
Overview of the Study on the Effect of Sucralose on Blood Glucose
*Corresponding Author(s):
Junzheng YangInstitute Of Consun Co. For Chinese Medicine In Kidney Diseases, Guangdong Consun Pharmaceutical Group, Guangzhou, China
Tel:+86 18790559526,
Email:yangjunzheng606403@163.com
Abstract
Sucralose is one kind of sweetener that is widely applied or used as an auxiliary material to cover up the bad taste of medicines, especially the unique bitter taste of Chinese patent medicines. Previous clinical studies found that Yi-Shen-Hua-Shi (YSHS) granule can significantly improve chronic glomerulonephritis, but because of its bitter taste, it is not conducive to long-term use in patients. Sucralose can improve the taste of Chinese patent medicines such as YSHS granules, but the effect of sucralose on blood glucose was not very clear, and whether the dosage of sucralose needs to be strictly controlled to avoid affecting blood glucose levels, especially in patients with diabetic nephropathy also should be discussed. For this purpose, we collected the clinical studies evaluating the effect of sucralose intake on blood glucose since 1996; the properties and metabolic characteristics of sucralose, the changes in blood glucose levels, and related biomarkers of glucose metabolism in different populations after single and long-term intake of sucralose were also summarized and reviewed.
Keywords
Blood glucose; Pharmacology; Sucralose; Sweetener; Yi-Shen-Hua-Shi granule
Introduction
Sucralose originated from a cooperative research project between Tate&Lyle Group and Queen Elizabeth College of London University in the late 1980s, and it passed the strict regulatory review process, including chemical characterization, validation of manufacturing methods and analytical methods, toxicology, pharmacokinetics, etc [1]. At present, sucralose is becoming the most frequently-used artificial sweetener in the world, accounting for 30% of the global low-calorie sweetener market ($2.29 billion) in 2016 [2]. It is also widely applied in the medical field and plays an important role in correcting the taste as a pharmaceutical auxiliary additive, such as suspension agent [3], oral liquid [4], orally disintegrating tablets [5] and granules [6].
Yi-Shen-Hua-Shi (YSHS) granule (NMPA Approval No. Z20090250) is a modern Chinese patent medicine developed by Guangzhou Consun Pharmaceutical Co., Ltd for the treatment of stage II and III chronic glomerulonephritis. The main effective substances include coumarins, triterpenoids, and flavonoids. Although the curative effect is remarkable [7-9], the prescription contains extremely bitter Chinese medicines such as Pinellia ternate and Coptis Chinensis, which seriously affects the long-term use of patients with chronic kidney disease. To overcome this shortcoming, YSHS granule product developers or researchers decided to use sucralose as a flavoring agent. The previous study found that 0.1g sucralose added to each sachet of medicine was the appropriate dose to cover up the bitter taste of YSHS granules. Since patients were required to take 3 sachets of YSHS granules daily (equivalent to a sucralose daily intake of 0.3g), we were concerned that the addition of sugar substitutes may cause potential blood glucose problems, such as the effect of a single dose of sucralose or the effect of the long term use of sucralose on the blood glucose of obesity patients, diabetic patients, and nephropathy patients. For this reasons, based on the retrieval formula “Sucralose[Title/Abstract]) & (Blood glucose[Title/Abstract]”, we obtained 21 clinical studies in the PubMed database that were directly related to the observation of blood glucose changes after the intake of sucralose and aimed to briefly summarize the properties and oral metabolic characteristics of sucralose, as well as the effects of sucralose on blood glucose in different patients at the single dose of sucralose and the long term use of sucralose.
