
Performance of the QuantiFERON-TB Gold In-Tube test to Monitor Treatment of Active Pulmonary Tuberculosis in Taiwan
*Corresponding Author(s):
Shou Chien ChenDepartment Of Family Medicine, Da-Chien General Hospital, Miaoli, Taiwan, Province Of China
Tel:+886 3735125,
Email:scchen818@yahoo.com.tw
Abstract
Background
Timely and effective monitoring of Tuberculosis (TB) treatment is an important strategy for prevention and control of TB. The aims of this study were to assess the performance of the QuantiFERON-TB Gold In-Tube (QFT-GIT) in diagnosis and monitoring response to anti-tuberculosis treatment in patients with active Pulmonary Tuberculosis (PTB).
Methods
We conducted a retrospective case-control study. Between March and September 2014, 28 cases with active PTB and 28 controls with no mycobacterial infection, matched by age within 3 years and week of visit to Taiwan Chest Hospital, were enrolled in the study. Serial testing by QFT-GIT at baseline and after 2 months of treatment was performed. A comparison of the performance of QFT-GIT with that of sputum culture status among study subjects was conducted.
Results
Compared to baseline, 25 (89%) cases had a decline in Interferon-gamma (IFN-γ) responses at 2 months culture-positive and the end of an intensive phase of anti-tuberculosis treatment, whereas three did not. Their IFN-γ responses declined significantly from baseline to 2 months (medium 2.11 vs. 0.88; P<0.005). The sensitivity of the QFT-GIT test for detection of pulmonary tuberculosis at cut-off points of 0.35 IU/ml, 0.20 IU/ml and 0.10 IU/ml was 71.4%, 78.6% and 82.1% respectively. The specificity at cut-off points of 0.35 IU/ml, 0.20 IU/ml and 0.10 IU/ml was 64.3%, 57.1%, and 53.6% respectively. The Positive Predictive Value (PPV) at cut-off points of 0.35 IU/ml, 0.20 IU/ml and 0.10 IU/ml was 66.7%, 64.7% and 63.9%, respectively.
Conclusion
Although our study indicates that QFT-GIT has moderate sensitivity and specificity, our results support the candidate of QFT-GIT assay as a potential tool for the diagnosis of tuberculosis and monitoring the efficacy of anti-tuberculosis treatment.
Keywords
INTRODUCTION
Tuberculosis (TB) is still an important public health problem throughout the world. In 2013, approximately 9.0 million people developed TB, with 1.5 million deaths [1]. However, early diagnosis of TB remains a complicated issue in the control and prevention of TB. Today, Tuberculin Skin Tests (TST) is one of the most commonly used methods for diagnosis of TB due to its low cost and convenience in most countries. However, there are several disadvantages to this method of TB diagnosis, such as poor specificity in people with Bacille Calmette-Guerin (BCG) vaccination and infection with Non-Tuberculous Mycobacteria (NTM), low sensitivity in immunocompromised persons and the requirement of two clinical visits to read the results [2,3]. Interferon gamma (IFN-γ) Release Assays (IGRAs) detect the ex vivo release of the key anti-tuberculosis cytokine, IFN-γ [3]. Previous studies have demonstrated that IGRAs may be an alternative for diagnosis of TB [4]. IGRAs include proteins that are more unique and specific to Mycobacterium tuberculosis (M. tuberculosis) than those in the Purified Derivative (PPD) and encoded by genes located in the Region of Difference 1 (RD 1) within the M. tuberculosis genome. These genes are not found in M. bovis BCG or most environmental Mycobacteria [5]. The QuantiFERON-TB Gold In-Tube test (QFT-GIT) assay (Cellestis, Carniege, Victoria, Australia) measures the IFN-γ concentration in whole blood after stimulation by specific tuberculosis antigens (e.g., Early Secreted Antigenic Target-6 [ESAT-6], Culture Filtrate Protein-10 [CFP- 10]) and TB7.7 antigen [6,7]. It is recognized as an efficient alternative test to detect the presence of Latent Mycobacterium Tuberculosis Infection (LTBI) [6-9]. Whether the QFT-GIT will be useful in monitoring responses to anti-tuberculosis treatment is unclear [7,10]. The potential prognostic use of IFN-γ responses has been studied in research describing Isoniazid (INH) treatment of LTBI and anti-tuberculosis treatment of active tuberculosis. In the case of LTBI, the prognostic use of IFN-γ is not yet clearly established. It has been reported that the IFN-γ responses after INH prophylaxis may be stronger [11], persistent [12], decreased [13], or dependent on the antigen used [14,15]. Similarly, in the treatment of active TB, some studies have observed post-treatment mitigation of the IFN-γ response [16-18], while others have reported persistent or even stronger IFN-γ responses after anti-tuberculosis treatment [19-22]. There is limited information regarding the effectiveness of the QFT-GIT test in Taiwan. The purpose of this study was to assess the performance of QFT-GIT test for diagnosis and monitoring in the treatment of active TB in Taiwan.
