
Pertuzumab and Trastuzumab Patch Testing In Acute Generalized Exanthematous Pustulosis (AGEP): A Case Report and Review of the Literature
*Corresponding Author(s):
Safae ChouraichiInova Schar Cancer Institute, Fairfax, Virginia, United States
Email:Safae.Chouraichi@inova.org
Introduction
We describe the use of pertuzumab and trastuzumab patch testing to determine the safety of rechallenging a patient with these medications after developing AGEP. The patient is a man in his 60s with HER2-amplified, RAS/RAF wild type and MMR-proficient metastatic sigmoid colon cancer, who developed facial erythema and oral ulceration two days after receiving treatment with atezolizumab infusion and pertuzumab/trastuzumab/hyaluronidase subcutaneous injection. By day six post-treatment, he developed diffuse erythematous papules coalescing into plaques on the trunk and extremities most prominent in a flexural distribution. Our patient had a negative patch test result for pertuzumab and trastuzumab, suggesting that the reaction was probably related to atezolizumab or hyaluronidase and ruling out pertuzumab and trastuzumab as causative agents.
Our report will be useful to oncologists and dermatologists who seek to determine causality in patients who develop cutaneous type IV mediated hypersensitivity reactions while receiving multiple drug therapy.
Background
Acute generalized exanthematous pustulosis (AGEP) is an acute pustular adverse drug reaction characterized by the rapid development of non-follicular, sterile pustules on an erythematous base, generally arising on the face and in intertriginous areas. While it is usually self-limited, AGEP can be associated with systemic symptoms including fever and leukocytosis. AGEP is most frequently attributed to antibiotics, in the majority of cases; however, other drugs have been implicated. The typical time interval between drug exposure and the onset of skin rash is approximately 48 hours [1]. Cutaneous patch testing is a testing modality used to isolate allergens that trigger allergic contact dermatitis, a type IV hypersensitivity reaction. As AGEP is also a type IV mediated hypersensitivity reaction, patch testing has been used to isolate the trigger of delayed drug hypersensitivity reactions including AGEP [2].
Case Report
A man in his 60s with HER2-amplified, RAS/RAF wild-type and MMR-proficient metastatic sigmoid colon cancer presented to the emergency department with severe dermatitis six days after receiving his first infusion of atezolizumab and pertuzumab/trastuzumab/hyaluronidase subcutaneous injection as part of a clinical trial. The patient was treated with prednisone 100 mg by mouth daily, which is the equivalent of 1 milligram per kilogram, with instructions to taper by 10 mg weekly. Soon after discharge, he was evaluated by dermatology and noted to have erythematous papules coalescing into plaques on the trunk and extremities most prominent in a flexural distribution (Figures 1-3). Punch biopsies were performed to further characterize the rash, which demonstrated sub corneal pustules consistent with acute generalized exanthematous pustulosis (AGEP) (Figure 4). Despite discontinuing anti-cancer therapy and the use of concurrent oral and topical steroids, his rash progressed and he reported decreased functional status, prompting hospital admission (Figure 5). While admitted he was noted to have leukocytosis with neutrophilia, acute kidney injury, elevated liver function tests, and hypocalcemia. Blood cultures were drawn and demonstrated Corynebacterium species. The patient was treated with methylprednisolone 125 mg intravenously once daily, and vancomycin intravenously with a trough level goal of 15-20 mcg/ml. On day three, the patient had significant clinical improvement with restored renal and hepatic function testing. The patient was discharged from the hospital on a steroid taper and a short course of oral doxycycline.
To determine if the patient could safely undergo treatment with an alternative anti-HER2 antibody therapy, we performed skin patch testing to rule out hypersensitivity to pertuzumab and trastuzumab. The patient required a slow prolonged corticosteroid taper to resolve his symptoms completely without exacerbations. The patch testing was performed thirteen days after the last corticosteroid dose.
Patch tests for commercialized form of trastuzumab and pertuzumab were performed by compounding a homogenous preparation of pertuzumab at a concentration of 30% w/w in petrolatum, and trastuzumab at a concentration of 30% w/w in petrolatum as described in published studies [3,4]. Testing with healthy controls was not feasible in a clinical setting; therefore, a literature review was conducted to ensure use of a non-irritant drug concentration in the patch testing. The drug products to be patch tested were placed on Finn chambers and fixed on the upper back. The patches were worn for a period of two days (48 hours), then removed and read, the first evaluation revealed no evidence of an initial cutaneous reaction. The final reading was performed on day four (96 hours after patch test application) and the results were read as negative.
- Investigations and Differential Diagnosis
Histopathology revealed prominent sub corneal neutrophilic pustules with a dermal lymphohistiocytic infiltrate, consistent with acute generalized exanthematous pustulosis.
Inpatient laboratory evaluation: WBC 23.43 x 103/µL, Absolute Neutrophil Count 21.21 x 103/µL, Creatinine 3.4 mg/dL, AST 47 U/L, ALT 58 U/L, Calcium 7.4 mg/dL.