Properties Of Sucralose
The chemical name of sucralose is 4, 1’, 6’-trichloro-4, 1’, 6’-Trideoxygenated galactose sucrose (C12H19Cl3O8) [10], as shown in figure 1. It has a disaccharide base with the substitution of 3 hydroxyl groups by chlorine, and the relative molecular weight is 397.63. The pure product appeared as a white or nearly white crystalline powder which is extremely soluble in water (solubility 28.2 g, 20 °C), ethanol, and methanol, and slightly soluble in diethyl ether [11]. Sucralose has the characteristics of high sweetness (600 times as much as sucrose [12]) and stability to light, pH, and high temperature. Thus, the sweetness level of the product can still be maintained after cooking, baking, and pasteurization [13]. Because sucralose does not contain calories and is almost not absorbed by the human body, it has been recommended by scholars as part of the weight loss strategy for patients with overweight, glucose intolerance, diabetes, and pregnancy diabetes [14,15]. The US Food and Drug Administration (FDA), the Canadian Ministry of Health, and the European Food Safety Administration set the daily acceptable intake of sucralose as 0.005g/kg, 0.009g/kg, and 0.015g/kg according to body weight, respectively [16-18].
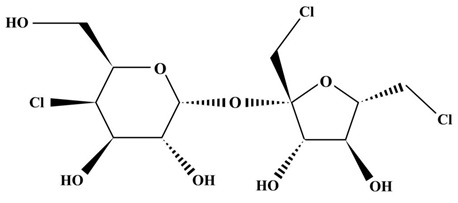 Figure 1: Schematic diagram of chemical structure of sucralose.
Figure 1: Schematic diagram of chemical structure of sucralose.
Oral Metabolic Characteristics of Sucralose
Sucralose was hard to absorb and had the short half-life period
During the whole development process of sucralose as a sweetener, a large number of studies have been conducted to evaluate its impact on physiology and general safety effect [19-21]. The results of these studies demonstrated that sucralose is not easy to absorb in the metabolism of the body. Animal experiments showed that after oral administration of sucralose, some of them are directly discharged from the feces in the form of original sucralose, and a small part of the residues are discharged through urine [22]. The metabolism of sucralose is similar to that of animals in the human body. After oral administration of 1mg/kg of sucralose, 78.3% of sucralose is directly excreted through feces, 14.5% is excreted through urine, and the main forms in urine are sucralose and a small amount of sucralose glucose glycolic acid [23]. Sucralose in the plasma reached the highest value about 2 hours after oral administration of sucralose, and its plasma half-life period was 13 hours, almost could be undetectable within 24 hours [24]. This feature of poor absorption and short half-life can be explained by the molecular structure of sucralose. As a relatively small polyhydroxylated molecule, sucralose is highly water-soluble rather than lipophilic, and most of it is not absorbed by the human body after oral administration. It is difficult for researchers to observe its bioaccumulation and gastrointestinal side effects. Therefore, sucralose is considered to be the best and ideal sweet substitute for patients with obesity, cardiovascular disease, and diabetes.
Sucralose could change the composition of intestinal microbiota
Many studies have shown that the imbalance of intestinal flora is related to the tendency of metabolic diseases such as obesity and diabetes [25]. Four studies reported that the level of insulin stimulating-polypeptide GLP generated by the intestine was significantly increased in the intake of sucralose in healthy people and patients with type 1 diabetes [26-29]. The researchers believed that the increase in blood glucose in obese female patients due to the intake of sucralose may be attributed to the imbalance of intestinal flora [30]. So far, five basic studies have consistently found that habitual consumption of sucralose leads to changes in intestinal microbiota; these studies were conducted in mice or rats, with doses ranging from 1.1 to 15mg/kg/d and lasting for 6 weeks to 6 months. The observed change was concentrated on the bacterial genus rather than the whole phylum. However, to further investigate which kind of bacterial genus is affected by the intake of sucralose, the results in different studies were inconsistent, and even the opposite change occurs in the same genus (i.e. bifidobacteria) [31,32].
