
Pharmacognostical and HPTLC Study of Unani Formulation Khamira Khas: A Cardiac Tonic
*Corresponding Author(s):
Shoeb Ahmed AnsariDrug Standardization Research Institute, PLIM Campus, Kamala Nehru Nagar, Ghaziabad, India
Email:shomedplay@gmail.com
Abstract
Despite the enormous use of Khamira Khas as cardiac tonic in Unani system of medicine, there has been a deficit of scientific affirmation of its purity. Its magnificent benefits impel the commercial manufacturers to carry out itsproduction at large scale to meet the demand. Sometimes large production in short time leads to negligence of quality check of the product. Hence there is inevitable needtoadopt a systematic approach to develop well designed methodologies and accurate standardization of herbal formulation Khamira Khas. Various standardization parameters such as microscopic studies, physico-chemical investigation (extractive values, moisture content, ash values, pH values) and HPTLC fingerprinting of the drug were carried out to assess the quality of the formulation. Heavy metals, aflatoxins and microbial load were also determined in order to ascertain its quality assurance which consequently aid in developing the scientific standards for the formulation.
Keywords
Heavy metals; HPTLC; Microscopy; Physico-chemical parameters; Standardization
INTRODUCTION
Medicinal plants have been known for millennia and are highly esteemed all over the world as a rich source of therapeutic agents for the prevention of diseases and ailments [1]. Over 60% of the world population relies on the use of traditional medicine, which is predominantly based on plant material [2]. In the health service sector, WHO also endorses and boosts up the exploration of traditional drugs by virtue of their facile availability, accessibility and affordability. However, paucity of pertinent authentication, rigorous quality control, documentation and standardization has created the main hurdle in the establishment of alternative drugs in the developed nations. The government of India has formal structures to regulate quality, safety, efficacy, practice and documentation of herbal medicine (National Policy on Indian system of Medicine and homeopathy-2002) [3]. Standardization of herbal drug is not an easy assignment as various components such as temperature, geographical localities, period and time of collection, age and part of the plant collected, method of collection and various other factors affect the bio efficacy and reproducible therapeutic effect [4]. In the manufacturing of poly-herbal formulations, adulteration, contamination and substitution or total absence or replacement of costly ingredients are dangerous practices that eventually affect the quality of the formulations. This has emphasized the necessity for the meticulous quality control and standardization of herbal preparations through proper scientific testing and evaluation methods [5].
The word Khamira represents the fermented confection in the Unani system of medicine, first established by the physicians of Mughal period. Khamira is a semi-solid sugar based preparation which is formulated by pouring a decoction of powdered herbal drugs to sugar or sugar & honey based qiwam (syrup) at a proper temperature. Khamiras usually act as a cardio-tonic and are also used in various other ailments like palpitations, weakness of heart, weakness of principal organs, cough, cold, catarrh, including respiratory and nervous disorders[6-8].Some of the findings have also disclosed the antioxidant and immunomodulator properties of Khamiras [9].
MATERIALS AND METHODS
Preparation of the formulation
All the ingredients were procured from the local market and identified botanically using pharmacognostical methods [10-11].The ingredients were further validated by comparing with the monographs available in API, Part I, Vol. III [12]. The formulation Khamira Khas was prepared as per the formulation composition given in NFUM, Part VI using the ingredients showed in table 1 [13].
|
S.No. |
Unani Name |
Botanical/English Name |
Part used |
Form |
Qty. |
|
1. |
Khas Hindi |
Vetiveriazizanioides (L.) Nash. |
Root |
Powder |
1 Kg |
|
2. |
Arq-e-Khas |
Vetiveriazizanioides(L.) Nash. |
Whole plant |
Distillate |
1.5 L |
|
3. |
Qand Safaid |
Cane sugar |
Crystal |
As such |
9 Kg |
Table 1: Formulation Composition of Khamira Khas.
