
Panax ginseng Meyer Herbal Preparation HRG80 for Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects
*Corresponding Author(s):
Alexander G PanossianPhytomed Ab, Bofinkvagen 1, Vaxtorp 31275, Sweden
Tel:+46 73330626,
Email:ap@phytomed.se
Abstract
A large body of evidence suggests that ginsenoside metabolites contribute substantially to the pharmacological effects of ginseng. These metabolites (rare ginsenosides) are present in minor amounts in white Ginseng and in larger amounts in steam processed red Ginseng. A recent study aimed to obtain evidence that rare ginsenosides significantly contribute in the overall efficacy of Ginseng. The efficacy of two Ginseng preparations containing approximately the same amounts of major ginsenosides but substantially different (7.8-fold) amounts of rare ginsenosides was compared in a three-arm, randomized, double-blinded, placebo-controlled, crossover trial. A hydroponically cultivated red Panax ginseng Meyer root preparation (HRG80®) was compared with traditionally harvested 6-year-old white P. ginseng (WG) and placebo for their ability to prevent symptoms of stress such as fatigue, impaired memory, reduced concentration, and attention deficit related to daily work in healthy subjects. The effects of HRG80® (daily dose: 418 mg of red ginseng powder, 63.5.mg of ginsenosides, and 52 mg of rare ginsenosides), PGS Arkopharma (daily dose: 764 mg of white ginseng powder, 19.8 mg of ginsenosides, and 6.1 mg of rare ginsenosides), and placebo capsules, taken orally once a day for 14 days in 50 tired but otherwise healthy subjects, were studied. The efficacy outcomes included the accuracy of processing in the d2 test for cognitive functions, an accuracy score obtained using a computerized memory test, and the perceived stress score. A statistically significant interaction effect between time and treatment was observed in the d2 attention and memory tests, indicating that HRG80 was more beneficial than placebo. The effects of WG were better than those of placebo, but the difference was not statistically significant. There was a significant difference between the effects of HRG80 and WG, which was observed after both single (day 1) and repeated administration (days 5 and 12). Importantly, the effective therapeutic daily dose of HRG80 (418 mg) was at least 10-fold lower than the commonly used effective dose red ginseng (4500–9000 mg per day).
Keywords
Cognitive; Occupational stress; Panax ginseng; Rare ginsenosides
BACKGROUND
Stress-induced fatigue
Fatigue is considered an alarm phase of the adaptive defense response of the neuroendocrine-immune system to stressors [1]. Fatigue, also known as weariness, tiredness, exhaustion or lethargy, is a common health complaint that may be generally defined as a lack of energy. Fatigue and sleepiness can affect performance at work and cause transportation and industrial accidents, resulting in considerable losses of property, personal injury and even death [2]. It is estimated that approximately 20% of Americans experience fatigue of sufficient intensity to interfere with the ability to enjoy a normal life, with physical causes being responsible for 20%-60% of fatigue cases and emotional causes being responsible for the balance.
Fatigue can have a physical and/or mental nature. Physical fatigue, also known as peripheral fatigue, results from repeated muscle actions followed by an inability to continue functioning at a level commensurate with normal ability. By contrast, mental fatigue represents a failure to complete mental tasks that require self-motivation and internal cues in the absence of cognitive failure or motor weakness. Mental fatigue decreases the efficiency of subjects’ occupational work or learning effectiveness in daily life. Mental fatigue may manifest in the form of decreased attention, reduced concentration, or somnolence. Acute fatigue is a normal phenomenon that disappears after a period of rest. Conversely, prolonged fatigue is often irrevocable, and the recovery mechanisms that alleviate acute fatigue are no longer effective. Chronic fatigue is caused by the prolonged accumulation of acute fatigue [3]. It is well known that social stresses frequently trigger acute mental fatigue and the onset of chronic fatigue syndrome [1]. Dealing with the COVID-19 viral outbreak has created a tremendous amount of constant stress directly related to the outbreak plus the implications of the lock down, and other restrictions that are put in place as a way to contain spreading of the virus. Stress is caused fighting the virus or worrying about a family member or yourself that you may be the next victim. There could not be more stress for people today who are losing their jobs without knowing if they have an income to meet the challenges of paying the mountain of bills piling up. Just the uncertainty of what the future will hold for millions of people can cause stress and stress-induced fatigue. The stress today may take a greater bodily toll than the virus itself.
Many people prefer to avoid problems related to decreased attention and reduced concentration during continuous mental work and resort to the use of stimulants (caffeine), sympathomimetics (ephedrine) and tonics such as cola [4]. However, regular consumers of coffee recognize that caffeine can disrupt sleep and that caffeine withdrawal is associated with various adverse effects including fatigue and headache. Cognitive performance is adversely affected by acute caffeine withdrawal, and even in the context of alertness reduced by sleep restriction, cognitive performance is not improved by caffeine in the absence of withdrawal effects. Because caffeine use is also associated with an increased risk for various cardiovascular diseases including high blood pressure and elevated plasma homocysteine levels, it has been suggested that caffeine consumption confers little benefit. Additionally, the diterpenes present in unfiltered coffee also appear to increase the risk of coronary heart disease [5,6]. The use of sympathomimetics might efficiently improve acute fatigue; however, their long-term use might accelerate the progression of chronic fatigue because sympathetic hyperactivity based on decreased parasympathetic activity is associated with mental fatigue induced by prolonged cognitive load [3].
Many plant extracts have exhibited good stimulating and anti-fatigue effects in experiments conducted in experimental animals. Of these, adaptogens (Panax ginseng, Rhodiola rosea, Eleutherococcus senticosus, Schisandra chinensis) have exhibited significant anti-fatigue effects including increased mental performance, particularly cognitive function, in placebo-controlled, double-blind studies [7]. These adaptogenic plants extracts can enhance resistance to stress and improve performance, concentration, and endurance during fatigue [8-15].
