
Parathyroid Gland Disease: An Intricate Interaction Between Physiology, Endocrinology, Pathology and Surgery
*Corresponding Author(s):
Elroy Patrick WeledjiProfessor Of Surgery, Faculty Of Health Sciences, University Of Buea, Cameroon, West Africa
Tel:237699922144,
Email:elroypat@yahoo.co.uk
Abstract
Diseases are essentially disorders of physiological function. However, most patients with hyperparathyroidism are discovered incidentally during routine biochemical screening that includes a serum calcium determination. As patients with chronic renal failure now live longer as a result of dialysis and renal transplantation, secondary hyperparathyroidism is becoming more common. The management requires the understanding of the physiology of calcium homeostasis and implications in the human body. The aim of surgery is to remove the abnormal glands and leave the patient normocalcaemic. The familial multiple endocrine neoplasia (MEN) syndrome often present with parathyroid hyperplasia and screening by hormonal and biochemical analysis is mandatory once an index case has been identified.
Keywords
Calcium; Homeostasis; HyperParathyroidism; Multiple Endocrine Neoplasia; Parathyroid Hormone; Surgery; Vitamin D
Introduction
Many diseases are natural experiments which can be of great value in showing how variables and systems act upon each other. Physicians recognize and distinguish diseases by symptoms and signs which are further disorders of function revealed by close observation or elicited by testing routines. Calcium is the most abundant cation in the human body, there being about 25moles (1kg), in an average 70 kg man. Almost all of this calcium is within bone, which consists essentially of complex salts of calcium and phosphate. However, both calcium and phosphorus (as phosphate) have important extra-skeletal functions. Parathyroid gland disease is mostly manifested as hyperparathyroidism. The elucidation of the aetiology of hyperparathyroidism is based upon understanding the physiology of calcium (Ca 2+) homeostasis and its importance in the human body. The short, medium/long-term control of calcium ion in the body is by the peptide parathyroid hormone, (PTH) and the steroid hormone Vitamin D. Calcitonin, a peptide hormone secreted by the parafollicullar C- cells of the thyroid gland which acts on bone to reduce the release of Ca 2+ to the ECF during episodes of hypercalcaemia may be protective against such abnormal rises but it does not seem to play any significant role in normal Ca 2+ control. There are usually 4 parathyroid glands, occasionally 3 or 5, anatomically arranged in pairs. The superior pair on the posterior surface of the thyroid gland developed from the fourth branchial pouch is intricately related to the recurrent laryngeal nerve as it courses under the inferior thyroid artery, but the inferior pair close to the lower pole and together with the thymus develop from the third pharyngeal pouch is mostly variable in position (anterior mediastinum, the thymus (10%), within the carotid sheath and intracapsular thyroid itself) may explain the cause of the ‘failed neck exploration’ (Figure 1). PTH is normally secreted in response to a lowering of the serum ionized calcium (n: 2.2-2.7mmol/l) by mobilizing calcium from bone and by encouraging calcium resorption and phosphate excretion at the renal tubule to keep constant the solubility product of calcium phosphate in the extracelluar fluid (ECF) for its important physiological activities. It also hydroxylates the 1 α part of the inactive vitamin D3 to the active 1, 25-dihydroxycholecalciferol at the renal tubule [1]. Activated vitamin D acts by increasing calcium and phosphate absorption from the gut for the medium and long-term control of total body calcium especially in children, during pregnancy and lactation where there are additional requirements for calcium [2]. The importance of the tightly controlled free extracellular Ca2+ which is the biologically- active fraction is because several vital processes are dependent on the appropriate calcium concentration: a) neuromuscular excitability: with a fall in Ca2+, excitable tissues become easier to stimulate which may be fatal due to cardiac arrhythmias, laryngeal spasm and respiratory arrest. These also result from increased permeability to sodium ions and membrane potential depolarization. By the same mechanism high [Ca 2+] depresses nerve and muscle activity; b) excitation-contraction coupling: since contraction depends on an increase in intracellular [Ca 2+] any decrease may reduce the strength of contraction in cardiac and smooth muscle; c) stimulus- secretion coupling: the release of secretory substances such as hormones and neurotransmitters depends on Ca 2+ entry following stimulation of the cell, and d) blood clotting is calcium dependent [3].