All the ingredients were taken of pharmacopoeial quality. The ingredient no.1 was cleaned and dried under shade to remove the moisture if any. It was ground to powder and sieved through mesh no.80. Ingredient no.2 & 3 were mixed together in a vessel and the content was boiled over a medium flame. At the boiling stage 2.5g of citric acid was added. The boiling of the content was continued until a qiwam (syrup) of 80% consistency was obtained. The vessel was removed from the flame and the ingredient no.1 was added immediately by thorough mixing. The mixture was vigorously beaten until it became homogenous and fluffy. Finally 2.5g sodium benzoate was added by thorough mixing. The content was allowed to cool at room temperature and the formulation so formed was stored in tightly closed glass container.
MICROSCOPY
2g of the drug was taken and stirred gently with hot water in a beaker. The supernatant was discarded and the process was repeated each time rejecting the supernatant and keeping the sediment until a clear or transparent solution was obtained. A little sediment was stained with iodine solution and mounted with 50% glycerin. A small amount of residue was treated separately with chloral hydrate solution, washed with distilled water and mounted in 50% glycerin. The various characters were observed under the microscope [10,14].
PHYSICO-CHEMICAL ANALYSIS
The physico-chemical parameters such as moisture content, water and ethanol extractive values, ash values, pH values were analyzed by standard methods [15,16].
HPTLC/ TLC FINGERPRINT ANALYSIS
Preparation of extract for HPTLC
After leaching out the sugar from the drug, 2g sample was extracted with 20ml of ethanol by refluxing for 20 minutes. The extract was filtered through Whatman No.1 filter paper. The filtrate was further concentrated up to 10 ml and used for HPTLC analysis.
HPTLC fingerprinting was carried out by applying the ethanol extraction aluminum TLC plate pre-coated with silica gel 60 F254 (E. Merck) using Desaga AS30 automatic sample applicator. The plate was developed up to a distance of 8cm in a twin trough glass chamber (10x10cm) using the solvent system of toluene: ethyl acetate (9: 1) as mobile phase. The plate was air dried at room temperature and observed underUV at 254nm & UV 366nm wavelengths. Later on the plate was derivatized by dipping it in 1% vanillin-sulphuric acid reagent followed by heating at 105°C till the colored bands appeared [17,18].
Estimation of Microbial Load
The microbial load viz. total bacterial count (TBC), total fungal count (TFC), Enterobacteriaceae, Escherichia coli, Salmonella spp., Staphylococcus aureus and Pseudomonas aeruginosa were estimated as per standard methods [19].
Estimation of Heavy Metals
The analysis of heavy metals like lead, cadmium, mercury and arsenic was carried out as per standardmethods [19-20].
Details of the Instrument and operating parameters
Atomic Absorption Spectrophotometer (AAS) model LABINDIA AA7000 was used for heavy metals analysis. The operating parameters were as follows:
Lead and Cadmium: Instrument technique - Flame atomization; wavelength (Lead) - 217 nm; wavelength (Cadmium) - 228.8 nm; slit width - 0. 5 mm; lamp current (Pb) - 4.0 mA; lamp current (Cd) - 3.0 mA; carrier gas and flow rate - air and acetylene, 1.1 L/min; sample flow rate - 2 ml/min. Mercury: Instrument technique - Cold vapour technique; wavelength - 253.7 nm; slit width - 0. 5 mm; lamp current - 3.0 mA; carrier gas and flow rate - argon, 1.1 L/min; sample flow rate - 5ml/min. Arsenic: Instrument technique - Cold vapour technique; wavelength - 193.7 nm; slit width - 0. 5 mm; lamp current - 6.0 mA; carrier gas and flow rate - acetylene, argon, 1.1 L/min; sample flow rate - 5ml/min. The hallow cathode lamps for Pb, Cd, Hg and As analysis were used as light source to provide specific wavelength for the elements to be detected.
Analysis of Aflatoxins
Aflatoxins B1, B2, G1 and G2 were analyzed as per Official Analytical Methods of the American Spice Trade Association (ASTA), 1997 [21].