Ginseng
Ginseng Radix et Rhizoma (P ginseng CA Meyer) is air-dried to generate white ginseng (WG, known in China asRen Shen) or steamed at 100ºC to generate red ginseng (Hon Shen), both of which contain ginsenosides Rg1 and Rb1 (dried drug)at levels of at least 0.40% [16].
Traditional and current uses
Ginseng root has been traditionally used in China, Korea, and Japan for thousands of years “to tonify the original qi greatly, resume pulse and secure collapse, tonify [the] spleen and replenish [the kidneys], engender fluid and nourish blood, tranquilize the mind and replenish wisdom” [17]. The species name was derived from the Greek words “Pan“(meaning “all“) and “axos“ (meaning “cure“), allowing a translation of “cure-all“ or panacea. The herbal root was named “ginseng”to represents two Chinese ideograms: “gin“ (pronounced ren) refers to “man“ and “seng“ (pronounced shen) refers to “essence“ because it is shaped like a man. It is believed to embody man's three mythical essences: body, mind and spirit [18].
The first medical records on efficacy of Ginseng, also known as Korean ginseng, are dated 200 CE, and its use was later summarized (in 1596 and 1959) in Chinese Materia Medica. Ginseng has been used to promote vitality and healthy aging, to enhance cognitive function, reverse general weakness, improve blood circulation, and restore normal pulse in subjects with palpitations. According to ancient records, long-term ginseng uses increases longevity and enhances mental and visual performance. However, ginseng has been used primarily as a tonic to invigorate weak bodies and help restore homeostasis, a modern term associated with adaptive stress syndrome, adaptability and adaptogen concepts [7,15,19].
The effectiveness of ginseng has been recognized in the West since the 18th century. Ginseng has been used as a general tonic or adaptogen to promote longevity andtreat fatigue and mental stress. Based on its multiple health applications,including increased energy, improved cognition, enhanced immunity, reduced blood sugar levels, better circulation and improved sexual health in men, Korean red ginseng is often considered an all-encompassing remedy by herbalists. Ginseng is one of the most popular nutraceuticals, with annual sales exceeding USD 200 million [18].
According to WHO, the medicinal uses of Ginseng Radix et Rhizoma are divided into three categories [20]:
- • Uses supported by clinical data, such as prophylactic and restorative treatment for enhancing mental and physical capacities, improving weakness, exhaustion, tiredness and loss of concentration, and aiding convalescence
- • Uses described in pharmacopoeias and in traditional systems of medicine such as the treatment of impotence and prevention of hepatotoxicity and gastric ulcers.
- • Uses not supported by experimental or clinical data including the treatment of nervous and metabolic disorders, infectious diseases, and other conditions.
In Europe, most ginseng preparations are used as tonicsto treat tiredness, weakness, and decreased mental and physical capacity [21].
Active constituents
Most of the pharmacological actions of ginseng are attributed to one type of tetracyclic triterpenoid glycosides (saponins), namely ginsenosides. Nearly 300 saponins have been reported in Panax species [22]. More than 200 saponins have been isolated from ginseng, including the roots (processed and native), leaves, stems, flower buds, berries and seeds. To date, 112 saponins have been reported as components of P. ginseng, including ginsenosides, which have been isolated from raw or processed ginseng, hydrolysates and semisynthetic saponins [23]. Most ginsenosides share a dammarane triterpenoid structure (Figure 1).
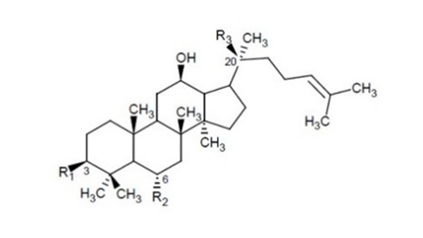
Figure 1: Chemical structures of ginsenosides in Korean red Panax ginseng preparation HRG80, and standard Korean white Panax ginseng preparation Arcopharma. R1, R2 and R3 = Glc–β-D-glucopyranosyl; Arap-α-L-arabinopyranosyl; Araf- α-L-arabinofuranosyl; Rha- α-L-rhamnopyranosyl, Table 1.
In total, 39 genuine ginsenosides have been identified and quantified in P. ginseng roots, leaves, stems, and berries using UPLC-QTOF/MS.The major ginsenosides in roots were M-Rb1 (0.47%), Rb1 (0.38%), Rf (0.46%), Ra2 (0.32%), Rg1 (0.31%), Re (0.3%), and Rgf (0.18%), whereas the content of other minor (rare) ginsenosides was less than 0.1% [24].
In our recent study, the content of rare ginsenosides in WG samples from Arkopham was 0.8%,compared with 12.43% in the hydroponically cultivated and processed preparation HRG80 (Figure 1 & Table 1).
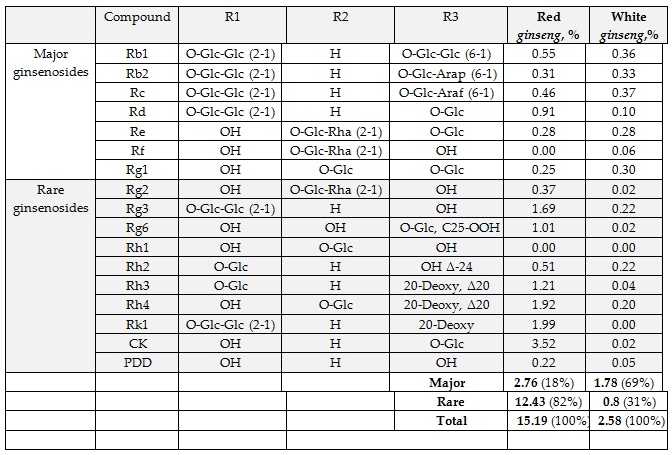
Table 1: Content (% of dry powdered root) in Korean red Panax ginseng preparation HRG80 and standard Korean white Panax ginseng preparation Arcopharma. R1, R2 and R3 = Glc–β-D-glucopyranosyl; Arap-α-L-arabinopyranosyl; Araf- α-L-arabinofuranosyl; Rha- α-L-rhamnopyranosyl.
