Ferraretti AP, Nygren K, Andersen AN, de Mouzon J, Kupka M, et al. (2017) Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open 2017: hox012.
- Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, et al. (2017) Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod 32: 1957-1973.
- Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology (2017) 2015 Assisted Reproductive Technology Fertility Clinic Success Rates Report. CDC, Atlanta, USA.
- Gerkowicz SA, Crawford SB, Hipp HS, Boulet SL, Kissin DM, et al. (2018) Assisted reproductive technology with donor sperm: national trends and perinatal outcomes. Am J Obstet Gynecol 218: 421.
- The Italian Assisted Reproductive Technology Register (2017) Italian Assisted Reproductive Technology National Summary Report.
- Lombardi L (2018) New Challenges for Human Reproduction: “Cross-Border Reproductive Care” and “Social Egg Freezing. Gender/Sexuality/Italy.
- Shenfield F, de Mouzon J, Pennings G, Ferraretti AP, Andersen AN, et al. (2010) Cross border reproductive care in six European countries. Hum Reprod 25: 1361-1368.
- Osservatorio Turismo Procreativo (2017) Conference. Rome, Italy.
- ESHRE (2017) ESHRE fact sheets. ESHRE, Europe, Belgium.
- Fertility Europe, European Society of Human Reproduction and Embryology (ESHRE) (2017) Evere, Grimbergen, A Policy Audit on Fertility. ESHRE, Europe, Belgium.
- Inhorn MC, Shrivastav P, Patrizio P (2012) Assisted reproductive technologies and fertility "tourism": examples from global Dubai and the Ivy League. Med Anthropol 31: 249-265.
- European Union (2016) Consolidated version of the Treaty on the Functioning of the European Union Part Three-Union Policies and Internal Actions Title XX - Environment Article 191 (ex Article 174 TEC). European Union, Spain, Italy.
- Practice Committee of American Society for Reproductive Medicine, Practice Committee of Society for Assisted Reproductive Technology (2013) Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril 99: 47-62.
- Stoop D (2012) From fresh heterologous oocyte donation to autologous oocyte banking. Facts Views Vis Obgyn 4: 271-282.
- European Commission (2004) Directive 2004/23/EC of the European Parliament and of the Council on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Official Journal of the European Union L102: 48-58.
- The Italian Assisted Reproductive Technology Register (2022) Italian Assisted Reproductive Technology National Summary Report.
Citation: Rizzello F, Coccia ME (2025) Overcoming Barriers to Reproductive Health and Rights. Introduction of Assisted Reproductive Technology with Egg/Sperm Donation in the Public Health Services in Italy. HSOA J Reprod Med Gynaecol Obstet 10: 206.
Copyright: © 2025 Francesca Rizzello, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