Discussion
Thus, the pathological overproduction of PTH depending on the cause is divided into three groups: primary (most common) secondary and tertiary. Primary hyperparathyroidism (10 HPT) characterized by a persistently elevated calcium and a low serum phosphate must be due to an autonomous production of PTH i.e. parathyroid adenoma (85%), parathyroid gland hyperplasia (10%) or a parathyroid carcinoma (2%). These are not inhibited by the prevailing circulating high Ca2+ level. Although the clinical features of primary hyperparathyroidism are due to hypercalcaemia, most patients are diagnosed incidentally during routine biochemical screening that involves serum calcium determination with the highest incidence in postmenopausal women. Many patients present with a complex of symptoms caused by long-standing hyperparathyroidism including muscle weakness (myopathy) and arthralgia, peptic ulcer disease (increased gastrin secretion), abdominal pain and constipation, renal calculi, psychiatric disorders, hypertension and the rare corneal band keratopathy. This is summarized with the well known tetrad of ‘stones’, ‘bones’, ‘moans’ and ‘abdominal groans’. The diagnosis is confirmed with biochemical test as a high serum calcium level coupled with a low serum phosphate is highly suggestive. An elevated serum PTH level coupled with a raised urinary calcium excretion completes the diagnosis. However, a normal serum intact PTH in the setting of hypercalcaemia does not rule out the disease [4]. In long-standing disease several radiological changes occur including subperiosteal bone resorption in the phalanges, generalized skeletal demineralization (‘pepperpot’ skull, osteitis fibrosa cystic, etc), renal calculi or nephrocalcinosis. Distinction must be made between other causes of hypercalcaemia such as metastatic skeletal carcinomatosis, sarcoidosis, myeloma, milk-alkali syndrome, vitamin D intoxication, tuberculosis (TB), thiazide diuretics, Paget’s disease. Protein electrophoresis (myeloma), bone scan, skeletal survey, parathyroid hormone assays, urinary calcium excretion, the Kveim and Tuberculin tests, if indicated should exclude the other causes. In a fit patient, surgery is treatment of choice even when the disease is asymptomatic. Parathyroid exploration is, however, a demanding operation due to difficulty in gland location with risk of failed neck exploration, recurrent laryngeal nerve injury (Figure 1), and post-operative hypocalcaemia. A sestamibi (thallium-technitium) isotope scan is useful to localize the adenoma preoperatively and infusion of methylene blue (5mg/kg in 500ml of 5% dextrose) 1 h before operation extending into the time of surgery, allows rapid parathyroid identification. An attempt should be made to identify all 4 glands (red-brown in colour, split-pea-sized and tongue- shaped) best recognized in a bloodless field. An adenomatous gland is usually enlarged and the diagnosis may be confirmed by frozen section analysis (Figure 2). Multiple adenomas (5%) should be removed and the normal glands left undisturbed. If all four glands are hyperplastic, three- and - a- half glands are removed, marking the remaining gland as functioning parathyroid tissue with a black silk suture should re-exploration be required later. If the remaining gland is inadvertently devascularised, it can be portioned into about 12 pieces and autotransplanted into the sternomastoid or flexor compartment of the forearm. Occasionally, thymectomy with amputation of the upper and lower thyroid poles is required if not all the parathyroids are identified. Symptomless hypercalcaemic patients are treated with a three- and- a- half gland excision, if their serum calcium is greater than 3.0 mmol/l, to prevent the renal, vascular and bone complications such as femoral neck fractures in postmenopausal women with HRT. CT scanning or selective venous sampling with a parathormone assay may be required to localize a gland following a failed neck exploration [4]. In rare cases, the autosomal condition of familial hypocalciuric hypercalcaemia with a normal parathyroid gland is responsible for a failed neck dissection. This should be diagnosed pre-operatively by a low 24hour urinary calcium and a family history, with possibly a failed neck exploration in a parent/sibling [5]. After surgery, a careful check on the serum calcium must be maintained as the circulating calcium levels can drop precipitously and cause symptomatic hypocalcaemia. This sudden drop in calcium level after surgery is due to increased absorption of calcium by bones- hungry bone syndrome. This is a common (5-13%) type of hypoparathyroidism with decrease in calcium and phosphorus. Oral or intravenous calcium supplements may be needed. Prolonged hypocalcaemia is treated with vitamin D supplements or 1α-hydroxycholecalciferol. Pre-operative loading with 1 α-hydroxycholecalciferol in patients with bone disease helps to manage postoperative hypocalcaemia and mitigate the dangers of prolonged hypocalcaemia such as cataract formation. Parathyroid carcinoma is rare but recognized by gland firmness, grey colour, attachment to neighbouring structures and markedly elevated calcium levels. Recurrent laryngeal nerve palsy is a late manifestation. If suspected. A frozen section helps to confirm the diagnosis and an en bloc removal with ipsilateral hemithyroidetomy is performed [6]. Secondary hyperparathyroidism (20 HPT) is physiological compensatory hypertrophy of all four parathyroid glands due to hypocalcaemia (e.g. in renal failure or vitamin D deficiency). It develops as a result of some of the complex metabolic changes associated with chronic renal failure, which tend to produce a low serum calcium level and an elevated serum phosphate level. This is probably due to a defect in the activation of vitamin D in the kidneys due to chronic kidney disease (CKD). PTH levels are raised but calcium levels are low or normal. PTH levels fall to normal after correction of the cause of the hypocalcaemia.