The nomenclature of the ginsenosides (Ra, Rb, Rc, etc.,) is associated with their decreasing polarity on TLC plates, from Ra to Rf, which correlates with the number of sugar moieties in the molecules of ginsenosides reflecting the grade of glycosylation.
In addition to ginsenosides, many other biologically active compounds have also been identified in P. ginseng, such as polysaccharides (Panaxans, ginsenans), aliphatic C17-polyacetylenes (panaxynol [also known as falcarinol], panaxydol, panaxytriol [0.002%–0.02%]), glycosides (isomaltol-α-d-glucopyranoside, ketopropyl-α-d-glucopyranoside, adenosine), alkaloids (N9-formylharman, ethyl β-carboline, perlolyrine, 1-carbobutoxy-β-carboline, 1-carbomethoxy-β-carboline), phenolic acids (maltol, salicylic acid vanillic acid, p-hydroxycinnamic acid), thiazole, and lignans (gomisin N, gomisin A, gintonin [a complex of lysophosphatidic acid and ginseng proteins]). In addition, numerous volatile monoterpenes and sesquiterpenes (including citral, limonene, eremophilene and β-elemene), phenolics, triglycerides, fatty acids, sugars, starch, pectins, amino acids, peptides, proteins and minerals were identified in ginseng roots [21,25-28].
Bioavailability of ginsenosides and their metabolism
The pharmacokinetics of different active ginseng compounds has been studied in both animals and humans [21]. The bioavailability of ginsenosides is low after their oral administration; however, various ginsenosides have varying pharmacokinetic behavior [29,30]. The highly glycosylated ginsenosides Rb1, Rb2, Rc, Rd., Re, Rg1 and Rg2 have poor stability in the gastrointestinal tract, and they are easily converted into monoglycosidesand aglycone ginsenosides (e.g., CK, Rh2, Rh1, F1) by gastric acid and/or the intestinal flora [29-36].
After oral administration, the concentrations of ginsenosides are high in blood, but their absorption is low. The absorption profile of ginsenosides in the intestinal mucosa and the availability of both intact ginsenosides and their metabolites from the intestines are exceptionally low [29,30]. The maximal concentration of ginsenosides in plasma is reached within 2 h, suggesting that they are rapidly absorbed and distributed in tissues. Rg1, Re, Rb1 and Rc reach the brain, but their concentrations rapidly decline over time [29,37]. Rg1 and Re are more readily distributed in the brain, and they are considered the main components that directly affect central nervous system neurons [37]. The plasma level of ginsenosides indicates that protopanaxadiol ginsenosides have higher concentrations and longer half-livethan protopanaxatriol ginsenosides [29,37]. After the biotransformation of ginsenosides, microbiota in the gut produces deglycosylated products [38,39]. The intestinal bacteria isolated from human feces and some food-derived microorganisms as well as fungi from soil around ginseng roots convert glycosylated ginsenosides to compound K [31,40-42]. Compound K was the only ginsenoside detected in plasma and urine after the oral administration of Rb1 [43]. The deglycosylated products are better absorbed than ginsenosides based on their greater ability to permeate biological membranes [44].
It was concluded that ginsenoside metabolites contribute substantially to the pharmacological effects of ginseng [21].
Rare ginsenosides and hydroponically cultivated ginseng HRG80
The term “rare ginsenosides” has been applied to ginsenosides naturally occurring in minor amounts in ginseng. They are generated from major highly glycosylated ginsenosides during thermal processing as well as by intestinal microflora (gut bacteria) or gastric juice in vivo [18,29,32,35].
Rare ginsenosides are chemically different from major ginsenosides in various ways:
- • Stereo chemical configuration of C-20 (R) compared with that in major ginsenosides (S) [18,23]
- • Lack of a hydroxyl group at C-20 [35] and
- • Lack of a sugar moiety at C-20 or C-3 (less glycosylated) of the tetracycline skeleton [35].
Rare ginsenosides have better bioavailability and presumably greater biological activity than their precursors containing more than one sugar moiety [18,29,30,35]. Rare ginsenosides have a wide range of bioactivities. For instance, ginsenoside CK was proved to protect against myocardial infarction and inhibit angiogenesis and the proliferation of various cancer cells [35,45,46]. Ginsenoside F2 exhibited anti-tumor and anti-obesity activities [47,48]. The rare ginsenoside 20(R)-Rg3 displayed more potent adjuvant activity than the major ginsenoside 20(S)-Rg3 [49].
High rare ginsenoside levels can be obtained in ginseng preparations by converting major ginsenosides using various methods such as heating, mild acid hydrolysis, alkali treatment and microbial and enzymatic biotransformation [34,50].
According to EP, the content of ginsenoside saponins in wild growing roots normally ranges 0.6–3.0% [51]. The content of active ginsenosides in a representative sample of white P. ginseng was approximately 2.6% of the root powder content (Figure 1 and Table 1). Seven compounds (Rg1, Rg2, Re, Rf, Rb1, Rb2, Rc and Rd) represented the major ginsenosides (1.78% dry root), comprising 69% of the root ginsenosides. They have poor bioavailability, they but easily metabolized/hydrolyzed by intestinal bacteria into active ginsenosides with higher bioavailability (Rh2, Rg3, compound K, Rg6, Rk1, Rk2, Rh1), which can be obtained via traditional cooking methods for P. ginseng roots.