BACKGROUND
The study was conducted in the Tainan Chest Hospital of the Ministry of Welfare and Health in Taiwan. Tainan Chest Hospital provides respiratory disease services comprising voluntary counseling and testing, medical care and laboratory testing. More than 4,320 people with respiratory disorder visit this hospital each year. Of these, 300 (7%) were diagnosed with tuberculosis.
MATERIALS AND METHODS
Cases
Controls
Study procedures
Approval for the study was obtained from the Institutional Review Board for the Protection of Human Subjects at Tainan Chest Hospital and National Cheng Kung University Hospital, Taiwan.
Laboratory tests
Additional specimens from 10 participants were sent to the Taiwan Centers for Disease Control (Taiwan CDC) for laboratory test replication (QFT); agreement between the results from the Taiwan CDC and Tainan Municipal Hospital was good.
Statistical analysis
Definition
RESULTS
| Variables | Total | Cases | Controls | P-value* |
| N=56 | N=28 | N=28 | ||
| Age (Yr) (Mean±SD) | 59.3±15.7 | 56.4±16.0 | 61.9±15.4 | 0.19 |
| Sex | ||||
| Male | 38 | 18 | 20 | 0.64 |
| Female | 18 | 10 | 8 | |
| BCG vaccination | ||||
| Yes | 39 | 19 | 20 | 0.60 |
| No | 17 | 9 | 8 | |
| QFT-GIT test | ||||
| Positive | 34 | 20 | 10 | 0.007 |
| Negative | 22 | 8 | 18 | |
| Symptoms | ||||
| Cough | ||||
| Yes | 47 | 22 | 25 | 0.50 |
| No | 9 | 6 | 3 | |
| Loss of weight | ||||
| Yes | 26 | 14 | 12 | 0.61 |
| No | 30 | 14 | 16 | |
| Night sweats | ||||
| Yes | 5 | 3 | 2 | 0.64 |
| No | 51 | 25 | 26 | |
| Co-morbidities | ||||
| CVDs | ||||
| Yes | 5 | 3 | 2 | 0.66 |
| No | 51 | 25 | 26 | |
| Diabetes | ||||
| Yes | 14 | 11 | 3 | 0.02 |
| No | 42 | 17 | 25 | |
| Family history of TB | ||||
| Yes | 8 | 3 | 5 | 0.48 |
| No | 48 | 25 | 23 | |
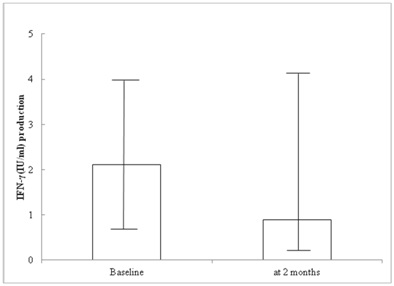
Figure 1: IFN-γ production using serial QTF-GIT assays (at baseline and 2 months after treatment initiation) in subjects with active tuberculosis on a standard regimen (n=28).