[7]. As patients now live longer as a result of dialysis and renal transplantation, secondary hyperparathyroidism (20 HPT) is becoming more common. Parathyroid surgeons thus often work in close liason with a renal unit because patients on dialysis provide a large population of the workload. Unlike primary hyperparathyroidism, the primary treatment is medical, aimed at reducing circulating serum phosphate levels and boosting the dietary intake of calcium. The condition usually resolves after renal transplantation [7]. Infrequently, long standing secondary hyperparathyroidism, most often in renal failure on dialysis may cause the parathyroid glands to develop an apparently autonomous parathyroid hyperplasia i.e., tertiary hyperparathyroidism (30 HPT). It is often only detected when patients develop a persistent hypercalcaemia after a successful renal transplantation. PTH is often grossly raised and subtotal parathyroidectomy is necessary at this stage [8].
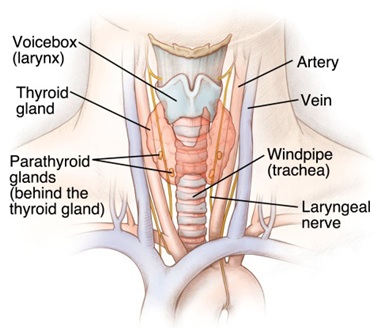 Figure 1: Schematic diagram of the topography of the parathyroid glands
Figure 1: Schematic diagram of the topography of the parathyroid glands
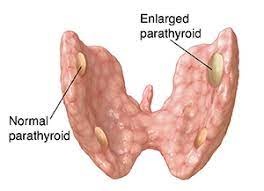 Figure 2: Schematic diagram of a parathyroid adenoma
Figure 2: Schematic diagram of a parathyroid adenoma
- Multiple Endocrine Neoplasia (MEN)
Patients with I0 HPT represent a population at increased risk for either of one of the two familial MEN syndromes (MEN1, MEN2). The rare MEN1 syndrome presents with tumours of the parathyroid glands, pancreatic islets and anterior pituitary and leads to premature death. All forms of MEN2 are autosomally inherited as a dominant gene and men and women are equally affected [9]. In MEN2a, there is familial occurrence of phaechromocytoma (frequently bilateral), medullary carcinoma of the thyroid (MCT) arising from the parafollicular C- cells and 10 HPT, although not all patients will develop all three abnormalities. The individual tumours may grow synchronously or metachronously and the clinical features are related to these functional adenomas or the mass in the abdomen or neck caused by the growing tumour. The pathogenesis of hyperparathyroidism remain unclear with hypercalcaemia and an elevated serum PTH level occurring in 10-25% of MEN2a patients [9]. The hypercalcaemia should always be excluded as a cause of recurrent, or complicated peptic ulcer disease [10]. Excision of the abnormal parathyroid gland is the main option in I0 HPT with an excellent prognosis [9]. MCT and phaechromocytomas are present in both MEN2a and MEN2b. The thyroid parafollicular cells and the adrenal medullary cells have a common embryological derivation from the neural crest. In some families only phaechromocytomas occur and in others only MCT but more often MCT precedes phaechromocytoma. MEN2b is a Scandinavian disease in which hyperparathyroidism is uncommon and in addition to phaechromocytoma and MCT, mucosal neuromas, a marfanoid habitus and sometimes ganglion neuromas in the gastrointestinal tract are added to the phenotype. It makes sense that serum gastrin, prolactin and calcitonin are also useful markers in patients with any clinical indications MEN syndromes [11]. Biphosphonates are effective osteoclasts inhibitors with 70-100% of patients becoming normocalcemic but duration of effects is usually several weeks and varies among patients and with the type of bisphosphonate [12]. The multiple endocrine neoplasia (MEN) syndromes have recently been reclassified into 4 groups: MEN1, MEN2 (formerly MEN 2a), MEN 3 (formerly MEN2b) and the recently identified MEN 4. MEN4 was first reported in 2006 and has limited data due to very small numbers of case reports [13]. They develop MEN1-associated tumours , but may have associated tumours of the kidneys, adrenals and reproductive organs. The phenotypic features of parathyroid neoplasia and pituitary adenomas tend to be smaller and less aggressive than MEN1- associated pituitary adenomas. Pancreatic neuroendocrine tumour () NET, including gastrinomas, have also been observed in MEN4 but with a decreased penetrance compared to MEN1 [13]. Understanding the genetics of each syndrome assist in determining screening time lines. Screening for these familial conditions by hormonal and biochemical analysis is mandatory once an index case been identified. Treatment for each manifestation are dependent on location, risk of recurrence or malignancy, hormone excess and surgical morbidity. Thus, treatment is complex and multidisciplinary management should include geneticists, genetic counselors, endocrinologists and endocrine surgeons [13].