Red ginseng powder in HRG80 contains 15.2% total ginsenosides (approximately 6-fold higher levelthan that WG), including 12.43% [15-fold higher level than that in WG) rare ginsenosides (CK, Rk1, Rh2, Rh3, Rh4, Rg2, Rg3, Rg6; Table 1).
Cultivation of P. ginseng in culture, including tissue (e.g., meristem, leaf tissue), cell and organ cultures (e.g., roots, hairy roots), is a promising approach to increase the content of targeted secondary metabolites under controlled conditions [52]. It is presumed that this approach is most suitable and effective for good manufacturing practices [52,53]. Soilless cultivation of ginseng in hydroponic and aeroponic cultures is most appropriate because of several reasons, including the fact that this method is safe and free of pesticides and agrochemicals [54-57]. Parameters including the nutrient solution composition, pH, aeration, temperature, light and numerous stressful factors are crucial for optimal plant growth and development and the increased biosynthesis of pharmacologically active secondary plant metabolites that play important roles in plant defense responses and survival [54,55,58]. Choi et al., cultivated P. ginseng hydroponically and observed higher ginsenoside and phenolic content in its leaves [54].
High levels of ginsenosides in HRG80 preparations were achieved by cultivating roots in stressful conditions, under which the roots produce large amounts of plant secondary metabolites and ginsenosides to adapt and survive [10]. Substantially (15.6-fold) higher rare ginsenoside content in ginseng roots was obtained by steaming the roots at 100ºC, a traditionally used processing method [10]. Importantly, the HRG80 capsules contain 50% inactive carriers; therefore, the total content of rare ginsenosides in capsules was 6.2%, which was 7.8-fold higher than that in WG (Arkopharma) capsules used as a comparator in a study on human subjects [10].
Pharmacological activity of ginsenosides, neuroprotective effect
Many studies have examined the botanical, chemical, pharmacological, and therapeutic properties of ginseng extracts, fractions, and isolated compounds in animal models and in vitro, and more than 6000 articles have been published since the 1950s [23]. Effects on the nervous, cardiovascular, immune, endocrine, reproductive, and detoxification systems and skin have been demonstrated [19,21]. Such investigations revealed that ginsenosides are constituents with a broad set of pharmacological activities (e.g., cytoprotective effects, anti-inflammatory effects, anti-microbial effects, anti-cancer effects) in various cell culture models and animal experiments [21,24,59-61].
Numerous in vivo and in vitro studies of ginseng have suggested its useful effects against cardiovascular and neurodegenerative diseases, central nervous system disorders, immunodeficiency and cancer, as well as effects against aging-related diseases [19]. In general, anti-oxidant, anti-inflammatory, anti-apoptotic and immune-stimulatory defense responses primarily underlie ginseng-mediated protective mechanisms [24, 59-61].
Ginsenosides have been found to exhibit immunomodulatory, anti-cancer, anti-fatigue, anti-aging, anti-diabetic, anti-depressant like and neuroprotective effects [62-68]. The effects of ginsenosides Rb1, Rg1, Rg2, Rg3, Ro, Rb3, Rd, Rf, Rh2 and 20 (S)-PTon the nervous system and behavior of animals were demonstrated in numerous studies [21]. Rb1, Rd, Re, Rg1, Rg2, Rg3, Rh1, Rh2, Rh3, PF11 and NTR1, as well as gintonin and compound K, have displayed potential activity against cognitive deficits [29]. Some of these compounds modulate glutaminergic and cholinergic neurotransmission and various intracellular signaling pathways with important roles in the regulation of cognitive functions. For example, compound K inhibits mTOR signaling in astrocytes, suggesting that it may be useful for treating or preventing several age-associated conditions including neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [29]. Compound K exerts anti-inflammatory effects in microglia by inhibiting the generation of free radicals and MAPK-mediated signaling. Compound K prevents mitochondrial dysfunction and activates antioxidant signal in neurons, leading to neuroprotection and preventing neuronal cell death [29].
The major ginsenosides Rg1 and Rb1 increase neural plasticity; in particular, Rg1 increases the proliferation and differentiation of neural progenitor cells in the hippocampus of adult mice and ischemia models in gerbils. This finding suggests its great value in treating Alzheimer’s disease and other neurodegenerative disorders characterized by neuronal loss. Ginsenosides Rg3, Rg1 and Rb1 can prevent neurodegeneration by modulating several adaptive stress-response signaling pathways involved in the regulation of brain cell survival [66]. Ginsenosides Rb1 and Rg1 induce the secretion of brain-derived neurotrophic factor, which promotes the growth, differentiation and survival of neuronal cells by increasing intracellular cyclic AMP levels via protein kinase, thereby augmenting new synapse formation and signaling [67]. Rg1 and Rb1 also upregulate brain anti-oxidant enzymes and inhibit apoptosis and calcium overload, which are important for neuroprotection [66].
Clinical studies
Effects on cognitive functions and anti-fatigue activity
The results of clinical trials suggesting beneficial effects of ginseng on stress and cognitive functions were critically summarized in several comprehensive and systematic review articles [21,69-72]. The outcomes obtained in clinical studies assessing the effects of ginseng extracts on cognitive functions, physical performance, and fatigues are conflicting. Most studieswere hampered by poor methodology, a lack of proper controls, and the absence of extract standardization. A meta-analysis of 12 Randomized Controlled Trials (RCTs) involving 630 participants (311 participants in the intervention group and 319 participants in the placebo group) revealed a lack of sufficient clinical evidence supporting the use of ginseng supplements to reduce fatigue and enhance physical performance because only a few RCTs with small sample sizes have been published to date [13].