The differential diagnosis in this case included Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), although the patient did not have significant fever, lymphadenopathy, or eosinophilia. Additionally, his RegiSCAR score was < 2, which made DRESS less likely [5]. The presence of sub corneal neutrophils on pathology could also be consistent with sub corneal neutrophilic dermatoses, such as pustular psoriasis or pemphigus foliaceous. However, the lack of a personal history of psoriasis, rapid onset, and recent drug exposure were consistent with AGEP.
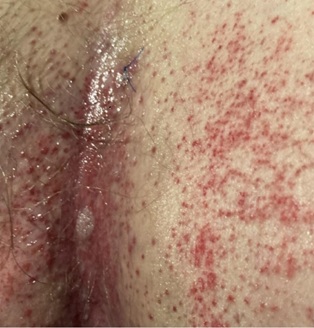 Figure 1: Non-blanching erythema of the inguinal crease with surrounding scattered erythematous papules and superficial well-demarcated erosions.
Figure 1: Non-blanching erythema of the inguinal crease with surrounding scattered erythematous papules and superficial well-demarcated erosions.
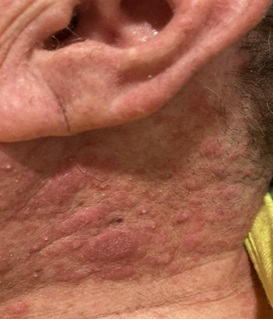 Figure 2: Left Lateral neck with erythematous papules and plaques intermixed with scattered non-follicular studded pustules.
Figure 2: Left Lateral neck with erythematous papules and plaques intermixed with scattered non-follicular studded pustules.
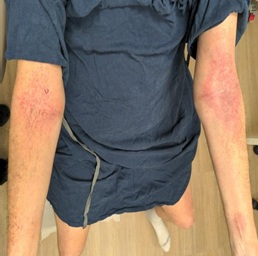 Figure 3: Bilateral antecubital fossae, day 16 after atezolizumab infusion and pertuzumab/trastuzumab/hyaluronidase subcutaneous injection with well-demarcated erythematous papules and background edematous erythema most concentrated in a flexural distribution.
Figure 3: Bilateral antecubital fossae, day 16 after atezolizumab infusion and pertuzumab/trastuzumab/hyaluronidase subcutaneous injection with well-demarcated erythematous papules and background edematous erythema most concentrated in a flexural distribution.
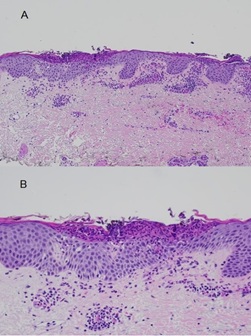 Figure 4: Histopathology sections stained by (H&E) at x 10 magnification (panel A) and x 40 magnification (panel B) illustrating a prominent sub corneal neutrophilic pustule with a dermal lymphohistiocytic infiltrate consistent with acute generalized exanthematous pustulosis.
Figure 4: Histopathology sections stained by (H&E) at x 10 magnification (panel A) and x 40 magnification (panel B) illustrating a prominent sub corneal neutrophilic pustule with a dermal lymphohistiocytic infiltrate consistent with acute generalized exanthematous pustulosis.
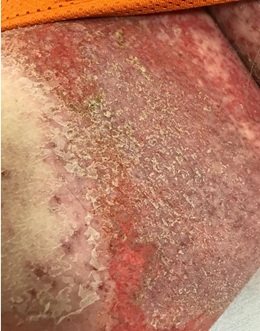 Figure 5: Inguinal crease, day 27 after atezolizumab infusion and pertuzumab/trastuzumab/hyaluronidase subcutaneous injection, beefy red edematous plaques with overlying desquamative asteatotic scale.
Figure 5: Inguinal crease, day 27 after atezolizumab infusion and pertuzumab/trastuzumab/hyaluronidase subcutaneous injection, beefy red edematous plaques with overlying desquamative asteatotic scale.
Discussion
We present the case of a patient who received treatment with combined atezolizumab and pertuzumab/trastuzumab/hyaluronidase and who subsequently developed drug induced AGEP.
AGEP is a rare type of severe cutaneous adverse reactions (SCARs). SCARs are a heterogeneous group of immunologically mediated delayed hypersensitivity reactions, which are more frequently triggered by drugs. Although rare, these events are potentially fatal. They consist predominantly of AGEP, Stevens-Johnson syndrome (SJS), Toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) [6].
The pathophysiology of AGEP has been investigated and several mechanisms have been proposed. Based on evidence from positive patch tests and lymphocyte transformation tests, AGEP is thought to be a type IV hypersensitivity reaction and involves CD4+T cells, CD8+ T cells, and inflammatory cytokines and chemokines. Drug-specific CD4+ and CD8+ T cells produce large amount of neutrophil chemoattractant interleukin-8 (IL-8). T helper 17 (Th17) cells may also play a role in the pathogenesis of AGEP through the recruitment, activation, and infiltration of neutrophils [7,8].
