
Parycalcitol + J131 - A New Route to Treatment Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Long-term Dialysis?
*Corresponding Author(s):
Radoslaw DrozdCentre For Nephrological Transplantation Regional Hospital In Szczecin, Szczecin, Poland
Tel: +48 501280927,
Email:adekpiotrdrozd@gmail.com
Abstract
Two cases with the dramatic course of secondary hyperparathyroidism in patients with chronic kidney disease on long-term dialysis. Has anything possible been done in management of these patients? Complications associated with impaired bone mineralization among patients with chronic kidney disease on long-term dialysis are observed frequently with an array of pathologic processes being found. Kidney osteodystrophy may be associated with either increased or decreased (adynamic bone disease, osteomalacia, aluminum-induced osteopenia) bone metabolism, as well as mixed forms related to the B2?microglobulin amyloidosis. Differential diagnosis of various types of osteopathy is difficult and is usually based on the histologic assessment of the bone biopsy. The most typical bone complication in patients with impaired kidney function is osetodistophy with increased bone metabolism, caused by secondary hyperparathyroidism clinically manifesting as osteitis fibrosa. High serum levels of PTH induce osteoclast and osteoblast activity. Early changes, with characteristic increase in the woven osteoid suggesting early, increased osteoplastic bone resorption may be found in a significant percentage of patients with GFR>60ml/min/1,73m2 of the body surface. Lower values of the GFR are associated with both faster bone synthesis and more active resorption with progressive increase in the intraosseous fibrosis and decreased bone mineralization. As the abnormalities progress, which is especially marked in patients on long-term dialysis, a rage of clinical symptoms, such as: severe bone and joint pain, bone deformation, pathological fractures, especially in the spinal region, calcifications of the soft tissues and vessels, including heart valves and lungs. In children, the most common abnormality is growth impairment. In some patient's skin calcifications, with subsequent necrosis, due to increased calcium deposition in small and medium arteries. The diagnosis is based on the typical clinical picture, biochemical parameters (calcium and phosphate ratio, parathormone levels, characteristic radiologic charges and sometimes, bone histology. Prevention and treatment of these complications includes effective dialysis, appropriate low-phosphate diet with limitation of the protein supply to the 0.8g/kg of the body mass, adequate calcium and active Vitamin D3 supply, introduction of the phosphate binding medications (sevelamer or lantan) as well as calcimimetic use (substances activating parathyroid gland calcium receptors inhibiting both its up-regulation and PTH secretion). In the severe cases, with insufficient effect of the treatment described above, parathyroidectomy is required after close ultrasound and scintigraphy-based assessment of these glands. However, even such treatment may be insufficient in some cases, as presented below.
CASE REPORTS
Here we would like to present two cases of the extremely advanced hyperparathyroidism with high bone turnover and dramatic course over the period of long-term follow-up [1]. In both, despite multidirectional treatment, the outcome of the therapy proved unsuccessful.
Case I
Patient M.H., born in 1951, under care of our center since February 1992, since the diagnosis of the chronic glomerulonephritis with nephrotic syndrome in the stadium of the kidney insufficient (creatinine levels of 1.8 mg/dL with normal calcium and phosphate levels). Kidney biopsy has never been performed in this patient, so no histologic data is available. In the treatment steroids and azathioprine was used, however without complete remission. Since 1993 in the treatment non-steroid anti-inflammatory drugs were introduced and angiotensin converting enzyme inhibitors - no improvement in the kidney function parameters was noted. Throughout the entire period of follow-up, the patient was treated with calcium carbonate and alphadiol. No monitoring of the parathyroid hormone levels was performed. In June 1997, in the routine abdominal ultrasound scan the tumor of the left kidney was found with subsequent nephrectomy.