Ten trials with good methodology (Jahad scale>3 in five of the eight trials) evaluated the effects of WG or red ginseng on psychomotor function [73-77]. Overall, six positive and two negative results were obtained in these studies. The authors concluded that there was a strong evidence of efficacy concerning psychomotor function. The authors reported the anti-mental fatigue effects of P. ginseng extract (G115) based on improvements in cognitive performance and memory in healthy volunteers in serial clinical studies [78-82]. However, no significant difference in efficacy was found between ginseng and placebo [71,78].
The results of several studies suggested that Korean red ginseng improves cognitive function (performance) in patients with Alzheimer’s disease when applied at daily doses of 4.5and 9.0g for at least 12 weeks [73,74,83,84]. Maximalimprovement was found around week 24.
The same dose of Korean red ginseng effectively enhanced cognitive function ina study of 20 healthy young males who were randomly assigned to receive a daily dose of 4500 mg of red ginseng or placebo for 2 weeks [85]. The cognition-enhancing effect of Korean ginseng powder was observed in a larger group of 90 healthy volunteers with mild cognitive impairment who received 3 g of Korean ginseng powder or placebo for 6 months. The subjects treated with ginseng displayed significant improvementsof visual learning and visual recall after 6 months compared with the outcomes of placebo-treated subjects [86].
Altered mood, impaired memory and reduced concentration are the major symptoms of chronic fatigue-associated disorders. The efficacy of P.ginseng in the treatment of mental and chronic fatigue was demonstrated in several studies. Thus, the anti-fatigue effects of 1200 mg of P.ginseng in a double-blind, placebo-controlled, crossover study were evaluated in night nurses, and the results were compared with the findings in nurses engaged in daytime work [87]. Ginseng restored ratings on tests of mood, competence and general performance, and the study concluded that ginseng had anti-fatigue activity [87]. The anti-fatigue effects of 2000 mg of P.ginseng was demonstrated in another randomized, double-blind, placebo-controlled trial of 90 subjects with chronic fatigue [12].
The ability of red ginseng to decrease industrial (occupational) fatigue and improve neuro-mental symptoms was demonstrated in 43-65-year-old taxi drivers [88]. The drivers were treated with red ginseng or placebo before and after work and their mental fatigue was measured using the F value, which was calculated as the reaction of the brain to visual irritation. The F value decreases in proportion to the severity of mental fatigue and aging. Based on the results, the authors concluded that red ginseng increases the F value and improves many neuro-mental symptoms.
Overall, ginseng is considered a promising treatment for fatigue. Ginseng may be a viable treatment for fatigue in people with chronic illnesses [11].
STUDY OF HRG80 IN STRESS-INDUCED FATIGUE
The efficacy of two ginseng preparations containing approximately the same amounts of major ginsenosides but substantially different (7.8-fold) amounts of rare ginsenosides (Table 1 and Figure 2) was compared in 50 tired but otherwise healthy subjects in a three-arm, randomized, double-blinded, placebo-controlled crossover trial [10]. The aim of the study was to assess the efficacy of a hydroponically cultivated red P. ginseng root preparation (HRG80) and a traditionally harvested six-year-old white P. ginseng standard preparation (WG) in comparison with placebo in preventing symptoms of stress. The efficacy measures included the accuracy of processing in the d2 test for cognitive functions, the obtained accuracy score of a computerized memory test (learning, memory and attention), and the Perceived Stress (PS) score (Figures 3 & 4) [89-90].
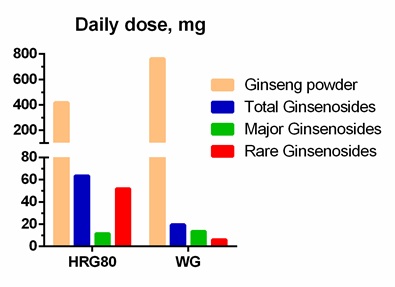
Figure 2: Daily doses of rare, major, and total ginsenosides in Korean red Panax ginseng preparation HRG80, and standard Korean white Panax ginseng preparation Arcopharma used in Marriage et al., study, 2020.
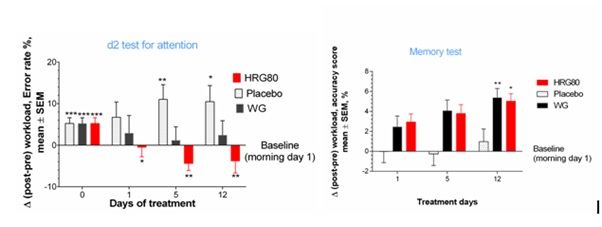
Figure 3: (a) Dynamic changes of post–pre-workload, accuracy score over the time. Changes in attention with time at Days 0,1,5 and 12 in placebo, ginseng HRG80 and ginseng control treatment groups. Between-group change comparison over time shows significant difference between ginseng HRG80 and ginseng control treatments (p< 0.0001, n = 50 in each group), ginseng HRG80 and placebo treatments, but no significant difference between positive and negative controls; (b) Changes of MT scores from baseline during whole study period; two-way ANOVA. Change in memory with time at Days 0,1,5 and 12 in placebo, ginseng HRG80 and ginseng control treatment groups. Between-group change comparison over time shows significant difference between ginseng HRG80 and placebo treatments (p< 0.0001, n = 50 in each group), ginseng control and placebo treatments, but no significant difference between two ginseng treatments. Significance of changes shown by symbols * p< 0.05, ** p< 0.01, *** p< 0.001l; ns, not significant.