In contrast, only one study evaluated the effect of sucralose intake on intestinal microbiota in humans. In this randomized controlled trial, 34 healthy volunteers received 0.78g/d sucralose capsules (equivalent to 2.6 times of YSHS granule) for 7 days [33]. Compared with the placebo, the intestinal microbiome was evaluated before and after the intervention, and there was no significant change in the intestinal microbiome of the sucralose group. It further suggests that the data obtained in rodent animal models may lack clinical relevance because of the huge differences in bacterial genera and species between mice and humans. At present, there is still not enough evidence to rule out the positive or negative effects of sucralose on human microbiota, and even the causal relationship between intestinal microbiota and disease remains to be determined.
The Single Dose Of Sucralose Has No Effect On Blood Glucose
Since FDA approved sucralose as a food additive, it can be found by reviewing many studies since 1996 that the daily single intake of sucralose in the range of 0.018 ~6 g dose not affect on the blood glucose level of healthy people, type 1 diabetes patients, type 2 diabetes patients, and obese diabetes patients. As shown in Table 1, this result is supported by a large number of experiments. In these studies, the dosage forms include capsule, self-made solution, and commercial beverage containing sucralose; the comparison method involved the use of sucralose alone or in combination with other sugar substitutes (acesulfame or aspartame); the administration routes included oral administration, intragastric administration, and duodenal infusion. There was only Pepino et al., study found that the level of blood glucose and insulin in obese participants increased significantly after a single oral administration of 0.048g of sucralose, and there were no changes in the levels of glucagon like peptide (GLP)-1 and Glucose Dependent Insulinotropic Polypeptide (GIP) [34]. It is worth noting that both GLP and GIP are hormone peptides that enhance insulin secretion in a glucose concentration-dependent manner. But in the study of Pepino et al., no increase in the expression of GLP and GIP was detected there seems to be a conflict with the significant increase in blood glucose and insulin levels that they observed [26]. We suggest large-scale double-blind experiments are needed for obese people to verify the phenomenon in the future. In addition to blood glucose, some clinical markers that may have direct or indirect effects on blood glucose, such as insulin, GLP, GIP, and C-peptide, are not been shown to be affected by the intake of sucralose in most studies, with the proportions of 89.5% (17/19), 80% (16/20), 100% (13/13) and 91.7% (11/12), respectively.
|
Year |
Dose |
Dosage form |
Administration |
Population characteristics |
Blood glucose |
Insulin |
GLP |
GIP |
C-peptide |
PYY |
Gastric emptying |
Appetite |
Glucagon |
Insulin sensitivity |
Insulin clearance |
Refer ence |
|
1996 |
1g/d |
Capsule |
Oral |
T1D |
No |
/ |
/ |
/ |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[35] |
|
1996 |
1g/d |
Capsule |
Oral |
T2D |
No |
/ |
/ |
/ |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[35] |
|
2009 |
0.08g/d |
Solution |
Gavage |
Healthy |
No |
No |
No |
No |
/ |
/ |
No |
/ |
/ |
/ |
/ |
[36] |
|
2009 |
0.8g/d |
Solution |
Gavage |
Healthy |
No |
No |
No |
No |
/ |
/ |
No |
/ |
/ |
/ |
/ |
[36] |
|
2009 |
0.046g/d |
Sugary cola |
Oral |
Healthy |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[37] |
|
2010 |
0.96g/d |
Normal saline |
Duodenum |
Healthy |
No |
/ |
No |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[38] |
|
2011 |
50mL/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
No |
/ |
/ |
No |
/ |
No |
/ |
/ |
/ |
[39] |
|
2011 |
6g/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