In histopathology from the excised material two neoplasms were found to be present in the kidney: stadium I carcinoma clalocellulare and stadium II carcinoma papillare. After a surgery the clinical progression of the kidney insufficiency was observed (creatinine levels - 3mg/dlm GFR of 23ml/min). Patient's and mental condition worsened significantly, with notable mood disorder (depression), negative attitude to further diagnostics and treatment. In the late December 2000 the patient was re-admitted with the end-stage kidney failure with necessity for the urgent hemodialysis. When hemodialysed, the patient was largely non-adherent, with poor compliance to the dietary and water restrictions, often presenting with hypervolemia. Moreover, she did not consent for the kidney transplantation. In the treatment, except for the hypotensive drugs the calcium and vitamin D3 supplements were used (Alphadiol 0,25ug and Calperos 3g/day). In July 2003 the PTH levels were 856pg/ml. In the thyroid ultrasound the enlarged parathyroid gland in the area of the upper part of the left thyroid lobe (mean diameter of 6,3mm) was noted. Further diagnostics (scintigraphy) or treatment (surgery) was not performed, due to the lack of patient's consent. The wasting progressed, with the loss of 15kg of the body mass with pains in the region of the thoracic and lumbar spine. Patient's posture changed notably – kyphosis became apparent. The patient continued to object to the surgery of the parathyroid adenoma, moreover she refused in-hospital treatment for initiation of calcimimetics and Renagel. In February 2009, chest computed tomography was performed; in the scans of the lower part of the neck enlarged thyroid gland was seen. In the right thyroid lobe calcified masses of the 0.5 and 0.9cm diameter were recorded, while in the left lower the focal lesion of 0.6 cm was observed. In the left lung the nodule (0.4cm) was seen, with no enlarged lymph nodes in the pulmonary hilar regions or in the mediastinum. In the CT scan result multiple lesions in the ribs (distortions with regenerative reaction) which may be associated with the rib fractures. Additionally, lesions in the vertebral bodies (Th8-L1), described as possible post-osteoporotic fractures, were found.
Patient consistently refused to consent for the surgery of the parathyroid adenoma. In 2009 treated with Mimpara and Ranagel, but with no clinical or biochemical improvement (PTH above 3000pg/ml, phosphates within the normal range). The patient died in October 2009 due to the progressive heart and respiratory failure (Figure 1).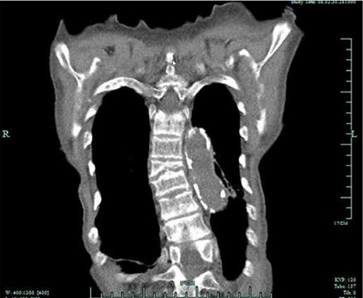 Figure 1: Chest skeletal system of fairst patients
Figure 1: Chest skeletal system of fairst patients
Case II
Patient S.L., female, born in 1953 with right kidney excised in 1959 due to the hydronephrosis with chronic left-sided pyelonephritis (1973) which progressed to the kidney failure ten years later. In 1985 the dialysis was introduced with kidney transplantation performed in May that year which functioned poorly and was removed in July of the same year. The hyperparathyroidism was observed during the follow-up, with thyroid gland resection and one-sided parathyroidectomy in February 1992 and later the same year the second parathyroidectomy. The excision was guided by the ultrasound scanning and the scintigraphy in both cases. In the early 90-ties we did not routinely assess the parathyroid hormone levels. In April 2002 PTH level was 2150pg/ml (the first result ever), with calcium and phosphate levels within the normal range. In the ultrasound scan of the neck, in the right thyroid stump the nodule, described as probable parathyroid gland, was found. This finding resulted in the next, third parathyroidectomy. It did not effect in the significant decrease of the PTH levels (500pg/ml). Osteoporosis was clinically apparent with pathological bilateral fracture of the femoral necks which necessitated implantation of the hip prostheses (firstly right-side, then the left one). At that time PTH levels were 1500pg/ml and increased to above 2000pg/ml in 2006. In the CT scan of the neck and chest the contrast enhancing nodule, sized 23x15 mm in the frontal mediastinum was observed, accompanied by the disseminated, characteristic for the hyperparathyroidism osteolytic lesion in the ribs, and the dense fluid in the sternoclavicular joints, more pronounced on the right. In scintigraphy the mediastinal tumor was confirmed and in June 2006 the patient was transferred to the department of Ge Transplant Surgery, Medical University, and Warsaw, where she underwent the excision of the lesion (fourth parathyroidectomy). After tumor excision the PTH levels dropped to almost 300pg/ml, however in September 2006 the levels of PTH rose again (above value of 1500pg/ml) with normal calcium and phosphate serum levels [2,3]. The patient underwent treatment with Mimpara 9300mg/day then 60 mg/day). In December 2006 the patient died due to progressive vascular-respiratory disorder in the mechanism similar to the patient described as the first case.