Conclusions
Many diseases are natural experiments and thus the important corollary to the physician’s ability to recognize and evaluate the abnormal, and take intelligent action to prevent, compensate for, and cure departures from normality. The management of parathyroid gland disease requires the understanding of the physiology of calcium homeostasis and implications in the human body. The clinical manifestations of hyperparathyroidism may be non-specific and thus require a high level of clinical suspicion. The aim of surgery is to remove the abnormal glands and leave the patient normocalcaemic. Patients presenting with primary hyperparathyroidism also represent a population at increased risk of the MEN syndrome.
References
- Mihai R, Farndon JR (2000) Parathyroid disease and calcium metabolism. British J of Anaesthesia 85: 29-43.
- Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, et al. (2016) Vitamin D, calcium homeostasis and aging. Bone Res 4: 16041.
- Burgoyne RD, Helassa N, McCue HV, Haynes LP (2019) Calcium sensors in neuronal function and dysfunction. Cold Spring Harb Perspect Biol 11.
- Walker MD, Silverberg SJ (2018) Primary hyperparathyroidism. Nat Rev Endocrinol 14: 115-125.
- Jalilian R, Binazar MJ, Mirza L (2017) Familial hypocalciuric hypercalcaemia and benefits of genetic confirmation: a case report and review. AACE Clinical Case Reports 3: 361-363.
- Marcocci C, Cetani F, Rubin MR, Silverberg S, Pinchera A, et al. (2008) Parathyroid carcinoma. Bone Miner Res 23: 1869-1880.
- Habas Sr E, Eledrisi M, Khan F, Elzouki ANY (2021) Secondary hyperparathyroidism in chronic kidney disease: pathophysiology and management. Cureus 13: 163888.
- Palumbo VD, Palumbo VD, Damiano G, Messina M, Fazzotta S, et al. (2021) Tertiary hyperparathyroidism: a review. Clin Ter 172: 241-246.
- Raue F, Kramps JL, Dralle H, Cougard P, Proye C, et al. (1995) Primary hyperparathyroidism in multiple endocrine neoplasia type 2a. J Intern Med 238: 369-373.
- Weledji EP (2016) A rare presentation of multiple endocrine neoplasia (MEN) type 2A syndrome. Annals Med and Surg 5: 35-37.
- Fardon JR, Geraghty JM, Dilley WG, Handwerger S, Leight GS (1987) Serum gastrin, calcitonin and prolactin as markers of multiple neoplasia syndrome in patients with primary hyperparathyroidism. World J Surg 11: 253-257.
- Hamdy NAT, McClosekey EV, Kanis JA (1994) Role of bisphosphonates in the medical management of hyperparathyroidism. Acta Chir Aust 26: 6-7.
- McDonell JE, Gild ML, Clifto-Bligh RJ, Robinson BG (2019) Multiple endocrine neoplasia: an update. Internal Medicine Journal 49: 954-961.
Citation: Weledji EP (2023) Parathyroid Gland Disease: An Intricate Interaction Between Physiology, Endocrinology, Pathology and Surgery. Archiv Surg S Educ 5: 045.
Copyright: © 2023 Elroy Patrick Weledji, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