/ |
/ |
/ |
/ |
No |
No |
No |
/ |
/ |
[40] |
|
2011 |
0.062g/d |
Aqueous solution |
Duodenum |
Healthy |
No |
No |
No |
/ |
/ |
No |
/ |
No |
/ |
/ |
/ |
[41] |
|
2012 |
0.06g/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[42] |
|
2012 |
0.046g/d |
Sugary soda |
Oral |
Healthy |
No |
/ |
↑* |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
[26] |
|
2012 |
0.046g/d |
Sugary soda |
Oral |
T1D |
No |
/ |
↑* |
No |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[26] |
|
2012 |
0.046g/d |
Sugary soda |
Oral |
Obese T2D |
No |
/ |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[26] |
|
2013 |
0.048g/d |
Aqueous solution |
Oral |
Obesity |
↑** |
↑** |
No |
No |
↑** |
/ |
/ |
/ |
No |
↓** |
↓* |
[30] |
|
2013 |
0.052g/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
No |
/ |
/ |
/ |
No |
/ |
/ |
/ |
/ |
[34] |
|
2013 |
0.159g/d |
Solution |
Oral |
Healthy |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[43] |
|
2015 |
0.024g/d |
Aqueous solution |
Oral |
Healthy |
↓** |
No |
↑* |
/ |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[27] |
|
2015 |
0.024g/d |
Aqueous solution |
Oral |
T2D |
No |
No |
No |
/ |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[27] |
|
2016 |
0.068g/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[28] |
|
2016 |
0.17g/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[28] |
|
2016 |
0.25g/d |
Aqueous solution |
Oral |
Healthy |
No |
No |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[28] |
|
2016 |
0.068g/d |
Sugary cola |
Oral |
Healthy |
No |
No |
↑** |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[28] |
|
2016 |
0.018g/d |
Sugary beverage |
Oral |
Healthy |
No |
No |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[28] |
|
2016 |
0.068g/d |
Syrup |
Oral |
Healthy |
No |
No |
No |
No |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[28] |
|
2019 |
0.048g/d |
Aqueous solution |
Oral |
Healthy |
No |
↑* |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
/ |
[44] |
Table 1: The effect of single intake of sucralose on the clinic markers of glucose metabolism.
"↑": Elevated; "↓": Decreased; No: no influence; Compared with placebo group/control group/health group (different controls in different studies), *P < 0.05, **P < 0.01. Abbreviation: T1D, type 1 diabetes; T2D, type 2 diabetes; GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide; PYY, peptide YY.
The Long Term Use Of Sucralose Has No Effect On Blood Glucose
Although the reports of long-term use of sucralose are quite limited (Table 2), the conclusions are all in keeping with the above findings of the effect of single dose of t on blood glucose; the effect of sucralose on blood sugar in healthy people, type 2 diabetes patients and obese diabetes patients are still within the normal range even it for 2 to 13 weeks, and more than half of the studies used the dose of sucralose were exceeded the daily dose of sucralose in YSHS granule.
|
Author |
Year |
Dose |
Dosage form |
Duration |
Administration |
Population characteristics |
Blood glucose |
Insulin |
GLP |
HbA 1c |
C-peptide |
Insulin sensitivity |
Acute insulin reaction |
Reference |
|
Baird |
2000 |
125-500mg/d |
Aqueous solution |
90d |
Oral |
Healthy |
No |
/ |
/ |
/ |
/ |
/ |
/ |
[24] |
|
Reyna |
2003 |
Not described |
Cookies |
4w |
Oral |
T2D |
No |
/ |
/ |
↓ |
/ |
/ |
/ |
[45] |
|
Grotz |
2003 |
0.667g/d |
Capsule |
13w |
Oral |
Obese T2D |
No |
No |
/ |
No |
No |
/ |
/ |
[46] |
|
Grotz |
2017 |
1g/d |
Capsule |
12w |
Oral |
Healthy |
No |
No |
/ |
No |
/ |
/ |
/ |
[47] |
|
Romo |
2018 |
0.036g/d |
Sugary beverage |
2w |
Oral |
Healthy |
No |
/ |
/ |
/ |
/ |
↓* |
↑* |
[48] |
|
Lertrit |
2018 |
0.2g/d |
Capsule |
4w |
Oral |
Healthy |
No |
↑** |
↑** |
/ |
/ |
↓* |
↓** |
[29] |
Table 2: The effect of long-term sucralose intake on clinical markers of glucose metabolism.