Two case histories of our patients indicate that despite full access to the modern medical care in some cases medics remain helpless in the respect of the inhibition of the progression of the secondary hyperparathyroidism in such patients.
Calcimimetics remain a fairly efficient treatment option, however its efficacy, despite often high does, is limited to PTH not exceeding 1000 pg/ml [4,5]. With higher levels of parathyroid hormone, the treatment of choice is parathyroidectomy. The clinical situation becomes even more complicated when the patient refuses to consent for the operation, or when the functions of the removed parathyroid glands are taken by the ectopic focuses (as noted in one of our patients). Theoretically we could consider if the co-administration of Cynacalcet with isotope (e.g. J131) would be helpful in such cases. Cynacacet, as the carrier with affinity to the calcium receptors, mainly in parathyroid glands, while J131 as the isotope inducing its involution. Such an association might be also used in other parathyroid pathologies (e.g. primary cancer of this gland).
The problem is also associated to the oral form of Cynacalcet which is available currently, as well as to the fact that calcium receptors are located also, however in minor quantities, in other tissues. This is the reason why at the moment such a co-administration is safe. However, it seems that the concept might be clinically tired, after adequate consent and cooperation with Amgen, which are the current owner and distributor of the Cynacalcet. In the last years another medication used for the prevention and treatment of the secondary parathyroid in patients with chronic kidney disease became available (parycalcytol - Zempler manufactured by Abbott) [6]. This drug is selectively activation the Vitamin D Receptors (VDR) in parathyroid glands, with no influence of the VDR in the gut [7,8]. It is used by intravenous injection; perhaps co administration of such a medication with J131 would be more real and beneficial for the patients, than j131-cynacalcet administration. You can block thyroid gland just before applying the paricalcitol J131 with e.g. Lugol's iodine. This would certainly require the adequate synthesis of the new co-formulation and a clinical trial (Figure 2).
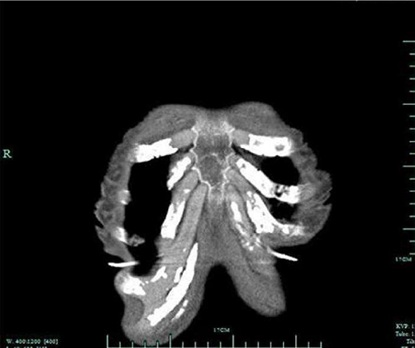 Figure 2: Second patient's thorax skeletal system.
Figure 2: Second patient's thorax skeletal system.
REFERENCES
- Brancaccio D, Cozzolina M, Cannela G, Messa P, Bonomini M, et al. (2011) Secondary hyperparathyroidism in chronic dialysis patients: results of the Italian FARO survey on treatment and mortality. Blood Purif 32: 124-132.
- Messa P, Macario F, Yaqoob M, Bouman K, Braun J, et al. (2008) The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 3: 36-45.
- Locatelli F, Cannata-Andia JB, Drueke TB, Horl WH, Fouque D, et al. (2002) Managment of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasuis on the control of hyperphosphatemia. Nephrology Dialysis Transplantation 17: 723-731.
- Maberti F, Saha H, Neyer U, et al. (2008) The Pan-European ECHO Study Investigator Group K/DOQI target achievement is improved with cinacalcet in clinical practice. ERA-EDTA XLV Congress, Stocholm, Sweden.
- Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, et al. (2006) Changes in serum calcium phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int 70: 351-357.
- Cunningham J, Locatelli F, Rodriguez M (2011) Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am SocNephrol 6: 913-921.
- Czekalski S, Oko A, Pawlaczyk K, et al. (2004) Przewlek?a niewydolno?? nerek. Aktualne metody hamowania progresji. Pol Arch Med Wewn CXI 97.
- Drueke T, Martin D, Rodriguez M (2007) Can calcimimeticsinhibt parathyroid hyperplasiphosa? Evidence from preclinical studes. Nephrol Dial Transplant 22: 1828.
Citation: Dziewanowski K, Drozd R (2019) Parycalcitol + J131 - A New Route to Treatment Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Long-term Dialysis? J Surg Curr Trend Innov 3: 021.
Copyright: © 2019 Krzysztof Dziewanowski , et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