Performance of QFT-GIT test
| QFT-GIT Test Cut-Off Value (IU/ml) | Cases | Controls | Sensitivity | Specificity | PPV | |
| N=28 | N=28 | (%) | (%) | (%) | ||
| ?0.35 | + | 20 | 10 | 71.4 | 64.3 | 66.7 |
| - | 8 | 18 | ||||
| ?0.20 | + | 22 | 12 | 78.6 | 57.1 | 64.7 |
| - | 6 | 16 | ||||
| ?0.10 | + | 23 | 21 | 82.1 | 53.6 | 63.9 |
DISCUSSION
With the exception of studies from Japan [8], Korea [26] and India [27], most other published studies [28-32] have reported the QFT-GIT assay to have moderate sensitivity (61-81%). In this study, we had similar findings, with a sensitivity of 71.4% and specificity of 64.3% to detect active PTB identified at the baseline QFT-GIT assessment. These findings are important, as the accuracy of IFN-γ responses has not been unequivocally established for the diagnosis of active TB. The previous study [33] shows the QFT-GIT has better performance than TST for the diagnosis of the tuberculosis. However, neither of them is stable in the diagnosis of TB.
Serial testing by QFT-GIT demonstrated an overall progressive weakening of the IFN-γ response during anti-tuberculosis treatment, and QFT-GIT assessment after 2 months of treatment could be an independent and sensitive indicator of the likelihood of failing to convert sputum culture status. Our study showed 11% of study subjects were persistent IFN-γ at 2 months culture-positive at the end of anti-tuberculosis treatment. A previous study [27] suggested that nearly half of the study cohort was still positive by QFT-GIT after 6 months of anti-tuberculosis treatment. In this study, we did not have data to follow-up after 6 months of anti-tuberculosis treatment. There are several possible explanations why immune responses to Even Specific Antigens (ESAT-6 and CFP-10) may not have dropped below pre-defined levels, resulting in positive tests after anti-tuberculosis treatment: 1) T-cell responses to ESAT-6 may persistent as a scar of previously treated or quiescent infection [21]; 2) the anti-tuberculosis treatment may only have helped infection revert to a stage of latency rather than conferring sterilizing immunity [34]; 3) it has been argued that in some individuals, a population of activated T-cells persists in the absence of direct mycobacterial antigen stimulation, even for several years after completing treatment [22]; 4) It is possible that a continued exposure to M. tuberculosis during anti-tuberculosis treatment, especially as the environmental burden is high and 5) there is inter-individual variation in the strength of the IFN-γ response that can be partly explained by genetic polymorphisms in the host [35]. Although the IFN-γ level measured by QFT-GIT assay decreased after successful anti-TB treatment in most patients, any of them exhibited QFT-GIT reversion to negativity. Thus, the reversion to negativity of QFT-GIT assay may not be a good surrogate for treatment response. Of course, the short follow-up time can affect.
The accuracy of the QFT-GIT assay varied according to the cut-off point. A cut-off of 0.35 IU/ml for diagnosis of active TB had moderate sensitivity (71.4%) and specificity (64.3%). If the cut-off point was set at 0.20 IU/l, the sensitivity increased to 78.6%, but the specificity decreased to 57.1%. Similarly, if the cut-off point was at 0.10 IU/ml, the sensitivity increased to 82.1%; however, the specificity was 53.6%. Consequently, when using the QFT-GIT assay for monitoring response to treatment, it may be necessary to revise the cut-off to be prognostically meaningful. Future studies will need to address this issue more directly using larger numbers of patients treated for active TB.