Details of Instrument and operating parameters
High Performance Liquid Chromatography (Thermo Fisher) was used for the analysis of aflatoxins. Column - Ultra C18, 250 X 4.6 mm, 5 µm particles; Mobile phase: Water: Acetonitrile: Methanol (65: 22.5: 22.5); Flow rate: 1 ml/min; Temperature: 35?; Detector: Fluorescence detector at 360 nm; Injection: 20 µl (Aflatoxins mixture and sample)
Analysis of pesticide residue
The analysis of pesticide residues was carried out as per the method described in AOAC, 2005 [20]. Pesticide residues were analyzed by employing Gas Chromatography-Mass Spectrometry (GC-MS) (Instrument-Agilent, detector-mass selective detector, column specification-DB5MS, carrier gas - helium, flow rate - 1ml/min, column length - 30 m, internal diameter - 0.25 mm, column thickness - 0.25 μm).
RESULTS AND DISCUSSION
Khamira Khas is a light brown semi-solid drug with pleasant aromatic smell and sweet taste.
Identification
Microscopy: Microscopy of khamira khas showed anatomical markers such as thin walled parenchyma cells (non lignified) and hexagonal parenchyma cells, tuft of fibre cells and fibre cells with broad lumen, pitted and reticulate vessel, large rounded parenchyma cell, large and thick walled sclerenchyma, sieve tube element, elongated cells with yellowish oil globule, cell filled with oil globules, druse crystals and starch granules, sclerenchyma sclereids (thick walled) with group of sclereids cells (Khas Hindi) (Figure 1).
 Figure I: Microscopy of Khamira Khas.
Figure I: Microscopy of Khamira Khas.
Physico-chemical parameters
The results of physico-chemical parameters of Khamira Khas (five samples) are tabulated in table 2. Quantitatively evaluated data revealed that the moisture content of the drug ranged between 9.02 - 9.76 % which is apt in case of Khamiras. Total ash content was not more than 0.70 % and acid insoluble ash remained in traces which indicated that siliceous matter was present in the drug samples in negligible amount. The water extractive valuesturned out to be on higher end, ranged between 82.15 - 82.88 % which indicated the presence of inorganic and more polar organic content and the ethanol soluble extractive values were also high and ranged between 71.20 - 72.35 % which indicated the extraction of polar constituents.
|
Parameters |
Results |
|
Ethanol extractive matter (w/w %) |
71.20 - 72.35 |
|
Water extractive matter (w/w %) |
82.15 - 82.88 |
|
Loss in weight on drying at 1050C (w/w %) |
9.02 - 9.76 |
|
Total ash (w/w %) |
0.62 - 0.70 |
|
Acid insoluble ash (w/w %) |
Traces |
|
pH of 1% aqueous solution |
6.30 - 6.42 |
|
pH of 10% aqueous solution |
5.25 - 5.36 |
|
Bulk density |
1.4322 - 1.4540 |
|
Reducing sugar (w/w %) |
52.45 - 53.55 |
|
Non-reducing sugar (w/w%) |
17.70 – 18.65 |
Table 2: Physico- chemical Parameters.
HPTLC studies of ethanol extract: The HPTLC data of ethanol extract is tabulated in table 3. The drug sample showed 02 bands under UV254nm and 05 major fluorescent band sunder UV 366nm. After derivatization of the plate with 1% vanillin - sulphuric acid reagent, 06 major colored bands were observed under visible light. (Figure 2).
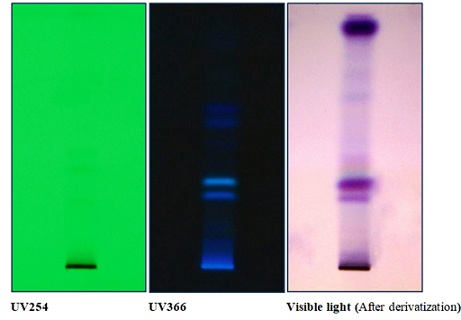 Figure 2: HPTLC of Khamira Khas.
Figure 2: HPTLC of Khamira Khas.
|
Solvent System |
Rf Values |
||
|
UV 254 nm |
UV366 nm |
Visible light (After derivatization with 1% vanillin-sulphuric acid reagent) |
|
|
Toluene: Ethyl acetate (9:1) |
0.26 green |
0.27 blue |
0.25 violet |
|
0.38 green |
0.32 turquoise |
0.30 violet |
|
|
|
0.47 blue |
0.33 cyan |
|
|
|
0.54 blue |
0.35 pink |
|
|
|
0.60 bue |
0.63 bluish purple |
|
|
|
|
0.88 violet |
|
Table 3: Rf values of ethanol extract.