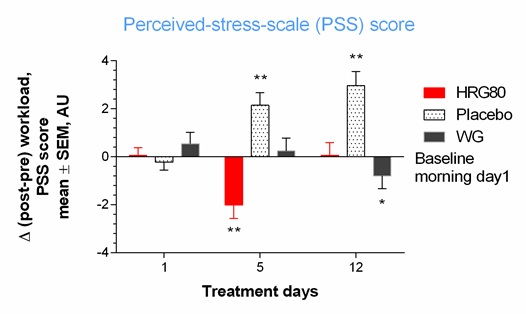
Figure 4: Change of total perceived-stress-scale (PSS) score from baseline during two weeks of treatment. Change in perceived stress with time at Days 0, 1, 5, and 12 in placebo, ginseng HRG80, and ginseng control treatment groups. Significance of changes shown by symbols * p< 0.05, ** p< 0.01, *** p< 0.001; ns, not significant. Between-group change comparison over time showed significant difference between ginseng HRG80 and ginseng control treatments (p = 0.0004, n = 50 in each group), ginseng HRG80, and placebo treatments, but no significant difference between positive and negative controls.
A statistically significant interaction effect between time and treatment (p < 0.0001) was observed in the attention d2 and memory tests, indicating that HRG80 was more beneficial than placebo. The effects of WG were better than those of placebo, but the difference was not statistically significant. HRG80 was associated with significantly better performance than WG (p < 0.0001) after both single (day 1) and repeated administration (days 5 and 12).
A statistically significant interaction effect between time and treatment was observed in the PS test, indicating that HRG80 was more beneficial than WG. Meanwhile, no difference in efficacy between HRG80 and WG was identified in the memory test. The number and types of adverse events were similar among the groups and no serious adverse events were observed.
STUDY HIGHLIGHTS
- • Red Korean ginseng is useful for ameliorating occupational stress-induced fatigue in healthy subjects.
Social stress often triggers acute mental fatigue and the onset of chronic fatigue syndrome as a result of the persistent accumulation of acute fatigue [3]. Mental fatigue is associated with decreased attention and reduced ability to concentrate, resulting in a failure to complete mental tasks, which could negatively affect work performance [3]. This is of primary importance for many healthy subjects involved in social networks, requiring regular computer use. Therefore, the participants of this study were individuals occupied in social services, including teleoperators, engineers and IT personnel who are permanently overloaded with cognitive tasks and exposed to social stress. The results of our study suggesting that ginseng HRG80 is a promising treatment for fatigue are in line with those of clinical studies of other adaptogenic plant extracts that exhibit anti-fatigue effects by increasing the mental performance and cognitive functions of healthy subjects and patients with chronic fatigue [8].
- • The primary objective of the study was to compare the efficacy and tolerability of the hydroponically cultivated red ginseng preparation HRG80 with placebo and WG as an active control with respect to effects on cognitive function, memory and stress-induced fatigue in healthy subjects.
- • HRG80 treatment exhibited significantly superior effects compared with those of WG and placebo regarding attention, memory and PS scores after single and repeated administration.
- • The effective therapeutic daily dose of ginseng HRG80 of 418 mg is at least 10-fold lower than the commonly used effective doses of red ginseng (4500-9000 mg per day).
- • A large body of evidence suggests that ginsenosidemetabolites contribute substantially to the pharmacological effects of ginseng [21]. These metabolites were found in minor amounts (rare ginsenosides) in WG but in larger amounts in steam-processed red ginseng. The results of a recently published clinical study provide evidence that rare ginsenosides significantly contribute to the overall efficacy of ginseng [10].
ACKNOWLEDGMENT
The authors would like to thank Enago (www.enago.com) for the English language review.
REFERENCES
- Watanabe Y, Kuratsune H (2006) Brain science on chronic fatigue. JMAJ 49: 19-26.
- Grandjean E (1979) Fatigue in industry. Br J Ind Med 36: 175-186.
- Mizuno K, Tanaka M, Yamaguti K, Kajimoto O, Kuratsune H, et al. (2011) Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav Brain Funct 7: 17.
- Lieberman HR (2001) The effects of ginseng, ephedrine, and caffeine on cognitive performance, mood and energy. Nutr Rev 59: 91-102.
- Higdon JV, Frei B (2006) Coffee and health: a review of recent human research. Crit Rev Food Sci 46: 101-123.
- Rogers PJ, Heatherley SV, Hayward RC, Seers HE, Hill J, et al. (2005) Effects of caffeine and caffeine withdrawal on mood and cognitive performance degraded by sleep restriction. Psychopharmacology (Berl) 179: 742-752.
- Panossian A (2017) Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann N Y Acad Sci 1401: 49-64.
- Panossian A, Wikman G (2009) Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol 4: 198-219.
- Dimpfel W, Schombert L, Keplinger-Dimpfel IK, Panossian A (2020) Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-Over Study. Pharmaceuticals (Basel) 13: 45.
- Mariage PA, Hovhannisyan A, Panossian AG (2020) Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals (Basel) 13: 57.
- Arring NM, Millstine D, Marks LA, Nail LM (2018) Ginseng as a Treatment for Fatigue: A Systematic Review. Altern Complement Med 24: 624-633.
- Kim HG, Cho JH, Yoo SR, Lee JS, Han JM, et al. (2013) Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial. PLoS One 8: 61271.
- Bach HV, Kim J, Myung SK, Cho YA (2016) Efficacy of Ginseng Supplements on Fatigue and Physical Performance: a Meta-analysis. J Korean Med Sci 31: 1879-1886.
- Inglis JE, Lin PJ, Kerns SL, Kleckner IR, Kleckner AS, et al. (2019) Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr Cancer 71: 21-40.
- Liao LY, He YF, Li L, Meng H, Dong YM, et al. (2018) A preliminary review of studies on adaptogens: comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin Med 13: 57.