There were several limitations in our study. First, the number of patients included in the study was small. However, this study provides important information regarding the role of QFT-GIT assays in the monitoring of active PTB treatment. Second, TST status may influence QFT-GIT results [36,37]. In our study, we did not evaluate the influence of TST status on the prognostic performance of QFT-GIT. Third, our study used mycobacterial culture as the gold standard for the diagnosis of TB. This methodology sometimes gives false negative results due to poor sputum sample collection or paucibacillary [38]. Therefore, our study may underestimate the performance of the QFT-GIT test for diagnosis of TB. Despite these limitations, our results support the utilization of the QFT-GIT assay as a potential tool to monitor the efficacy of anti-tuberculosis treatment in cases of active PTB.
In conclusion, our study indicates that QFT-GIT has moderate sensitivity and specificity; however, our results support the candidate of QFT-GIT assay as a potential tool for the diagnosis of tuberculosis and monitoring the efficacy of anti-tuberculosis treatment.
ACKNOWLEDGEMENT
We are grateful to the staff of Tainan Chest Hospital, Ministry of Welfare and Health, Taiwan, for their skilled interviewing and collection of study information.
REFERENCES
- World Health Organization (2014) Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland.
- Detjen AK, Keil T, Roll S, Hauer B, Mauch H, et al. (2007) Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis 45: 322-328.
- Davies PDO, Lalvani A, Thillai M, Gordon SB (2014) Clinical Tuberculosis (5th edn), Taylor & Francis Group, Abingdon, UK.
- Lu P, Chen X, Zhu LM, Yang HT (2016) Interferon-Gamma Release Assays for the Diagnosis of Tuberculosis: A Systematic Review and Meta-analysis. Lung 194: 447-458.
- Dilektasli AG, Erdem E, Durukan E, Eyübo?lu FÖ (2010) Is the T-cell-based interferon-gamma releasing assay feasible for diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country? Jpn J Infect Dis 63: 433-436.
- Carvalho AC, Pezzoli MC, El-Hamad I, Arce P, Bigoni S, et al. (2007) QuantiFERON-TB Gold test in the identification of latent tuberculosis infection in immigrants. J Infect 55: 164-168.
- Dheda K, Pooran A, Pai M, Miller RF, Lesley K, et al. (2007) Interpretation of Mycobacterium tuberculosis antigen-specific IFN-gamma release assays (T-SPOT.TB) and factors that may modulate test results. J Infect 55: 169-173.
- Kobashi Y, Obase Y, Fukuda M, Yoshida K, Miyashita N, et al. (2006) Clinical reevaluation of the QuantiFERON TB-2G test as a diagnostic method for differentiating active tuberculosis from nontuberculous mycobacteriosis. Clin Infect Dis 43: 1540-1546.
- Lalvani A (2007) Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 131: 1898-1906.
- Menzies D, Pai M, Comstock G (2007) Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 146: 340-354.
- Wilkinson KA, Kon OM, Newton SM, Meintjes G, Davidson RN, et al. (2006) Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis 193: 354-359.
- Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, et al. (2006) Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. J Occup Med Toxicol 1: 7.
- Higuchi K, Harada N, Mori T (2008) Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology 13: 468-472.
- Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, et al. (2007) Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am J Respir Crit Care Med 175: 282-287.
- Goletti D, Parracino MP, Butera O, Bizzoni F, Casetti R, et al. (2007) Isoniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: a pilot study. Respir Res 8: 5.
- Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, et al. (2001) Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosisinfection in healthy urban Indians. J Infect Dis 183: 469-477.
- Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, et al. (2004) Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis 38: 754-756.
- Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah D, et al. (2006) Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis 6: 66.
- Ulrichs T, Anding R, Kaufmann SH, Munk ME (2000) Numbers of IFN-γ-producing cells against ESAT-6 increase in tuberculosis patients during chemotherapy. The International Journal of Tuberculosis and Lung Disease 4: 1181-1183.
- Wu-Hsieh BA, Chen CK, Chang JH, Lai SY, Wu CH, et al. (2001) Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin Infect Dis 33: 1336-1340.
- Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, et al. (2007) Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am J Respir Crit Care Med 175: 282-287.
- Pai M, Joshi R, Bandyopadhyay M, Narang P, Dogra S, et al. (2007) Sensitivity of a whole-blood interferon-gamma assay among patients with pulmonary tuberculosis and variations in T-cell responses during anti-tuberculosis treatment. Infection 35: 98-103.
- Bjerrum S, Oliver-Commey J, Kenu E, Lartey M, Newman MJ, et al. (2016) Tuberculosis and non-tuberculous Mycobacteria among HIV-infected individuals in Ghana. Trop Med Int Health 21: 1181-1190.
- Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A (2008) Predictive value of a whole-blood IFN-gamma assay for the development of active TB disease. Am J Respir Crit Care Med 177: 1164-1170.
- Gerogianni I, Papala M, Klapsa D, Zinzaras E, Petinaki E, et al. (2008) Whole-blood interferon-gamma assay for the diagnosis of tuberculosis infection in an unselected Greek population. Respirology 13: 270-274.
- Kang YA, Lee HW, Hwang SS, Um SW, Han SK, et al. (2007) Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest 132: 959-965.
- Katiyar SK, Sampath A, Bihari S, Mamtani M, Kulkarni H (2008) Use of the QuantiFERON-TB Gold In-Tube test to monitor treatment efficacy in active pulmonary tuberculosis. Int J Tuberc Lung Dis 12: 1146-1152.
- Dewan PK, Grinsdale J, Kawamura LM (2007) Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis 44: 69-73.
- Goletti D, Carrara S, Vincenti D, Saltini C, Rizzi EB, et al. (2006) Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin Microbiol Infect 12: 544-550.
- Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, et al. (2011) Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 37: 100-111.
- Nishimura T, Hasegawa N, Mori M, Takebayashi T, Harada N, et al. (2008) Accuracy of an interferon-gamma release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis 12: 269-274.
- Vincenti D, Carrara S, Butera O, Bizzoni F, Casetti R, et al. (2007) Response to region of difference 1 (RD1) epitopes in human immunodeficiency virus (HIV)-infected individuals enrolled with suspected active tuberculosis: a pilot study. Clin Exp Immunol 150: 91-98.
- Lu P, Chen X, Zhu LM, Yang HT (2016) Interferon-Gamma Release Assays for the Diagnosis of Tuberculosis: A Systematic Review and Meta-analysis. Lung 194: 447-458.
- Nakielna EM, Cragg R, Grzybowski S (1975) Lifelong follow-up of inactive tuberculosis: its value and limitations. Am Rev Respir Dis 112: 765-772.
- Sallakci N, Coskun M, Berber Z, Gürkan F, Kocamaz H, et al. (2007) Interferon-gamma gene+874T-A polymorphism is associated with tuberculosis and gamma interferon response. Tuberculosis (Edinb) 87: 225-230.
- Igari H, Watanabe A, Sato T (2007) Booster phenomenon of QuantiFERON-TB Gold after prior intradermal PPD injection. Int J Tuberc Lung Dis 11: 788-791.
- Leyten EM, Prins C, Bossink AW, Thijsen S, Ottenhoff TH, et al. (2007) Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific interferon-gamma assay. Eur Respir J 29: 1212-1216.
- Ndzi EN, Nkenfou CN, Gwom LC, Fainguem N, Fokam J, et al. (2016) The pros and cons of the QuantiFERON test for the diagnosis of tuberculosis, prediction of disease progression, and treatment monitoring. International Journal of Mycobacteriology 5: 177-184.
Citation: Chen KT, Wang PH, Chien ST, Chen SC (2016) Performance of the QuantiFERON-TB Gold In-Tube test to Monitor Treatment of Active Pulmonary Tuberculosis in Taiwan. J Community Med Public Health Care 3: 022.
Copyright: © 2016 Kow Tong Chen, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