Evaluation of quality (WHO) parameters
In order to assess the quality of drug samples, the microbial loads viz. Total Bacterial Count (TBC), Total Fungal Count (TFC), Enterobacteriaceae, Escherichia coli, Salmonella spp., Staphylococcus aureus and Pseudomonas aeruginosa were analyzed and found to be in permissible limit (Table 4). The heavy metals lead, cadmium, mercury and arsenic were found to be below detection Limit (LOD) in the drug samples (Table 5). Moreover, aflatoxins B1, B2, G1 and G2 were below the Limit of Quantification (LOQ). Similarly pesticide residues such as organo chlorine group, organo phosphorus group, alachlor, aldrin, chlordane, DDT, endosulfan, heptachlor, lindanewere also below the Limit of Quantification (LOQ). The study indicates that the drug samples were free from heavy metals, aflatoxin and pesticide contaminations (Table 6).
|
Parameters |
Results |
WHO Limits for internal use |
|
Total Bacterial Count (TBC) |
4x 103cfu/g |
1x105cfu/g |
|
Total Fungal Count (TFC) |
1x103cfu/g |
|
|
Enterobacteriaceae |
Absent |
NIL |
|
Escherichia coli |
Absent |
NIL |
|
Salmonella spp. |
Absent |
NIL |
|
Staphylococcus aureus |
Absent |
NIL |
|
Pseudomonas aeruginosa |
Absent |
NIL |
Table 4: Microbial Load.
|
Sl. No |
Element |
Values |
WHO Limits for internal use |
|
1. |
Lead |
<LOD |
10 ppm |
|
2. |
Cadmium |
0.3 ppm |
|
|
3. |
Arsenic |
3.0 ppm |
|
|
4. |
Mercury |
1.0 ppm |
Table 5: Analysis of Heavy Metals.
|
S. No. |
Parameter analyzed |
Results |
|
1. |
Alachlor |
< LOQ |
|
2. |
Aldrin (Aldrin and dieldrin combined expressed as dieldrin) |
|
|
3. |
Azinophos-methyl |
|
|
4. |
Bromopropylate |
|
|
5. |
Chlordane (cis, tans and oxychlordane) |
|
|
6. |
Chlorfenvinphos |
|
|
7. |
Chlorpyrifos |
|
|
8. |
Chlorpyrifos-methyl |
|
|
9. |
Cypermethrin (and isomers) |
|
|
10. |
DDT (all isomers, sum of p,p’-TDE (DDD) expressed as DDT) |
|
|
11. |
Deltamethrin |
|
|
12. |
Diazion |
|
|
13. |
Dichlorvos |
|
|
14. |
Dithiocarbamates (as CS2) |
|
|
15. |
Endosulphan (sum of isomers &Endosulphansulphate) |
|
|
16. |
Endrin |
|
|
17. |
Ethion |
|
|
18. |
Fenitrothion |
|
|
19. |
Fenvalerate |
|
|
20. |
Fonofos |
|
|
21. |
Heptachlor(sum of Heptachlor & Heptachlor epoxide) |
|
|
22. |
Hexachlorobenzene |
|
|
23. |
Hexachlorocyclohexane isomer (other than γ) |
|
|
24. |
Lindane (γ – Hexachlorocyclohexane) |
|
|
25. |
Malathion |
|
|
26. |
Methidathion |
|
|
27. |
Parathion |
|
|
28. |
Parathion methyl |
|
|
29. |
Permethrin |
|
|
30. |
Phosalone |
|
|
31. |
Piperonylbutoxide |
|
|
32. |
Pirimiphos methyl |
|
|
33. |
Pyrethrins (sum of isomers) |
|
|
34. |
Quintozen (sum of Quintozene, pentachloroaniline and methyl pentachlorophenylsulphide) |
Table 6: Pesticide residues.