While the presentation, timing, and histopathology were consistent with AGEP, identifying the causal agent in this case represented a diagnostic challenge with important clinical ramifications. In most cases of AGEP, withdrawal of the responsible drug is the major therapeutic intervention. However, in this case, the culprit medication was likely one of his anti-cancer therapies; discontinuation of these agents had important implications with respect to his cancer treatment and overall prognosis. A case of AGEP secondary to atezolizumab was identified in the literature [9], and no cases of AGEP secondary to pertuzumab or trastazumab have been identified. Given the patient’s negative patch testing and successful re-challenge to pertuzumab and trastuzumab, we deduced that atezolizumab was the most likely causative agent. Although performing a patch test with atezolizumab could have provided definitive confirmation, it would have placed additional financial burden on the patient. Additionally, since the patient has been removed from the clinical trial, there was no clinical indication to test with atezolizumab, as the patient will not be able to receive it outside of the trial. Nonetheless, this limits the conclusiveness of our findings.
A review of safety data for atezolizumab and risk of SCARs was completed in Europe. As of 31 July 2020, a cumulative analysis in patients who received atezolizumab identified 99 cases of SCARs worldwide of which 36 cases were confirmed by histopathology or specialist diagnosis. Out of the reported cases, 35 cases were identified as erythema multiforme, 25 cases as SJS, 12 cases as toxic skin eruption, 8 cases as TEN, 7 cases as bullous dermatitis, 6 cases as generalized exfoliative dermatitis exfoliative, 4 cases as DRESS, and 2 cases as skin necrosis. The incidence rate of SCARs, regardless of severity, in company-sponsored clinical trials was 0.7% in atezolizumab monotherapy (N=3178) and 0.6% in combination therapy (N=4371) [10]. A rare case of histopathologically confirmed AGEP triggered by atezolizumab has also been reported in a patient with lung adenocarcinoma.
Re-challenging the patient with a suspected drug was not considered due to the possible recurrence of severe AGEP. Instead, we elected to use patch testing to restrict the potential development of a recurrent cutaneous reaction mimicking AGEP clinically and histologically to the patch test site, such as an acute localized exanthematous pustulosis [11]. However, rather than patch testing with atezolizumab, we elected to use trastuzumab and pertuzumab. A growing body of evidence supports the use of HER2-directed therapy in patients with HER2-overexpressing metastatic colon cancer [12,13]. Thus, we reasoned that a negative patch test with pertuzumab and trastuzumab would further implicate atezolizumab as the drug responsible for this patient’s AGEP and support retreatment with the HER2-directed regimen, which the patient resumed without rash recurrence.
Conclusion
Our case highlights the utility of patch testing for aiding in the determination of the causative agent in patients who develop cutaneous drug reactions including AGEP in response to multiple drug therapies.
References
- Szatkowski J, Schwartz RA (2015) Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 73: 843-848.
- Moreau A, Dompmartin A, Castel B, Remond B, Leroy D (1995) Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 34: 263-266.
- Legendre P, Bonnaure A, Bellay R, Lester MA, Boivin PN (2019) 3PC-055 Cytotoxic agents: skin tests for the diagnosis of drug hypersensitivity European Journal of Hospital Pharmacy. 26: A61-A62.
- Barbaud A, Gonçalo M, Bruynzeel D, Bircher, European Society of Contact Dermatitis (2001) Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 45: 321-328.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, et al. (2013) RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 169: 1071-1080.
- Adler NR, Aung AK, Ergen EN, Trubiano J, Goh MSY, et al. (2017) Recent advances in the understanding of severe cutaneous adverse reactions. Br J Dermatol. 177: 1234-1247.
- Kabashima R, Sugita K, Sawada Y, Hino R, Nakamura M, et al. (2011) Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J Eur Acad Dermatol Venereol. 25: 485-488.
- Tokura Y, Mori T, Hino R (2010) Psoriasis and other Th17-mediated skin diseases. J UOEH. 32: 317-328.
- Chang HC, Ko PH, Chang YS, Chou PC (2021) Acute generalized exanthematous pustulosis induced by atezolizumab in a patient with lung adenocarcinoma. Lung Cancer. 159: 175-176.
- Medicines and Healthcare products Regulatory Agency (UK) (2021) Atezolizumab (Tecentriq) and other immune-stimulatory anti-cancer drugs: risk of severe cutaneous adverse reactions (SCARs). Drug Safety Update. 14: 2.
- Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, et al. (2001) T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 107: 1433-1441.
- Parikh A, Atreya C, Korn WM, Venook AP (2017) Prolonged Response to HER2-Directed Therapy in a Patient with HER2-Amplified, Rapidly Progressive Metastatic Colorectal Cancer. J Natl Compr Canc Netw. 15: 3-8.
- Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, et al. (2019) Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 20: 518-530.
Citation: Chouraichi S, Wadlow R, Allais B, Ronkainen S (2023) Pertuzumab and Trastuzumab Patch Testing In Acute Generalized Exanthematous Pustulosis (AGEP): A Case Report and Review of the Literature. J Clin Stud Med Case Rep 10:207
Copyright: © 2023 Safae Chouraichi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