"↑": Elevated; "↓": Decreased; No: no influence; Compared with placebo group/control group/health group (different controls in different studies), *P < 0.05, **P < 0.01. Abbreviation: T2D, type 2 diabetes; GLP-1, glucagon-like peptide-1; HbA1c, glycosylated hemoglobin.
The experimental design of these studies is also more rigorous. For example, Grotz’s study is only carried out in young men, which can avoid the potential effects of menstruation-related hormone changes on blood glucose control, and the possibility of avoiding a reduction in the sensitivity of older populations to changes in blood glucose levels affecting outcomes [47]. Different from the previously observed dosage forms (such as solution and capsule), Reyna et al. added sucralose as a sweetener in the improved biscuit for a 4-week evaluation [45]. Baird et al., also designed a 9-day single high dose (10mg/kg) experiment, a 17-day 2-5mg/kg dose increase experiment, and a 90-day 125 mg/day, 250 mg/day, and 500 mg/day dose repeat experiment to observe and evaluate the effect of sucralose on the blood glucose [24].
It is worth noting that the dosage of sucralose in Baird's research [24] and Grotz's research [47] actually exceeded the expected human intake. Grotz et al., can only require healthy subjects to eat three times a day, it is impossible to count whether the food contains sucralose; the results of the study demonstrated that sucralose has no effect on fasting or postprandial blood glucose, insulin, C-peptide, and HbA1c. Baird et al. found that healthy volunteers had good tolerance to sucralose at a single dose of up to 10mg/kg/day and a repeated dose of 5mg/kg/day for 13 weeks; the analysis of blood samples of volunteers who had taken 5 mg/kg/day sucralose for 12 consecutive weeks showed that the bioaccumulation of sucralose in blood was insufficient, indicating that the level of sucralose in blood would not increase with long-term high dose exposure [24]. Even though the intake of sucralose in the diet was much higher than expected, none of the subjects reported adverse effects, and the general signs, biochemical markers, and hematological data were not affected by sucralose intake. In the safety study of sucralose, about No adverse effects were found in rodents at 1500 mg/kg/day sucralose were taken for 104 consecutive weeks (which is equivalent to 16 times of the adult dosage prescribed by the World Health Organization after conversion) [21, 49,50], this dose is considered to be the highest non-adverse effect level of sucralose. According to these studies and the extensive animal safety database, there is no evidence demonstrating that frequent or long-term intake of sucralose at the maximum expected intake will have adverse effects on human health.
Conclusion
Taken altogether, in all studies with a daily intake of more than 0.3g (daily dose of sucralose in YSHS granule), no significant impact was found on fasting blood glucose or area under the curve after glucose tolerance. In which the observed maximum daily dose was 6 g (equivalent to 20 times of YSHS granule), and the longest period of administration was 13 weeks. The subjects covered healthy individuals, type 1 diabetes patients, type 2 diabetes patients, and obesity patients. The reason for the low effect of sucralose on blood glucose may be related to its high water solubility, difficult absorption, and short half-life period. These studies provide an important basis for the process optimization of sucralose as a drug flavoring agent, especially for extremely bitter Chinese patent drugs such as YSHS granule, so as to facilitate the safe application of patients with abnormal blood glucose.
Acknowledgement
None.
Conflict of Interests
There is no conflict of interest in this article.
Funding
None.
References
- Magnuson BA, Roberts A, Nestmann ER (2017) Critical review of the current literature on the safety of sucralose. Food Chem Toxicol 106: 324-355.
- Business Wire (2020) Global zero-calorie sweetener market projected to be worth USD 2.84 billion by 2021 [Internet]. Technavio, UK.
- Aceves SS, Dohil R, Newbury RO, Bastian JF (2005) Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol 116: 705-706.
- Johns P, Dowlati L (2003) Determination of acesulfame and sucralose in oral electrolyte maintenance solution by liquid chromatography. J Aoac Int 86: 79-85.