SUMMARY AND CONCLUSION
Standardization of the traditional medicines is essential to maintain their purity and efficacy. Thus, in modern era more attention and stress have been given on the standardization of Unani formulations. The HPTLC fingerprinting and evaluated pharmacognostical&physico-chemical parameters of Khamira Khas will be productive for fixing pharmacopoeial standards of the drug. All the quality parameters were found to be in permissible limits which indicate that Khamira Khasis free from toxic materials and is safe for human consumption. Hence, the present study holds high significance to ensure authenticity and quality of the drug.
ACKNOWLEDGEMENT
The authors are extremely thankful to the Director-General, CCRUM, New Delhi for their valuable guidance, encouragement and providing necessary research facilities to carry out the studies.
REFERENCES
- Sharma A, Shanker C, Tyagi LK, Singh M, Rao CV (2008) Herbal medicine for market potential in India: An overview. Acad J Plant Sci 1: 26?
- World Health Organization (2019) WHO global report on traditional and complementary medicine. World Health Organization, Geneva, Switzerland.
- Singh B, Kumar M, Singh A (2013) Evaluation of implementation status of national policy on Indian systems of medicine and homeopathy 2002: Stakeholders’ perspective. Ancient Science of Life 33: 103-108.
- Kumari R, Kotecha M (2016) A review on the standardization of herbal medicines. International Journal of Pharma Sciences and Research 7: 97-106.
- World Health Organization (1998) Quality control methods for medicinal plant materials. World Health Organization, Geneva, Switzerland.
- Rehman HZ (1991) Kitabul Murakkebat. Aligarh, Litho Colour Prints:65.
- KabiruddinM (1967) Bayaz-e-Kabir. Delhi, Dafter-ul-Masehi; 2: 55-67.
- Khan GJ (1995) Makhazanul Murakkebat. Delhi, Ajaz Publication House: 104-110.
- Alam MA, Ahmed Z, Ansari AH, Ahmed S, Mand D, et al (2014) Iksir-e-Badan (Elixir): Unique influence from Unani medicine – A Review. International Journal of Herbal Medicine 1: 40.
- Wallis TE (2005) Textbook of pharmacognosy. (5thedn). CBS Publishers & Distributors Pvt. Ltd., New Delhi, India.
- Trease GE, Evans WC (1989) Pharmacognosy. (13thedn). Baillière Tindall, London, UK.
- Ministry of Health & Family Welfare, Govt. of India (2001) Ayurvedic pharmacopoeia of India. Ministry of Health & Family Welfare, Govt. of India.
- Ministry of Health & Family Welfare, Govt. of India (2011) National formulary of Unani medicine, Ministry of Health & Family Welfare, Govt. of India.
- Johansen DA (1940) Plant microtechnique. McGraw Hill Book Company Inc., New York and London: 181-186.
- Ministry of Health and Family Welfare, Government of India (1987) Physicochemical standards of Unani Formulations Part 2, CCRUM, Ministry of Health& Family Welfare, New Delhi, India.
- World Health Organization (1998) Quality control methods for medicinal plant materials, World Health Organization, Geneva, Switzerland.
- Sethi PD (1996) High performance thin layer chromatography.(1stedn), CBS Publishers and Distributers, New Delhi, India.
- Wagner H and Bladt S (1984) Plant drug analysis: A thin layer chromatography atlas, Springer-Verlag, 2nd, Germany.
- WHO (1998) Quality control methods for medicinal plant materials, WHO, Geneva.
- Anonymous (2005) Official methods of analysis. In: Horwitz W, Latimer G W (eds.). AOAC International; Maryland, chapter 3: 10-11.
- Anonymous (1997) Official analytical methods of the American Spice Trade Association (ASTA). Inc. 4th, New Jersey, USA.
Citation: Ansari SA, Khan AS, Ahmed MW, Asif M, Meena R, at al. (2020) Pharmacognostical and HPTLC Study of Unani Formulation Khamira Khas: A Cardiac Tonic. J Altern Complement Integr Med 6: 118.
Copyright: © 2020 Shoeb Ahmed Ansari, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