- Ginseng radix (2008) In: European Pharmacopoeia. monograph.
- Pharmacopoeia of the People’s Republic of China (2010).
- Yue PY, Mak NK, Cheng YK, Leung KW, Ng TB, et al. (2007) Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin Med 2: 6.
- Radad K, Gille G, Liu L, Rausch WD (2006) Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 100: 175-186.
- Radix Ginseng (1999) In: WHO monographs on selected medicinal plants. World Health Organization 1: 168-182.
- EMA/HMPC/321232/2012 (2014) Assessment report on Panax ginseng C.A. Meyer, radix Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use).
- Yu L, Chen Y, Shi J, Wang R, Yang Y, et al. (2019) Biosynthesis of rare 20(R)-protopanaxadiol/protopanaxatriol type ginsenosides through Escherichia coli engineered with uridine diphosphate glycosyltransferase genes. J Ginseng Res 43: 116-124.
- Shin Byong-Kyu, Kwon SW, Park JH (2015) Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res 39: 287-298.
- Lee JW, Choi BR, Kim YC, Choi DJ, Lee YS, et al. (2017) Comprehensive Profiling and Quantification of Ginsenosides in the Root, Stem, Leaf, and Berry of Panax ginseng by UPLC-QTOF/MS. Molecules 22: 2147.
- Ru W, Wang D, Xu Y, He X, Sun YE, et al. (2015) Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.). Drug DiscovTher 9: 23-32.
- Choi SH, Jung SW, Lee BH, Kim HJ, Hwang SH, et al. (2015) Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol 6: 245.
- Kim HJ, Jung SW, Kim SY, Cho IH, Kim HC, et al. (2018) Panax ginseng as an adjuvant treatment for Alzheimer's disease. J Ginseng Res 42: 401-411.
- Im DS, Nah SY (2013) Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin 34: 1367-1373.
- Jakaria M, Haque ME, Kim J, Cho DY, Kim IS, et al. (2018) Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget 9: 33601-33620.
- Pan W, Xue B, Yang C, Miao L, Zhou L, et al. (2018) Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 129: 272-282.
- Zhou SS, Xu J, Zhu H, Wu J, Xu JD, et al. (2016) Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Rep 6: 22474.
- Xue P, Yao Y, Yang XS, Feng J, Ren GX (2017) Improved antimicrobial effect of ginseng extract by heat transformation. J Ginseng Res 41: 180-187.
- Shen H, Leung WI, Ruan JQ, Li SL, Lei JP, et al. (2013) Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin Med 8: 22.
- Zheng MM, Xu FX, Li YJ, Xi XZ, Cui XW, et al. (2017) Study on Transformation of Ginsenosides in Different Methods. Biomed Res Int 2017: 8601027.
- Quan K, Liu Q, Wan JY, Zhao YJ, Guo RZ, et al. (2015) Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci Rep.5:8598.
- Yu S, Zhou X, Li F, Xu C, Zheng F, et al. (2017) Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci Rep 7: 138.
- Zhang Y, Lin L, Liu GY, Liu JX, Li T (2014) Pharmacokinetics and brain distribution of ginsenosides after administration of sailuotong. Zhongguo Zhong Yao Za Zhi. 39:316–321.
- Liu H, Yang J, Du F, Gao X, Ma X, et al. (2009) Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug MetabDispos 37: 2290-2298.
- Lee J, Lee E, Kim DH, Lee J, Yoo J, et al. (2009) Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol 122: 143-148.
- Kim D-H (2018) Gut microbiota-mediated pharmacokinetics of ginseng sapon. J Ginseng Res 42: 255-263.
- Chi H, Kim DH, Ji GE (2005)Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol Pharm Bull. 28:2102-2105.
- Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett. 27: 765-771.
- Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K (1998) Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 50: 1155-1160.
- Hasegawa H, Uchiyama M (1998) Antimetastatic efficacy of orally administered ginsenoside Rb1 in dependence on intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Med 64: 696-700.
- Tsutsumi YM, Tsutsumi R, Mawatari K, Nakaya Y, Kinoshita M, et al. (2011) Compound K, a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/PI3K pathway. Life Sci 88: 725-729.
- Shin KO, Seo CH, Cho HH, Oh S, Hong SP, et al. (2014) Ginsenoside compound K inhibits angiogenesis via regulation of sphingosine kinase-1 in human umbilical vein endothelial cells. Arch Pharmacol Res 37: 1183-1192.
- Mai TT, Moon J, Song Y, Viet PQ, Phuc PV, et al. (2012) Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett 321: 144-153.
- Siraj FM, SathishKumar N, Kim YJ, Kim SY, Yang DC (2014) Ginsenoside F2 possesses anti-obesity activity via binding with PPARγ and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J EnzymInhib Med Ch 30: 9-14.
- Wei XJ, Chen J, Su F, Su XY, Hu TJ, et al. (2012) Stereospecificity of ginsenoside Rg3 in promotion of the immune response to ovalbumin in mice. Int Immunol 24: 465-471.
- Yang XD, Yang YY, Ouyang DS, Yang GP (2015) A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 100: 208-220.
- European Directorate for the Quality of Medicines & Health Care (2008) Ginseng radix. In European Pharmacopoeia, Monograph 01/2008:1523; European Directorate for the Quality of Medicines & Health Care: Strasburg, France.
- Adil M, Jeong BR (2018) In vitro cultivation of Panax ginsengA. Meyer. Ind Crops Prod 122: 239-251.
- Davies KM, Deroles SC (2014) Prospects for the use of plant cell cultures in food biotechnology. Curr Opin Biotechnol 26: 133-140.