- Venkatesh GM, Stevens PJ, Lai JW (2012) Development of orally disintegrating tablets comprising controlled-release multiparticulate beads. Drug Dev Ind Pharm 38: 1428-1440.
- Si M, Ma Z, Zhang J, Li X, Li R, et al. (2021) Qingluoyin granules protect against adjuvant-induced arthritis in rats via downregulating the CXCL12/CXCR4-NF-kappaB signalling pathway. Pharm Biol 59: 1441-1451.
- Zhao J, Chan YC, He B, Duan TT, Yu ZL (2019) A patent herbal drug Yi-Shen-Hua-Shi granule ameliorates C-BSA-induced chronic glomerulonephritis and inhabits TGFbeta signaling in rats. J Ethnopharmacol 236: 258-262.
- Tan Z, Si Y, Yu Y, Ding J, Huang L, et al. (2022) Yi-Shen-Hua-Shi Granule Alleviates Adriamycin-Induced Glomerular Fibrosis by Suppressing the BMP2/Smad Signaling Pathway. Front Pharmacol 13: 917428.
- Liang M, Zhu X, Zhang D, He W, Zhang J, et al. (2022) Yi-Shen-Hua-Shi granules inhibit diabetic nephropathy by ameliorating podocyte injury induced by macrophage-derived exosomes. Front Pharmacol 13: 962606.
- Grotz VL, Munro IC (2009) An overview of the safety of sucralose. Regul Toxicol Pharmacol 55: 1-5.
- Iizuka K (2020) Is the Use of Artificial Sweeteners Beneficial for Patients with Diabetes Mellitus? The Advantages and Disadvantages of Artificial Sweeteners. Nutrients 14: 4446.
- Schiffman SS, Rother KI (2013) Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev 16: 399-451.
- Barndt R, Jackson G (1990) Stability of sucralose in baked goods. Food Technol (Chic) 44: 62-66.
- Hunter SR, Reister EJ, Cheon E, Mattes RD (2019) Low Calorie Sweeteners Differ in Their Physiological Effects in Humans. Nutrients 11: 2717.
- Palatnik A, Moosreiner A, Van SSO (2020) Consumption of non-nutritive sweeteners during pregnancy. Am J Obstet Gynecol 223: 211-218.
- US Food & Drug Administration (2019) Additional information about high-intensity sweeteners permitted for use in food in the United States. U S Food & Drug Administration, USA.
- Health Canada (2019) List of permitted dweeteners (lists of permitted food additives). Health Canada, Canada.
- Risdon S, Battault S, Romo AR, Roustit M, Briand L, et al. (2021) Sucralose and Cardiometabolic Health: Current Understanding from Receptors to Clinical Investigations. Adv Nutr 12: 1500-1513.
- US Department of Health and Human Services Food and Drug Administration (1988) Food Additives Permitted for Direct Addition to Food for Human Consumption: Sucralose: Final Rule. US Department of Health and Human Services Food and Drug Administration, USA.
- Kille JW, Tesh JM, McAnulty PA, Ross FW, Willoughby CR, et al. (2000) Sucralose: assessment of teratogenic potential in the rat and the rabbit. Food Chem Toxicol 38: 43-52.
- Goldsmith LA (2000) Acute and subchronic toxicity of sucralose. Food Chem Toxicol 38: 53-69.
- John BA, Wood SG, Hawkins DR (2000) The pharmacokinetics and metabolism of sucralose in the rabbit. Food Chem Toxicol 38: 111-113.
- Knight I (1994) The development and applications of sucralose, a new high-intensity sweetener. Can J Physiol Pharmacol 72: 435-439.
- Baird IM, Shephard NW, Merritt RJ, Smith GH (2000) Repeated dose study of sucralose tolerance in human subjects. Food Chem Toxicol 38: 123-129.
- Qin J, Li Y, Cai Z, Li S, Zhu J, et al. (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55-60.