- Choi SY, Cho CW, Lee Y, Kim SS, Lee SH, et al. (2012) Comparison of ginsenoside and phenolic ingredient contents in hydroponically-cultivated ginseng leaves, fruits, and roots. J. Ginseng Res 36: 425-429.
- Park KW, Yang DS (2002) Production of functional Korean ginseng by selenium supplement in hydroponic system. XXVI International Horticultural Congress: The Future for Medicinal and Aromatic Plants. 629: 307-311.
- Kim GS, Lee SE, Noh HJ, Kwon H, Lee SW, et al. (2012) Effects of natural bioactive products on the growth and ginsenoside contents of Panax ginseng cultured in an aeroponic system. J Ginseng Res 36: 430-441.
- Kim YJ, Lee OR, Kim KT, Yang DC (2012) High frequency of plant regeneration through cyclic secondary somatic embryogenesis in Panax ginseng. J Ginseng Res 36: 442-448.
- Hwang CR, Lee SH, Jang GY, Hwang IG, Kim HY, et al. (2014) Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J Ginseng Res 38: 180-186.
- Joo SS, Yoo YM, Ahn BW, Nam SY, Kim YB, et al. (2008) Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation.Biol Pharm Bull 31:1392-1396.
- Leung KW, Wong AS (2010) Pharmacology of ginsenosides: a literature review. Chin Med 5: 20.
- Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC (2018) Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res 42: 123-132.
- Chen XJ, Zhang XJ, Shui YM, Wan JB, Gao JL (2016) Anticancer activities of protopanaxadioland protopanaxatriol-type ginsenosides and their metabolites, Evid Based Complement Alternat Med 2016: 5738694.
- Dao-Tong HE, Wang B, Chen LM (2012) Research progress on pharmacological effects of ginsenoside. J Liaoning Univ Tradit Chin Med 14: 118-121.
- Ren Y, Wang JL, Zhang X, Wang H, Ye Y, et al. (2017) Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res Bull 134: 211-219.
- Ahmed T, Raza SH, Maryam A, Setzer WN, Braidy N, et al. (2016) Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res Bull 125: 30-43.
- Cheng Y, Shen LH, Zhang JT (2005) Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin 26: 143-149.
- Liang W, Ge S, Yang L, Yang M, Ye Z, et al. (2010) Ginsenosides Rb1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res 1357: 19-25.
- Ardah MT, Paleologou KE, Lv G, Menon SA, Abul Khair SB, et al. (2015) Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol Dis 74: 89-101.
- Geng J, Dong J, Ni H, Lee MS, Wu T, (2010) Ginseng for cognition. Cochrane Database Syst Rev 12: CD007769.
- Lee MS, Yang EJ, Kim JI, Ernst E (2009) Ginseng for cognitive function in Alzheimer's disease: a systematic review. J Alzheimers Dis 18: 339-344.
- Choi J, Kim TH, Choi TY, Lee MS (2013) Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One 8: 59978.
- Ong W-Y, Farooqui T, Koh H-L, Farooqui AA, Ling EA (2015) Protective effects of ginseng on neurological disorders. Frontiers in Aging Neuroscience 7: 129.
- Lee ST, Chu K, Sim JY, Heo JH, Kim M (2008) Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord 22: 222-226.
- Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, et al. (2008) An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur J Neurol 15: 865-868.
- Kennedy DO, Scholey AB, Wesnes KA (2001) Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutr Neurosci 4: 295-310.
- Kennedy DO, Scholey AB (2003) Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav 75: 687-700.
- Scholey AB, Kennedy DO (2002) Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol 17: 35-44.
- Kennedy DO, Reay JL, Scholey AB (2007) Effects of 8 weeks administration of Korean panax ginseng extract on the mood and cognitive performance of healthy individuals. J Ginseng Res 31: 34-43.
- Reay JL, Kennedy DO, Scholey AB (2005) Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol 19: 357-365.
- Reay JL, Kennedy DO, Scholey AB (2006) Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J Psychopharmacol 20: 771-781.
- Wesnes KA, Ward T, McGinty A, Petrini O (2000) The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle-aged volunteers. Psychopharmacology (Berl) 152: 353-361.
- Kennedy DO,Scholey AB (2003) Ginseng: Potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav 75: 687-700.
- Fu LM, Li JT (2011) A systematic review of single Chinese herbs for Alzheimer's disease treatment. Evid Based Complement Alternat Med 2011: 640284.
- Heo JH, Lee ST, Oh MJ, Park HJ, Shim JY, et al. (2011) Improvement of cognitive deficit in Alzheimer’s disease patients by long term treatment with korean red ginseng. J Ginseng Res 35: 457-461.
- Yeo HB, Yoon HK, Lee HJ, Kang SG, Jung KY, et al. (2012) Effects of Korean red ginseng on cognitive and motor function: a double-blind, randomized, placebo-controlled trial. J Ginseng Res 36: 190-197.
- Park K, Jin H, Rhee HY, Kim S, Lee SE, et al. (2013) A randomized, double-blind, placebo-controlled clinical trial of Korean ginseng as a functional food in mild cognitive impairment. Alzheimers Dement 9: 804.
- Hallstrom C, Fulder S, Carruthers M (1982) Effects of Ginseng on the Performance of Nurses on Night Duty. Comparative medicine East and West 6: 277-282.
- Kaneko H, Nakanishi K (2004) Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci 95:158-162.
- Steinborn MB, Langner R, Flehmig HC, Huestegge L (2018) Methodology of performance scoring in the d2 sustained-attention test: Cumulative-reliability functions and practical guidelines. Psychol Assess 30: 339-357.
- Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24: 385-396.
Citation: Lemerond T, Panossian AG (2020) Panax ginseng Meyer Herbal Preparation HRG80 for Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects. J Altern Complement Integr Med 6: 100.
Copyright: © 2020 Terrence Lemerond, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