- Brown RJ, Walter M, Rother KI (2012) Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care 35: 959-964.
- Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, et al. (2015) Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur J Clin Nutr 69: 162-166.
- Sylvetsky AC, Brown RJ, Blau JE, Walter M, Rother KI (2016) Hormonal responses to non-nutritive sweeteners in water and diet soda. Nutr Metab (Lond) 13: 71.
- Lertrit A, Srimachai S, Saetung S, Chanprasertyothin S, Chailurkit LO, et al. (2018) Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: a randomized, double-blind, placebo-controlled trial. Nutrition 56: 125-130.
- Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S (2013) Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 36: 2530-2535.
- Donia MBA, Masry EME, Rahman AAA, McLendon RE, Schiffman SS (2008) Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J Toxicol Environ Health A 71: 1415-1429.
- Wang QP, Browman D, Herzog H, Neely GG (2018) Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. Plos One 13: 199080.
- Thomson P, Santibanez R, Aguirre C, Galgani JE, Garrido D (2019) Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br J Nutr 122: 856-862.
- Wu T, Bound MJ, Standfield SD, Bellon M, Young RL, et al. (2013) Artificial sweeteners have no effect on gastric emptying, glucagon-like peptide-1, or glycemia after oral glucose in healthy humans. Diabetes Care 36: 202-203.
- Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, et al. (1996) Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care 19: 1004-1005.
- Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, et al. (2009) Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296: 735-739.
- Brown RJ, Walter M, Rother KI (2009) Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 32: 2184-2186.
- Ma J, Chang J, Checklin HL, Young RL, Jones KL, et al. (2010) Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 104: 803-806.
- Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, et al. (2011) Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr 65: 508-513.
- Brown AW, Bohan BM, Onken KL, Beitz DC (2011) Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr Res 31: 882-888.
- Steinert RE, Frey F, Topfer A, Drewe J, Beglinger C (2011) Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr 105: 1320-1328.
- Wu T, Zhao BR, Bound MJ, Checklin HL, Bellon M, et al. (2012) Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr 95: 78-83.
- Stellingwerff T, Godin JP, Beaumont M, Tavenard A, Grathwohl D, et al. (2013) Effects of pre-exercise sucralose ingestion on carbohydrate oxidation during exercise. Int J Sport Nutr Exerc Metab 23: 584-592.
- Arauz AYG, Hernandez NB, Palomera LF, Suarez RA, Leon KLD, et al. (2019) A Single 48 mg Sucralose Sip Unbalances Monocyte Subpopulations and Stimulates Insulin Secretion in Healthy Young Adults. J Immunol Res2019: 6105059.
- Reyna NY, Cano C, Bermudez VJ, Medina MT, Souki AJ, et al. (2003) Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. AM J Ther 10: 438-443.
- Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, et al. (2003) Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc 103: 1607-1612.
- Grotz VL, Sunyer XP, Porte DJ, Roberts A, Richard TJ (2017) A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul Toxicol Pharmacol 88: 22-33.
- Romo AR, Salinas CAA, Cordova GXB, Diaz RAG, Valdes PA (2018) Sucralose decreases insulin sensitivity in healthy subjects: a randomized controlled trial. Am J Clin Nutr 108: 485-491.
- Mann SW, Yuschak MM, Amyes SJ, Aughton P, Finn JP (2000) A combined chronic toxicity/carcinogenicity study of sucralose in Sprague-Dawley rats. Food Chem Toxicol 38: 71-89.
- Mann SW, Yuschak MM, Amyes SJ, Aughton P, Finn JP (2000) A carcinogenicity study of sucralose in the CD-1 mouse. Food Chem Toxicol 38: 91-97.
Citation: Lin Z, Li M, Meng L, Li M, Duan T, et al. (2023) Overview of the Study on the Effect of Sucralose on Blood Glucose. J Altern Complement Integr Med 9: 319.
Copyright: © 2023 Ziyang Lin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

